1 Introduction
Recently voltammetry was used in the reduction mode to study the production of NA (nitroso-arginine), a product of endothelial NO-synthase by the endothelial cells of rat corpus cavernosum [1]. It was observed that the l-arginine pathway was used during rat penis vasodilatation in normal physiological conditions. Authors [2] reported that rat corpus cavernosum endothelium used exclusively in vivo the endothelium derived relaxing factor (EDRF) derived from the l-arginine pathway. They discovered that the cGMP pathway was the only system to mediate the penile erection in rats [2]. In rat other vascular beds, the most important relaxing factors come either from the l-arginine pathway, from prostacyclin pathway or from reactive oxygen species (ROS) pathway [3,4]. A remaining question was to know the nature of the mediator of vasodilatation in rat corpus cavernosum when the l-arginine pathway was inhibited. As soon as 1988, Taylor and Weston proposed an additional factor to EDRF which was called endothelial derived hyperpolarizing factor (EDHF) [5]. EDHF caused relaxation with simultaneous increase of the membrane potential, which differentiated it from EDRF. EDHF was described as an endothelium-derived non-nitric oxide (NO) and non-prostacyclin factor that mediated hyperpolarization of vascular smooth muscle via calcium-sensitive K+ channels aperture. Considerable works have been done to identify this factor and its true nature was still matter of debate, as seen in literature and in the recent congress of Antwerp [6]. Its nature seemed to be dependent of the vascular bed. EDHF might have a predominant role in small resistance arterioles in rats. At this time there was no information on the nature of EDHF in rat corpus cavernosum. In other species like mice, the cAMP pathway played a role in corpus cavernosum [7] and hydrogen peroxide was described in mice corpus cavernosum as an endothelium-derived relaxing factor (EDRF) and hyperpolarizing factor (EDHF) using in vitro experiments [8]. The source of hydrogen peroxide was proposed as either eNOS or NAD(P)H oxidases. The two enzymes were present in endothelial cells. NAD(P)H oxidases were present in endothelial cell membranes. It secreted superoxide [9]. Superoxide dismutase (SOD) transformed into H2O2 in the extracellular space. H2O2 was a mild oxidant using the cGMP pathway. Then H2O2 could be a serious candidate to vasodilatation in rat penis. Prostacyclins did not use the cGMP pathway and were not candidates for vasodilatation in rat penis. Studies showed a cross-talk between EDRF and EDHF in the regulation of endothelium mediating vasorelaxation [10]. The inhibition of EDRF in rat corpus cavernosum could reveal EDHF if present and detectable by voltammetry. As candidate, H2O2 was described as electroactive either in oxidation or in reduction. But in oxidation H2O2 peak potential was located near catecholamine, ascorbate or NO peaks depending of the different microelectrodes [11–13]. ROS were generally electroactive, but it is quite difficult to identify and quantify generated free radicals in vascular tissues at the same time. Amperometric techniques at fixed potential have been developed for and H2O2 with different microelectrodes [14–17]. But their selectivity for ROS in vivo is a major problem due to a fixed potential and to very low current measurements (a few pA).
Recently, reductive differential pulse voltammetry (RDPV) showed the ability to detect , H2O2 and NA using the electroactive domain in reduction that was not used with microelectrodes by others, excepted for oxygen measurements on platinum in vivo [18,19]. Here the oxygen interferences were absent, due to the carbon surface of the microelectrode, which reacted slowly with oxygen and gave a poor detection limit. RDPV gave simultaneous identification of a compound by its peak potential and quantification by the height of this peak. The sensitivity limit was the μM range with currents in nA range. The advantage of RDPV was the absence in vivo of endogen peaks, excepted NA peak if present. This is not the case in the oxidative domain where many endogen molecules are electroactive in vivo.
The present study outlines RDPV in vascular endothelium of corpus cavernosum of living rats using a micro carbon electrode during simultaneous vasodilatation by acetylcholine and inhibition of the l-arginine pathway by l-NMA. The identification of new endogen RDPV peaks was realized either in vitro by their peak potential values or in vivo by intracavernous injection of pharmacological agents acting on these peaks presence, apparition, increase and disappearance.
2 Material and methods
2.1 Materials
Acetylcholine (Ach), l-NMA, l-arginine, bradykinin, arachidonic acid, indometacin, reduced nicotinamide dinucleotide (NADH), reduced nicotinamide dinucleotide phosphate (NADPH), diethyldithiocarbamate (DDTC), catalase and SOD were purchased from Sigma (Saint-Quentin-Fallavier, France). H2O2 was from Merck (Noisy, France). Diphenyl-iodonium (DPI) was from Calbiochem (France).
