Version française abrégée
Les pratiques agricoles influencent fortement la distribution et l'abondance des espèces dans les agrosystèmes. Il est communément admis que l'agriculture biologique est plus favorable à la biodiversité et procure de meilleurs habitats d'alimentation pour l'avifaune que l'agriculture conventionnelle. Des travaux récents en Méditerranée émettent cette hypothèse pour la riziculture, qui s'est développée dans des régions de haute importance pour l'avifaune aquatique. La culture conventionnelle, qui se caractérise principalement par l'usage de fertilisants chimiques et de divers pesticides interdits en culture biologique, est en effet susceptible de modifier les réseaux trophiques des rizières. L'impact des deux types de pratiques culturales a été testé sur les communautés macroinvertébrées des rizières de Camargue. Dix parcelles cultivées de façon biologique et neuf de façon conventionnelle ont été échantillonnées sur trois périodes lors de la saison culturale 2001. Trente-neuf familles d'invertébrés ont été recensées dans les parcelles biologiques et 37 dans les parcelles conventionnelles, pour un total de 40 familles. Les Tubificidae ont uniquement été rencontrés dans les parcelles conventionnelles et six autres familles (Erpobdellidae, Lymnaeidae, Physidae, Planorbidae, Dryopidae et Psychodidae) étaient fortement dominantes dans ces parcelles. Sept familles (Baetidae, Caenidae, Coenagrionidae, Libellulidae, Corixidae, Dysticidae et Hydrophilidae) étaient plus abondantes en culture biologique. Les effectifs de neuf des 11 familles de diptères, dont les Chironomidae, n'étaient pas différents entre les deux traitements. Les invertébrés prédateurs, essentiellement représentés par les Dysticidae, Coenagrionidae et Libellulidae dans les échantillons, représentaient 18, 40 et 70 % des communautés dans les parcelles biologiques en juin, juillet et août respectivement et moins de 1,5, 8 et 12 % dans les parcelles conventionnelles, où les communautés étaient dominées par des herbivores stricts.
Deux des variables explicatives prises en compte pour l'analyse, la période et l'utilisation de l'insecticide fipronil sont celles qui expliquent le mieux les variations d'abondance, pour une majorité de familles. Cet insecticide, utilisé dans le monde entier contre divers ravageurs des cultures et parasites d'animaux domestiques, était en 2001 le seul à être homologué en riziculture pour lutter contre les larves de chironomes. La période au cours de la saison culturale est apparue comme le principal facteur explicatif pour les Coenagrionidae, les Libellulidae, les Baetidae et les Ephydridae. L'insecticide est le facteur dominant pour six familles et le second pour quatre autres, son impact étant négatif pour six d'entre-elles (Dysticidae, Hydrophilidae, Libellulidae, Coenagrionidae, Baetidae, Corixidae et Ephydridae). L'abondance des Tubificidae et de certains mollusques (Lymnaeidae et Physidae) est positivement corrélée à l'utilisation d'herbicides.
L'estimation de la biomasse de macroinvertébrés disponible confirme le rôle des rizières comme source de nourriture pour les oiseaux d'eau pendant la période culturale. Globalement, la biomasse invertébrée (poids sec) est trois fois plus élevée dans les parcelles conventionnelles que dans les parcelles biologique en juin (, , , ) ; deux fois en juillet (, , , ) et 1,5 fois en août (, , , ). Néanmoins, les mollusques n'entrent pas dans le régime alimentaire de la majorité des oiseaux fréquentant les rizières pendant la saison culturale, notamment les hérons groupe emblématique des rizières, qui se nourrissent essentiellement de gros coléoptères, de larves d'odonates et d'amphibiens. En conséquence, quelle que soit la période, mais surtout en début de saison, la biomasse des proies disponibles pour ce groupe est plus élevée dans les parcelles biologiques que dans les autres.
