1 Introduction
Excess fat mass, which is characterized by an increase of fat cell size and number, is accompanied by the propensity of obese individuals to exhibit various features of the metabolic syndrome, i.e. insulin resistance leading to type 2 diabetes, hypertension and dyslipidemia. Moreover, based upon large scale epidemiological studies, a relationship is found between BMI and the incidence of cancer, e.g., breast cancer. Together, these observations imply that adipocytes and/or other cell types present within adipose tissue are able to cross-talk with other organs through secreted products. Although adipocytes have been recognized as secretory cells for a long time [1] and the number of secreted proteins is estimated at a few hundred [2], the cloning of the ob gene and the role of leptin [3,4] have definitely assessed adipose tissue as an endocrine organ. As we will see below, alterations in the production of leptin and other secreted proteins have substantial effects on body adiposity and components of the metabolic syndrome. Recently, the importance of macrophages in the adipose tissue of obese animals and individuals has been recognized and has increased our understanding of the initiation of a metabolic syndrome and the cross-talk within adipose tissue [5–9].
Another important aspect of adipose tissue biology is that adipocyte precursor cells are endowed with secretory properties whose products, associated with circulating hormones, participate in their own differentiation process, termed adipogenesis. Lastly, it is now recognized that the stromal-vascular compartment of adipose tissue contains various precursor cells and stem cells which give rise to different lineages in vitro and in vivo. This may ultimately be responsible for the self-renewal of adipocyte progenitor cells observed when sub-cutaneous adipose tissue expands continuously, as is the case in patients suffering from morbid obesity.
2 Secreted factors from preadipocytes and adipogenesis
The circulating hormones implicated in adipogenesis have been characterized and shown to be active in murine and human pre-adipocytes, e.g., glucocorticoids and insulin [10]. However, extracellular factors secreted from pre-adipocytes and triggering intracellular signalling pathways are also implicated in the early events of adipogenesis. In vitro studies mainly performed with murine pre-adipocyte clonal lines have shown that three redundant ligand/receptor systems are functional and concur to the up-regulation of CAAT/enhancer binding proteins (C/EBPs) β and δ whose gene invalidation in vivo impairs severely adipose tissue formation [11] (Fig. 1). The first system described involves prostacyclin which, after synthesis from arachidonic acid, is released and binds to a cell surface receptor IP-R. In the presence of arachidonic acid, cycloxygenase inhibitors (aspirin, indomethacin) as well as antibodies added externally and directed toward prostacyclin decrease by 40–70% adipogenesis [12–15]. Thus prostacyclin is able, after secretion, to act as a paracrine/autocrine effector in this process. Of note is carbacyclin, a stable analogue of prostacyclin, which promotes ex vivo and in vivo the formation of adipocytes within a few hours [16].
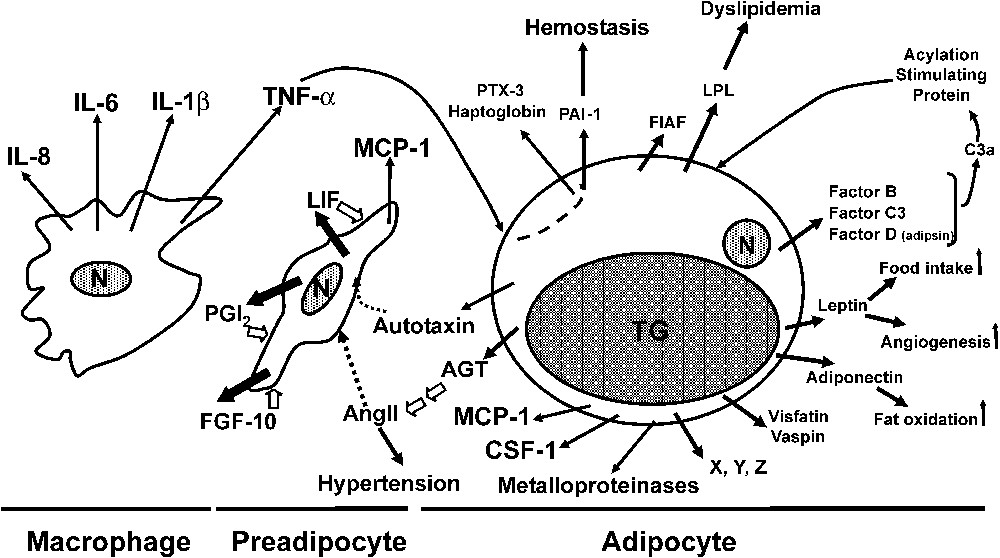
Secreted factors from preadipocytes and adipocytes involved in adipogenesis and the metabolic syndrome. AGT, angiotensinogen; AngII, angiotensin II; CSF-1, colony stimulating factor-1; FIAF, fasting-induced adipose factor; FGF-10, fibroblast growth factor-10; IL, interleukin; LIF, leukaemia inhibitory factor; LPL, lipoprotein lipase; MCP-1, monocyte chemoattractant protein-1; N, nucleus; PAI-1, plasminogen activator inhibitor-1; PGI2, prostacyclin; PTX3, pentraxin 3, TNF-α, tumour necrosis factor-α; TG, triglycerides. Intracellular signalling pathways are indicated by white arrows.