2.2 Voltammetry
A microelectrode was built using a microcarbon fibre (8–12-μM diameter, Le Carbone Lorraine, France) inserted in a glass microelectrode and stretched in a microforge according to previous works [20]. It was commercially available (MFC1, Tacussel, Lyon). The reference and auxiliary electrodes were respectively Ag/AgCl and platinum. All measurements were performed with three electrode systems: one apparatus (PRG5, Tacussel, France), which was automated with relay cards and AD/DA cards, inserted in a microcomputer or an integrated apparatus (Autolab, PGSTAT 10, Roucaire, France). These two apparatus gave identical results. RDPV was performed with the following parameters: pulse height = 0.1 V, pulse delay = 0.1 s, potential swept = 0.04 V s−1. The starting potential was vs. Ag/AgCl. The swept were made in reduction. No Faraday cage was needed for measurements according to nA current range. Standardizations for H2O2 was realized in vitro in phosphate buffered saline (PBS) at 20 °C at oxygen atmospheric pressure at vs. Ag/AgCl (range 1–200 μM). With these microelectrodes, the sensitivity limit was μM for H2O2. Other compounds can be detected in reduction. For NO the limit of detection was μM at vs. Ag/AgCl. Superoxide was also detected at μM at vs. Ag/AgCl. Oxygen was undetectable under 1 mM at vs. Ag/AgCl. The observed signal in vivo for RDPV was the NA peak at vs. Ag/AcCl, as previously described [1].
2.3 Animals
Male adult rats (Sprague Dawley) were 432–544 g (Janvier, Le Genest-Saint-Isle, France). They were anesthetized with urethane (1.45 g kg−1, ip) and were on a homemade heating table during measurements. The penis was exposed and a small incision (1–2 mm) was realized. Then the voltammetric microelectrode and a micro glass-device for intracavernous injection of pharmacological agents were introduced with micromanipulators (Prior, Phymep, France) in the corpus cavernosum near the voltammetric microelectrode under binocular observation (Zeiss, France). Each drug was dissolved in PBS at 1 mM (37 °C); 40–50 μl were injected each time. DPI was dissolved in dimethylsulphoxide (DMSO). Intracavernosal injection of a compound was made at least in three different animals. Vasodilatation of penis was monitored using laser Doppler flowmetry. Briefly, a microprobe was put on the penis near the microelectrode (PF418 master probes and Periflux 4001 master, Perimed, France). It gave on line the blood flow in flow units. In a first series ( rats) RDPV was realised every minute with simultaneous laser Doppler flowmetry after intracavernous injection of acetylcholine (50 μl, 100 μM); Then 30 min later, a mixture of acetylcholine plus l-NMA was injected ic (50 μl Ach 100 μM, l-NMA 1 mM). In a second series ( rats), RDPV was realised every minutes after intracavernous injection of the same mixture of acetylcholine and l-NMA; then at 30 min a compound () was injected ic. In each series RDPV was monitored 2 h in vivo. Each rat received one compound and this compound was tested on three rats.
3 Results
In the first group, NA peak was present after acetylcholine injection as previously described [1]. The simultaneous injection of acetylcholine and l-NMA gave the disappearance of NA peak with appearance of a new peak at vs. Ag/AgCl (see Fig. 1). This new peak was stable during the experiment. Its peak potential is identical to the peak value of H2O2. According to standardization, H2O2 concentration was () in vivo. Laser Doppler flowmetry gave blood flow units multiplied by 10.9 compared with the basal value at 6 min after ic injection of Ach and l-NMA. The voltammetric peak height and blood flow units were correlated during measurements (, , measuring time: 0–15 min after ic, rats).

An example of voltammetric signals in a rat corpus cavernosum. (A) Basal signal after ic injection of acetylcholine. (B) Signal 1 min after ic injection of a mixture of acetylcholine and l-NMA. (C) Signal 5 min after ic injection of a mixture of acetylcholine and l-NMA.
In the second group, the identity of the peak and the biological system responsible were studied. Results are summarized in Table 1. Briefly, in vivo injection of H2O2 near the microelectrode tip in corpus cavernosum enhanced dramatically this peak. Injection of catalase made the peak disappeared with appearance of NA peak (see Fig. 2A). This peak at was attributed to NA, a product of eNOS, according to previous studies [1,21–23]. SOD had no effect on the H2O2 peak. l-Arginine made the H2O2 peak disappear and the NA peak come again. DDTC injection made this H2O2 peak disappear and a new peak at vs. Ag/AgCl appeared (see Fig. 2B). This potential value corresponded to as previously demonstrated in vitro [19]. NAD(P)H increased H2O2 peak. DPI made the H2O2 peak disappearance. Two vasodilatators in penis bradykinin and acetylcholine enhanced the H2O2 peak when eNOS was inhibited. Indometacin, a cyclo-oxygenase inhibitor and arachidonic a prostanoid precursor had no effect on the H2O2 peak.