Le fipronil, insecticide non sélectif, apparaît comme le principal responsable des différences observées dans les communautés d'invertébrés entre modes culturaux. L'absence d'effet significatif des traitements sur les chironomes paraît attribuable à l'impact négatif de l'insecticide sur les invertébrés prédateurs de ces diptères.
1 Introduction
Occupying more than 40% of the European Union area, agriculture plays a substantial role in nature conservation [1–3]. The way in which farmlands are managed can strongly influence the distribution and abundance of species [4,5], and it is commonly stated that organic farming is more favourable for biodiversity and creates better feeding habitats for avifauna than conventional chemical-based cultivation [6]. This is the case for rice cultivation in the Mediterranean [7,8], which takes place in sites of highest importance for avifauna [9]. In the Camargue (Rhone River delta, southern France), one of the most important breeding grounds for waterbirds in Europe [10], drastic changes have occurred from the 1940s, inducing the loss of 40 000 ha of natural areas related to the extension of agriculture, salt exploitation, and industry [11]. Human management of most remaining wetlands, used as private hunting estates, lead to the simplification of aquatic ecosystems and a loss in diversity [11]. Nowadays, natural habitats and crops respectively cover 50% the area, with rice farming representing 16% of cultivated land. Flooded during the breeding period of birds and when natural marshes can partially dry off, rice fields complement or substitute them as feeding habitats.
However, the use of insecticides for pest control, but also of herbicides and fertilisers, can modify trophic webs and alter the development of rice field animal communities, especially invertebrates in various parts of the world [12–15]. Conventionally cultivated rice fields are thus often assumed to be of lower value as feeding habitats for birds compared to organic ones [7]. A study carried out in Camargue (Rhone River delta) showed that, in spring and summer, waterbirds feeding in rice fields were more numerous in organic plots than in the others; the authors suggested that this may be explained by differences in prey availability [16]. To test this hypothesis, the effects of both practices on fauna were compared in Camargue paddies, during the 2001 cropping season. We focused on macro-invertebrates, which constitute, with amphibian larvae, the main prey of herons, the most abundant bird group foraging in this agrosystem in spring and summer [16].
2 Material and methods
2.1 Rice cultivation in the study area
In Camargue, rice fields are usually ploughed and harrowed in January. At the end of April–beginning of May, seedling usually takes place on the same day as flooding, or within one week after. Germination starts between seven and 15 days later and plants emerge from water approximately one month after sowing. The growth of stem takes place until August and harvest generally occurs at the end of September.
Irrigation water is pumped from the Rhone River for all rice fields. Conventional culture is mainly characterized by the use of chemical fertilizers and pesticide application, which are not allowed in organic culture. In conventional fields, insecticide application occurs twice during the season: very soon after flooding for chironomid control, and late August for pyralid control. In 2001, fipronil, used worldwide to control various crop and veterinary pests [17], was the only insecticide authorised for the control of chironomid larvae in rice fields. Fipronil is applied as a seed coating. Herbicides are sprayed soon after flooding and then once or twice until mid-June; their main targets are Echinochloa spp and Cyperacea. Fungicides and anti-algae are added in some plots, at the same period.
2.2 Sampling
Macro-invertebrates were monitored in ten organic and nine conventional rice fields (1.5 to 2 ha) run by farmers. Flooding and seedling took place the last week of April and the first week of May in conventional and organic fields, respectively. All the plots were sown with indica long-A-type rice. The first N application was made at the beginning of May and repeated in July, in combination or not with P and K.
Sampling was conducted during the first week of June, July, and August. These were previously identified as key periods in terms of use by waterbirds [16]. Macro-invertebrates were collected using a square-sampler [15]. The frame of the trap (0.1 m2) was placed over eight quadrates along a transect (2 m between plots, from 5 to 19 m from the border of the field). These positions were slightly changed between the three sampling occasions. The vegetation was systematically removed, washed in the trap and discarded. Benthos and sediment (the first 2–3 cm of the layer) were pushed into the net using a broom trap [14]. Macro-invertebrates () were collected after washing on 5- and 1-mm sieves. They were preserved in 70% ethanol for laboratory identification, and were then dried at 60 °C for 72 h and weighed. During the sampling, amphibian larvae were also collected in the traps, dried and weighed.