Prostacyclin, and its stable analogue carbacyclin, are able to induce a rise in cAMP levels and the activation of the protein kinase A (PKA) pathway. Most importantly, after differentiation, prostacyclin is no longer produced and IP-R appears non-functional [15]. The second ligand/receptor system involves leukaemia inhibitory factor (LIF) and LIF receptor which are also developmentaly regulated. Pre-adipocytes, but no longer adipocytes, secrete bioactive LIF, and an antagonist of LIF receptor inhibits by ∼60% adipogenesis. Early expression of C/EBPβ and C/EBPδ is rapidly stimulated following exposure to LIF through the activation of the ERK pathway [17].
The third ligand/receptor system involves secreted fibroblast growth factor-10 (FGF-10) which, after binding to its cognate cell surface receptor [18], up-regulates C/EBPβ expression. Thus it is clear that extensive redundancy is taking place to ensure adipogenesis, in accordance with the need of adipose tissue to ensure physiological functions. In vivo data are in good agreement with these in vitro observations. Prostacyclin receptor knockout mice appears as a key player during the gestation/suckling period in promoting the enhancing effect of linoleic acid (C18:2 ), a precursor of arachidonic acid and thus of prostacyclin, on fat tissue development [19]. Not unexpectedly, a lack of LIF gene expression does not prevent this development as LIF-related cytokines, such as interleukin-6 (IL-6), cardiotropin-1 (CT-1) and ciliary neurotrophic factor (CNTF), can compensate in LIF-knockout mice by acting through the LIF receptor which recognizes these various ligands. Unfortunately, invalidation of the LIF receptor gene is lethal, precluding any study on adipose tissue development. Lastly, the development of this tissue is markedly reduced in mice invalidated for the FGF receptor gene [18]. The paucity of data, if any, on the factors secreted by human pre-adipocytes, prevents the drawing of any conclusion similar to rodent data. However, the importance of the linoleic acid/arachidonic acid/prostacyclin pathway in excessive adipose tissue development is suggested by the striking relationship observed during the last four decades between the dramatic increase in both the linoleic and arachidonic content of ingested fats and the increasing prevalence of overweight and obesity in infants, i.e. at a time where a positive energy balance is not yet an ongoing process [20,21].
3 Secreted factors from adipocytes
An amazing number of proteins and other factors secreted from adipocytes have been characterized over the last fifteen years. However, we will limit our discussion to the secreted factors which have been shown to play a role in the development of a metabolic syndrome and which participate in the cross-talk between cells within adipose tissue and which are implicated in adipose tissue expansion. Secretion of pro-inflammatory proteins will be briefly mentioned as they are discussed elsewhere in this issue.