Effect of intracavernosal injection of various compounds on hydrogen peroxide concentrations
| Various compounds | H2O2 concentration (% initial value). Time 5 min after ic | Half-life of H2O2 disappearance or apparition (sec) after ic | Nature of other peak after H2O2 disappearance |
| Catalase | 0 | 12 | NA |
| SOD | unchanged | zero | no other peaks |
| l-Arginine | 23 | 244 | NA |
| H2O2 | 256 | 123 | no other peaks |
| NADPH | 154 | 487 | no other peaks |
| NAD | 141 | 539 | no other peaks |
| DDTC | 0 | 58 | superoxide |
| Acetylcholine | 137 | 282 | no other peaks |
| DPI | 8 | 185 | no other peaks |
| Bradykinin | 146 | 220 | no other peaks |
| Indometacin | unchanged | zero | no other peaks |
| Arachidonic acid | unchanged | zero | no other peaks |
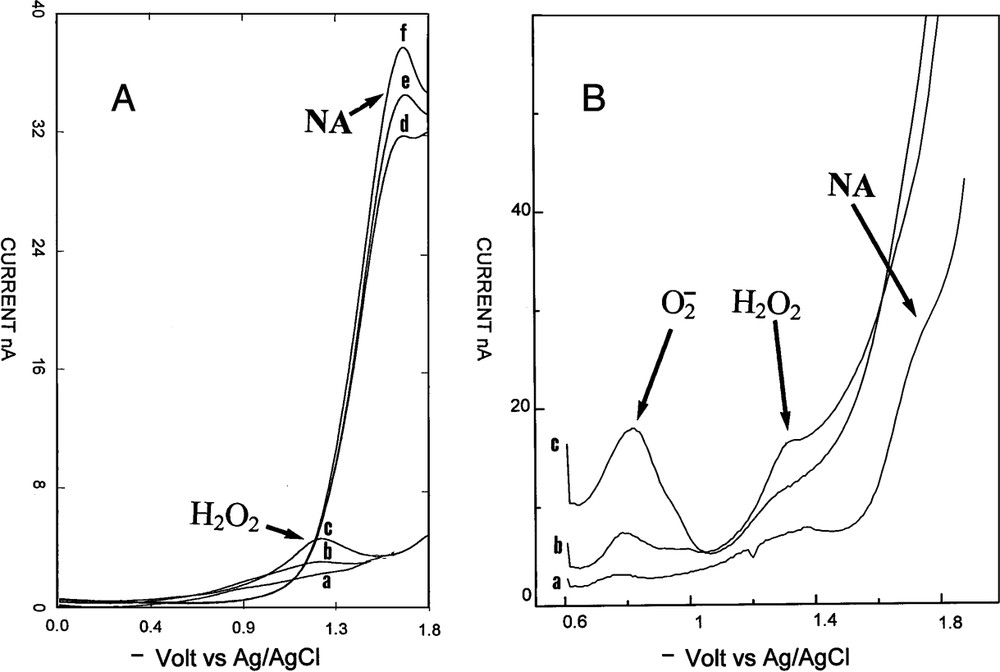
An example of variation of hydrogen peroxide in a rat corpus cavernosum. (A) Voltammetric signal after injection of acetylcholine and l-NMA: (a) before injection, (b) 2 min, (c) 5 min. Then injection ic of catalase: (d) 1 min, (e) 2 min, and (f) 3 min after injection. (B) Simultaneous injection ic of acetylcholine, l-NMA and DDTC: (a) before injection, (b) 1 min, (c) 2 min after injection.
4 Discussion
4.1 Identification of the endogen peak
RDPV was realized on line in rat corpus cavernosum. The microelectrode tip was located in the extra vascular space in contact with the endothelial cells of the sinus. The microelectrode detected compounds at the contact of its active tip. When the microelectrode tip was removed of the cell surface contact the signal immediately disappeared. Measurements corresponded to punctual concentrations at the interface of the microelectrode tip and endothelial cells membranes. A substance was detected when its concentration was superior to the sensitivity limit of RDPV for this molecule. Before penis-induced vasodilatation, no signals were observed in RDPV, indicating either an absence of electroactive compounds or an insufficient concentration of electroactive products. This is highly favourable for the study of vasodilatation by voltammetry. Simultaneous injection of acetylcholine and l-NMA produced vasodilatation followed by the laser Doppler apparatus and appearance of a new peak. Its precise potential localization permitted to attribute this signal to H2O2. It was observed that molecules had the same peak potential either in vitro or in vivo. According to the fact that only one endogen molecule, NA, have been observed in vivo as electroactive in reduction on carbon microelectrode [18–20], the identification of H2O2 is quasi certain. Injection of H2O2 near the microelectrode tip confirmed this identity. The fact that injected H2O2 induced vasodilatation was another important point. The third proof was found by the injection of catalase, which was specific for H2O2. Catalase gave an immediate disappearance of the endogenous peak. Microelectrodes were easily standardized in vitro for H2O2 in PBS without oxygen interferences according to an endogen level below the sensitivity limit. The observed concentrations in rat corpus cavernosum (0.115 μM) during vasodilatation were close to the concentrations observed in rat brain for H2O2 using microdialysis [24,25]. The stability, the repeatability, and the high current values of these peaks with always the same potential permitted to follow and quantify H2O2 concentrations in the extracellular space without any problems.