2.3 Data analysis
Analyses were carried with STATISTICA 6.0 Statsoft. Abundance data were log-transformed, , to normalise residuals. Homogeneity of variance was assessed by Levene tests. For each macro-invertebrate family, the mean number of individuals per plot was compared between organic and conventional plots by a two-factor ANOVA (period, treatment). When differences were significant, LSD Post hoc tests were carried out. The effects of 11 parameters on abundance were also tested: period; use of insecticide; use of three different herbicide groups respectively; use of fungicide; N; P and K; water conductivity (mS); water temperature (°C); depth (cm). The ten different herbicides used in the conventional study fields were regrouped into categories (Ha, Hb, and Hc), in accordance with their combined use in the same plot: (Ha) bentazone, 2.4 MCPA, propanil, cyhalofop-butyl, carbetamide, oxadiazon; (Hb) molinate and azimsulfuron; (Hc) bensulfuron-methyl and pretilachlor. Two fungicides were also used in some of the plots, soon after flooding (5–10 days): one contains chlorothalonil, the other combines fungicide, bactericide and virucide: chlorydrate of poly (imino-imido biguanidine) and N-alkyl dimethyl benzyl ammonium. They were considered as a single modality (treated/not treated).
For each family, Akaike's Information Criterion [18] was used in order to identify parsimonious models. Only those families for which abundance was significantly different between treatments for at least one period, according to ANOVA results, were selected for this analysis. We then explored the effects of each variable on family abundance (linear regression), using a Generalized Linear Model (GLM) implemented in STATISTICA 6.0 Statsoft. Comparison of mean biomass of potential food available for waterbirds between cultural types was measured by t-tests.
3 Results
3.1 Orders and families recorded
Among the eight invertebrate orders recorded, four (Ephemeroptera, Heteroptera, Coleoptera, Odonata) were more abundant in organic fields at one of the three periods of measurement at least and two (Achaeta, Gastropoda) in conventional ones (Fig. 1). For Oligochaeta as for Diptera, there was no significant difference between treatments, whatever the period.
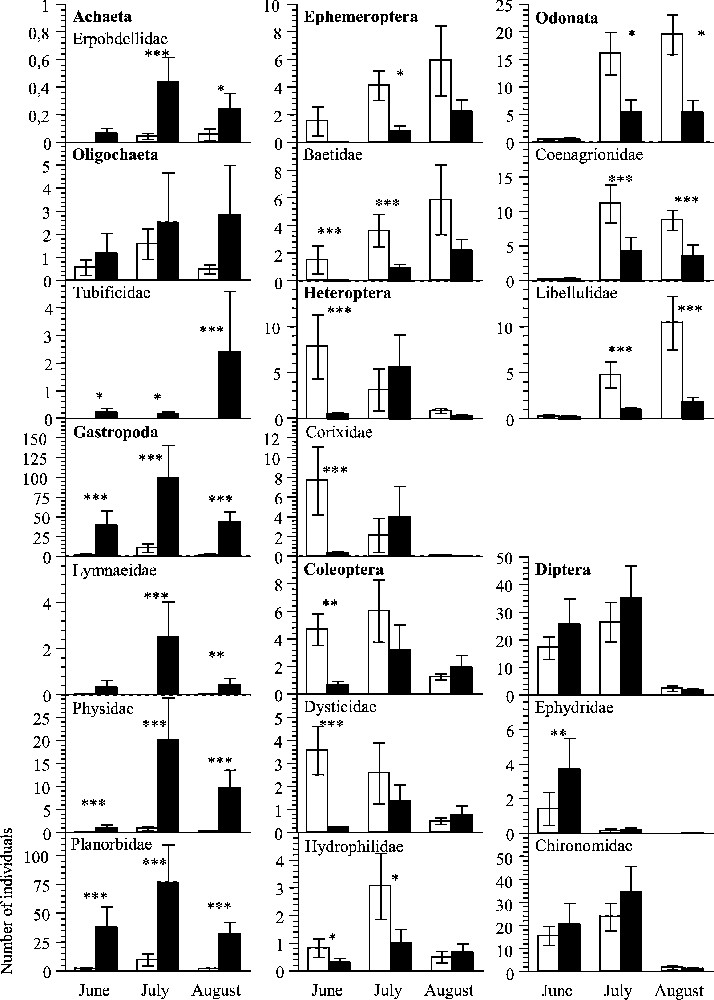
Mean abundance of invertebrate taxa in rice fields on three sampling periods. All orders recorded, the 12 families retained in the AIC, and Chironomidae are illustrated. Open bar, organic fields; black bar, conventional fields. Two-factor ANOVA and LSD Post hoc tests were carried out. Differences between pairs of treatments are significant at p<0.05 (∗), p<0.01 (∗∗), p<0.001 (∗∗∗) or not significant.