3.1 Leptin
Leptin (16 kDa) is predominantly but not exclusively expressed in adipocytes, the subcutaneous adipose depot being the major source. Among adipokines secreted from fat cells, its key role is to provide a message from the periphery to the central nervous system (CNS). As such, it regulates energy balance – intake and expenditure – by inhibiting orexigenic pathways and stimulating anorexigenic pathways of the hypothalamus as described in innumerable publications and reviews [22–27]. In vitro and in vivo data support the idea that insulin indirectly stimulates leptin secretion via its increasing effects on glucose utilization in adipocytes. Interestingly, when high-fat meals are compared with high-carbohydrate/low-fat meals, leptin levels are reduced. This suggests a mechanism by which a high fat/high caloric diet favors overconsumption of calories and the subsequent weight gain.
Leptin levels are known to be associated with fat mass and are elevated in obese animals and individuals. Thus leptin resistance rather than the lack of circulating leptin occurs and explains the reasons why leptin therapy in obese patients has been unsuccessfull. It is now well accepted that the key role of leptin in the regulation of energy homeostasis is to function as a signal of negative energy balance and low energy stores. In the case of leptin deficiency in hyperphagic mice due to mutations in the ob gene, treatment with recombinant leptin restores metabolic, neuroendocrine, reproductive and immune functions. The presence of leptin receptor in various tissues – including the long form ObRb able to transduce the leptin-generated signal – suggests that leptin can also have direct peripheral effects in addition to central effects. This issue still remains controversial despite an impressive number of studies but it seems likely that a direct effect takes place in the immune system. However, under most experimental conditions which demonstrate peripheral effects, the levels of free circulating and bioactive leptin, i.e. not associated to the leptin transporter Ob-Re, are increased by several orders of magnitude. Consequently, it cannot be excluded that leptin could well trigger additional cytokine-related signalling pathways, e.g., IL-6, CNTF and LIF pathways. In addition to central and possibly peripheral effects, leptin has also the capacity to act locally where, similarly to vascular endothelial growth factor (VEGF), it exhibits potent angiogenic properties which contribute to adipose tissue growth [28,29].
3.2 Adiponectin
Adiponectin – also known as complement-related protein 30 (Acrp30), adipose most abundant gene transcript (apM1) and adipoQ – is a 30 kDa protein secreted from adipocytes and present in the circulation as dimer/trimer and higher-order complexes [24,30]. The adiponectin monomer contains a collagenous tail and a globular head domain. The subunits are linked through intermolecular disulfide bonds. Of note, a mutant form of adiponectin, lacking the critical cysteine residue (Cys39Ser) in the collagenous domain, exhibits higher bioactivity than the wild-type form [31]. Contrary to leptin, adiponectin levels are decreased in obese animals and patients. Adiponectin is a multifunctional protein which exerts pleiotropic insulin-sensitizing effects against cardiovascular disease. It lowers hepatic glucose production [32] and increases glucose uptake and fatty acid oxidation in skeletal muscle [33]. When ob/ob (leptin deficient) mice are overexpressing the Cys39Ser mutant form of adiponectin, the animals remain morbidly obese but exhibit normal glucose and lipid levels, emphasizing that adiponectin plays a major metabolic role at the periphery. Clearly, use of adiponectin for treatment of the metabolic syndrome is of great interest but relies on unanswered questions regarding the relationships between its structure(s) and function(s). For instance, it cannot be excluded that minor changes in total circulating levels routinely measured by commercial kits may correspond to more important changes in those of bioactive form(s) of adiponectin.
3.3 Resistin
Resistin is a 10 kDa protein secreted from rodent adipocytes which has been implicated in the development of insulin resistance [34]. In humans, contrary to rodents, macrophages appear as the main source [35]. Although normalization of resistin levels through antisense technology restores insulin sensitivity in mice, its functional role in humans remains elusive and requires further studies.