4.2 Biological origin of H2O2
The origin of H2O2 was studied by injection of pharmacological products. H2O2 production in vivo was well known and different enzymatic origins could be responsible of its secretion. Xanthine oxidase, eNOS, P450 enzymes and NAD(P)H oxidases were candidates to synthesize H2O2 in vivo in various cells. In endothelial cells eNOS and NAD(P)H oxidases have been described to produce H2O2. Precedent work on eNOS demonstrated that was not produced at a detectable level by voltammetry in pure enzymatic preparation, even in the absence of the substrate. The enzyme, eNOS, was not the source of extracellular release of H2O2 in this system, as observed in endothelial cells [1,7]. Intracavernous injection of DDTC, a SOD inhibitor, gave H2O2 peak disappearance along with the appearance of a new peak. This new peak corresponded to , which was identified by its peak potential. This was the first time that was observed in vivo by RDPV. This was due to the high concentration value of H2O2 in vivo according to the equimolar transformation of into H2O2 by SOD. SOD injection had no effect on H2O2 peak in vivo. These facts could be explained by the fact that was produced inside the cells and that H2O2 was only present in the extracellular fluid where is located the microelectrode [26]. Another point was the presence of extracellular SOD, which immediately scavenged to H2O2 without needing an exogenous source of SOD. DDTC experiments showed that was the source of H2O2. DDTC can penetrate into endothelial cells. It is not the case for injected SOD. Substrates and inhibitor of NAD(P)H oxidases were tested. DPI, a specific NAD(P)H oxidases inhibitor, inhibited immediately this production. This indicated an enzymatic synthesis for H2O2 by NAD(P)H oxidases. NADH and NADPH injections enhanced the H2O2 peak, indicating NAD(P)H oxidases system for H2O2 production. The vasodilatation measured by laser Doppler flowmetry supported the idea that H2O2 was a vasodilatator in the corpus cavernosum bed. This was observed in other rat vascular tissues. H2O2 was found in pulmonary arterial walls where it is secreted by endothelial cells using NAD(P)H oxidases localized in the cells membrane [27,28]. It was observed in calf or bovine pulmonary arteries a cyclic guanosine monophosphate (cGMP)-mediated relaxation occurring through an increased intracellular H2O2 generation. It was also observed in rat aorta [29]. NA was not the sole agent of penile vasodilatation, H2O2 is another mediator of vasodilatation using cGMP pathway. Two molecules of the prostanoid system were tested. Indometacin and arachidonic acid had no effects on the H2O2 peak, eliminating the prostanoid pathway for its production.
4.3 Proposed mechanism of vasodilatation in rat penis
In normal physiological conditions the l-arginine pathway was the sole used for vasodilatation in rat corpus cavernosum. When this system was blocked, another system using endothelial NAD(P)H oxidases was used with production of H2O2, a potent physiological vasodilatator. Intracavernous injection of l-arginine made H2O2 disappear with simultaneous apparition of NA, the product of eNOS. The presence of l-arginine imposed the synthesis of NA. NA is the mediator of vasodilatation in normal conditions [1]. There was a switch mechanism between the two systems depending of the availability of eNOS to synthesize NA. An absence of l-arginine or the inhibition of eNOS switched the eNOS system to the NAD(P)H oxidases system. The ability of voltammetry to monitor NA, H2O2 and was very useful for continuous on line measurements in tissue. In mice, H2O2 was presented as EDHF and it could be the case in rat corpus cavernosum. NA was presented as EDRF in the same tissue; if true, voltammetry could monitor simultaneously EDRF and EDHF in a living animal. Rat corpus cavernosum was a very useful model for such studies.
5 Conclusion
In conclusion, this study showed on-line in vivo determination and identification of H2O2. Voltammetry demonstrated that was the primary source of endogenously produced vasoactive H2O2. The NAD(P)H oxidases endothelial system was the source of this endogen vasodilatator, which replaced the eNOS system when it was unable to function normally.