We found 39 invertebrate families in conventional plots, 37 in conventional ones and 40 in total. The number of families reached 30 and 22 in organic and conventional plots respectively, in June; 37 and 36 in July; 26 and 29 in August. For 17 families, abundance was found to significantly differ between organic and conventional plots, on one of the three sampling dates at least (Table 1). Among them, Tubificidae were only recorded in conventional plots and six other families (Erpobdellidae, Lymnaeidae, Physidae, Planorbidae, Dryopidae, and Psychodidae) were highly dominant in these plots (10 to 100 times more in conventional than in organic). In contrast, seven families (Baetidae, Caenidae, Corixidae, Dysticidae, Hydrophilidae, Coenagrionidae, and Libellulidae) were more numerous in organic plots (but always less than four times more). Nine out of the 11 Diptera families, including the Chironomidae, showed no difference in abundance between treatments.
Total number of invertebrates recorded in organic (org) and conventional (cvn) plots during the study
| Taxa | Families | Org | Cvn | Families | Org | Cvn |
| (a) Significant differences | (b) No difference | |||||
| Achaeta | Erpobdellidae | 7 | 61 | |||
| Oligochaeta | Tubificidae | 0 | 242 | Lumbricidae | 178 | 301 |
| Gastropoda | Lymnaeidae | 2 | 262 | Succineidae | 4 | 10 |
| Physidae | 78 | 2507 | ||||
| Planorbidae | 900 | 11 877 | ||||
| Ephemeroptera | Baetidae | 787 | 263 | |||
| Caenidae | 43 | 5 | ||||
| Heteroptera | Corixidae | 705 | 333 | Gerridae | 103 | 131 |
| Veliidae | 9 | 35 | Hydrometridae | 6 | 0 | |
| Mesoveliidae | 7 | 6 | ||||
| Pleidae | 17 | 2 | ||||
| Coleoptera | Dryopidae | 3 | 54 | Elmidae | 7 | 7 |
| Dytiscidae | 476 | 191 | Gyrinidae | 6 | 0 | |
| Hydrophilidae | 314 | 161 | Haliplidae | 29 | 7 | |
| Helophoridae | 11 | 8 | ||||
| Hydrochidae | 1 | 1 | ||||
| Hydroscaphidae | 1 | 1 | ||||
| Odonata | Coenagrionidae | 1442 | 668 | Aeshnidae | 32 | 31 |
| Libellulidae | 1111 | 250 | ||||
| Diptera | Culicidae | 131 | 62 | Dolichopodidae | 2 | 7 |
| Ephydridae | 111 | 311 | Ceratopogonidae | 23 | 9 | |
| Psychodidae | 1 | 63 | Chironomidae | 2929 | 4470 | |
| Limoniidae | 12 | 1 | ||||
| Rhagionidae | 48 | 42 | ||||
| Sciomyzidae | 2 | 5 | ||||
| Stratiomyidae | 4 | 3 | ||||
| Tabanidae | 3 | 19 | ||||
| Tipulidae | 2 | 5 | ||||
| Trichoptera | Leptoceridae | 1 | 0 |
The strong difference in numerical abundance found for some families between organic and conventional plots had an impact on functional composition. Given the diet of each and every species recorded in the samples, invertebrate predators were essentially represented by Dysticidae, Coenagrionidae, and Libellulidae. They formed 18, 40 and 70% of the communities in June, July and August, respectively, in organic plots. For the same months, they represented respectively less than 1.5, 8 and 12% in conventional plots, where the communities were dominated by strict herbivores during the three periods.
3.2 Factors influencing the abundance of macro-invertebrate families
For five families (Caenidae, Veliidae, Dryopidae, Culicidae, Psychodidae), the value given by GLM was lower than 0.10. We therefore considered that their abundance could not be explained by the model and did not take them into account (Table 2). Two variables, ‘period’ (especially between the 1st and the 3rd samplings) and ‘fipronil insecticide use’, appeared to have a major influence on abundance (Table 2). The sampling period was the main explanatory factor for Coenagrionidae and Libellulidae, with few individuals recorded during the first period of measurement, and for Baetidae and Ephydridae almost absent during the 2nd and the 3rd periods. Insecticide treatment appeared to be the main factor influencing half of the twelve families and the second after the ‘period’ for three others. Pesticide use was negatively correlated with the abundance of six of them: Baetidae, Corixidae, Dysticidae, Hydrophilidae, Coenagrionidae, and Libellulidae.
Beta regression coefficients of variables selected by GLM and of the models
| Taxon | 1–3 | 2–3 | Ins | N | PK | Ha | Hb | Hc | Fg | Cd | Wh | |
| Achaeta | ||||||||||||
| Erpobdellidae | −0.21 | 0.23 | 0.20 | −0.18 | −0.23 | 0.18 | ||||||
| Oligochaeta | ||||||||||||
| Tubificidae | 0.15 | 1.19 | −0.14 | 0.31 | 0.31 | 0.45 | 0.67 | 0.32 | ||||
| Gastropoda | ||||||||||||
| Lymnaeidae | −0.17 | 0.52 | −0.20 | 0.40 | 0.36 | −0.32 | −0.17 | −0.11 | 0.15 | 0.51 | ||
| Physidae | 0.32 | −0.36 | −0.13 | 0.32 | 0.33 | 0.40 | −0.38 | 0.11 | 0.50 | |||
| Planorbidae | −0.21 | 0.40 | −0.13 | −0.13 | 0.26 | −0.18 | 0.25 | −0.34 | 0.20 | 0.56 | ||
| Ephemeroptera | ||||||||||||
| Baetidae | 0.29 | −0.26 | −0.23 | 0.14 | −0.24 | 0.21 | 0.15 | 0.23 | ||||
| Heteroptera | ||||||||||||
| Corixidae | −0.18 | −0.11 | −0.24 | 0.15 | 0.18 | 0.21 | 0.19 | 0.21 | ||||
| Coleoptera | ||||||||||||
| Dysticidae | −0.17 | −0.65 | 0.22 | 0.22 | 0.27 | 0.16 | 0.11 | 0.10 | 0.22 | |||
| Hydrophilidae | 0.19 | −0.36 | −0.53 | 0.22 | 0.29 | 0.18 | 0.19 | |||||
| Odonata | ||||||||||||
| Coenagrionidae | 0.56 | −0.31 | −0.34 | −0.20 | −0.27 | 0.29 | −0.14 | −0.16 | 0.48 | |||
| Libellulidae | 0.50 | −0.12 | −0.42 | −0.16 | −0.10 | −0.21 | 0.18 | −0.12 | −0.11 | 0.10 | 0.42 | |
| Diptera | ||||||||||||
| Ephydridae | −0.56 | 0.20 | −0.21 | −0.11 | 0.21 | 0.24 | 0.21 | 0.17 | −0.12 | 0.13 | 0.31 |
Herbicides and fungicides were also found to play an important role in the abundance of some families. The use of each and every herbicide group was highly positively correlated with the abundance of two mollusc families (Lymnaeidae and Physidae) and of Tubificidae. On the contrary, fungicides were found to have a strong negative impact on Lymnaeidae and Physidae.
3.3 Food biomass available for birds
Throughout the cultural cycle, the total biomass (dry weight) of macro-invertebrates collected remained significantly higher in conventional than in organic plots, although the difference between treatments decreased with time (Table 3). Total macro-invertebrate biomass was three times higher in conventional (cvn) than in organic plots (org) at the beginning of June (, , , ); it was twice as high at the beginning of July (, , , ) and 1.5 times higher at the beginning of August (, , , ).
Total biomass (dry weight in kg/ha) of orders per period and cultural type
| Period | Organic plots | Conventional plots | ||||
| 1 | 2 | 3 | 1 | 2 | 3 | |
| Achaeta | 0 | 0.2 | 0.3 | 0.4 | 2.8 | 1.7 |
| Oligochaeta | 7.1 | 20.7 | 6.2 | 17.0 | 36.6 | 45.8 |
| Gastropoda | 4.5 | 26.2 | 4.4 | 110.8 | 281.5 | 133.4 |
| Ephemeroptera | 1.0 | 2.8 | 4.1 | 0.1 | 0.6 | 1.8 |
| Heteroptera | 13.6 | 5.4 | 1.4 | 0.8 | 10.8 | 0.7 |
| Coleoptera | 16.5 | 21.2 | 4.4 | 2.7 | 12.3 | 8.3 |
| Odonata | 2.9 | 100.3 | 121.6 | 3.0 | 38.1 | 41.4 |
| Diptera | 10.8 | 16.6 | 1.4 | 18.0 | 24.6 | 1.3 |
Considering that gastropods are not preyed upon by the majority of birds foraging in rice fields during spring and summer, i.e. herons [8], we repeated the calculation without taking this group into account. When gastropods were excluded, the food biomass available for water birds was then equivalent between treatments at the beginning of the season (, , , ), but 50% higher in organic plots in July (, , , ) and August (, , , ).
Herons are essentially feeding on large Coleoptera, Odonata and amphibian larvae in this habitat [7,16,19]. When the same analysis was restricted to Coleoptera and Odonata, the biomass available for birds was then four times greater in organic than in conventional in June (, , , ) and twice in July (, , , ) and August (, , , ). The addition of amphibian larvae biomass representing respectively 4% and 25% in conventional and organic rice fields, to that of Coleoptera and Odonata, accentuated the difference.
4 Discussion
Strong differences in the abundance of the most numerous families were registered between organic and conventional plots. For the majority of invertebrate families, especially predators, insecticide treatment was found to be the main explanatory factor. The diminution of differences between treatments observed for families as Dysticidae and Baetidae at the end of the cultivation period, due to an increased abundance in conventional plots (Fig. 1), may then be explained by the remanence effect of the insecticide, estimated to 20 days [17]. On the other hand, a direct impact of the insecticide on the taxa, which reach a peak of abundance late in the season, such as Odonata, is unlikely. Favourable trophic conditions in organic rice fields the previous months could therefore explain the difference observed between treatments in July and August, as Coleoptera and Ephemeroptera are dominant prey in the diet of Odonata [15].
The reduction of predators in treated fields, may have given rise to cascade effects [20]. This could stem from a diminution of prey abundance, induced by an increase in primary consumers competition. The increase in competition for resources from gastropods toward other grazers could in turn influence the limitation of predators feeding on grazers, such as Coleoptera. A similar mechanism could explain the maintenance in August of strong differences in Achaeta densities between organic and conventional plots. Studies showed that an increase in the number of Erpobdellidae (only Achaeta family recorded in this study), could be correlated with an increase in the abundance of their prey [21], notably Tubificidae [22]. Pesticides, by limiting the predator number, could have indirectly favoured gastropods (Lymnaeidae, Planorbidae and Physidae), also known to be unaffected by both insecticides [23] and herbicides [24].
Gastropoda together with Diptera larvae dominated the conventionally cultivated plots as previously observed in the Ebro delta rice fields [25]. One of the consequences was a marked difference in the functional composition between the two cultivation practices carried out in August, although differences between conventionally and organically farmed fields tapered off along the cultivation period. The ratio predators/non predators reached 3 in organic plots, but less than 0.08 in conventional ones. The increase of this ratio with time from flooding date corresponds to observations made in other types of temporary wetlands [26] and has already been noticed in Camargue rice fields [15]. The proportions of predators in organic rice fields are comparable to those observed in macro-invertebrate communities of natural temporary ponds [27].
Predators are considered as a key group in the rice field invertebrate assemblages [15,28], and their reduction may lead to an increase in the density of their prey [14]. As the toxic impact of fipronil on Diptera cannot be questioned [17,29], this reduction of predators may explain the surprising lack of difference in Chironomid abundance between organic and conventional fields. Similar results observed for this group with other non selective pesticides in rice fields [13] support this hypothesis.
The estimation of available food biomass confirmed the potential role of rice fields as feeding areas for waterbirds, throughout the cultivation period [9,30]. When all macro-invertebrates are considered, the hypothesis previously raised of a lower value of conventional rice fields as foraging habitats for avifauna compared to organic ones [7,16] cannot be ascertained by our study. The total biomass of macro-invertebrates collected was higher in conventional plots and especially during the breeding season when the demand to feed the chicks is maximal. However, prey biomass available for herons, the most abundant birds feeding in this agrosystem, was more abundant in organic plots than in the others. Moreover, the dominance of some invertebrate groups in conventional plots may have consequences on other aspects of community functioning. For example, the high abundance of snails, some of which are intermediate hosts of trematodes, could increase the rate of parasitism in birds the definitive hosts. These links between parasitism, ecosystems and human activities have been highlighted in a recent review [31].
The results of our study on macro-invertebrate assemblages in rice paddies are obviously insufficient to clarify fully the respective advantages and disadvantages of organic and conventional cultivation practices. However different studies have already shown the negative effects of pesticides on aquatic communities, either in natural marshes sited within an agricultural zone [32] or in rice fields [14,33]. The necessity to maintain treatment levels of chemicals (insecticides, herbicides, fertilizers) as high as they are now in the Camargue, has been widely questioned by agronomists for years. Conventional rice cultivation is known to be associated with particular problems [34,35] that cannot be excluded by pesticides authorized for rice cultivation in the Camargue currently. Moreover, contaminants originating from rice cultivation but which are no longer authorised, are still regularly found at high levels in strictly protected and/or adjacent sites, with sometimes damaging consequences [36], and are also found in the trophic chain [36,37].
Our results illustrate how an underestimation of the role of key species [38,39], in this case predatorous invertebrates [14,28], can potentially have not only ecological, but also agronomic consequences. The insecticide, identified as largely responsible for the differences observed in macro-invertebrate assemblages between treatments, was nevertheless found to be inefficient in the control of its desired target, as its negative impacts on predators counterbalance its direct effect.
Acknowledgements
This research was funded by the Sansouïre and the MAVA Foundations and the ‘Centre français du riz’. We would like to thank J.-C. Mouret and R. Hammond, who provided us with data on field management.