3.4 Lipoprotein lipase
Lipoprotein lipase (LPL) is a 55 kDa enzyme which can be considered as the prototype of secreted proteins from adipocytes [36]. Trying to detail its properties and the regulation of its activity is out of the scope of this review. LPL is a glycoprotein secreted by exocytosis from intracellular vesicles. Of note, insulin up-regulates LPL mainly at a post-translational level and calcium triggers its folding to active dimers [37]. Following transendothelial transport, it binds to proteolycans on the luminal side of the endothelium and becomes fully active in association with apolipoprotein C2 present on the surface of triglyceride-rich lipoprotein particles. LPL then gives rise to monoglycerides and fatty acids. Although not proven, LPL could act in concert with the acylating stimulating protein (ASP), thought to control fatty acid levels in the interstitial space, i.e. between endothelial cells and adipocytes (see below).
3.5 Acylation stimulating protein
The acylation stimulating protein (ASP) is a 8.9 kDa protein produced by adipose tissue after secretion from adipocytes of complement factor C, factor B and factor D (adipsin). Successive proteolytic events result in the formation of C3a which then gives rise to C3a desArg termed ASP. Although ASP is present in the circulation, its primary action appears local. In vitro, it stimulates triglyceride synthesis and glucose transport in adipocytes whereas in vivo C3 knockout mice, unable to produce ASP, exhibit a delayed post-prandial lipid clearance; these animals are hyperphagic but resistant to a high-fat diet, show increased energy expenditure and improved insulin sensitivity [38]. ASP may represent a metabolic link between the local environment of adipocytes and the regulation of a key adipocyte function, i.e. triglyceride storage. Recently, increases in ASP and factor C3 associated to a decrease in adiponectin levels in obese versus non-obese children have been observed, in the absence of lipid abnormalities at that age, suggesting a predisposition towards enhanced fat storage and decreased fat oxidation in the obese children [39]. Reducing ASP production from adipocytes is of potential interest against diet-induced weight gain, but will require further studies on its nutritional regulation. Interestingly, the orphan G protein-coupled receptor C5L2 has been recently characterized as a functional receptor for ASP [40]. However, C5L2 has a rather ubiquitous tissue distribution, with the highest levels in spleen. Thus finding C5L2 antagonists to modulate triglyceride storage in adipose tissue remains an interesting but difficult challenge.
3.6 Fasting-induced adipose factor
Recently a new gene has been identified, encoding the fasting-induced adipose factor (FIAF), also known as PPARγ angiopoietin-related protein (PGAR), angiopoietin-like protein 4 (ANGPLT4) or hepatic fibrinogen/angiopoietin-related protein (HFARP). FIAF encodes a secreted, angiopoietin/fibrinogen-like protein which is not exclusively produced in adipose tissue, but highly expressed under fasting conditions [41,42]. In mouse and human blood plasma, FIAF is present as the native 45 kDa protein and predominantly in a 32 kDa truncated form though a smaller 30 kDa form can also been observed [43]. Interestingly, the FIAF protein is proteolytically processed during adipocyte differentiation and its role in regulating lipid storage in adipose tissue has recently been proposed as FIAF is a potent lipoprotein-lipase (LPL) inhibitor in vitro and in vivo. Under fasting conditions, it is assumed that the high levels of circulating FIAF impairs LPL activity at a time where the circulating levels of LPL substrates, i.e. triglyceride-rich lipoprotein particles (chylomicrons and very low density lipoproteins), are very low. Conversely, its post-prandial decrease should allow LPL to hydrolyze triglyceride substrates in adipose tissue [44].
3.7 Visfatin and Vaspin
Visfatin (52 kDa) and Vaspin (45 kDa) are two newly identified adipokines which have been reported to be highly enriched in the visceral fat of humans and mice and whose plasma levels increase during weight gain [45]. Visfatin is identical to pre-B cell colony-enhancing factor (PBEF) known to be expressed in lymphocytes present in adipose tissue, whereas vaspin is a member of serine protease inhibitor family. Both proteins have a glucose-lowering effect. In rodents, visfatin mimics the effect of insulin but, considering its plasma concentration, its contribution in lowering glycemia should be small. In humans, a detailed study on 189 subjects shows no significant correlation between plasma levels and various parameters of insulin sensitivity. Moreover, the visfatin gene expression is similar between visceral and subcutaneous adipose tissue, whereas a significant correlation exists between this expression and BMI or percent body fat [46]. Taken together, these results indicate that rodent data can hardly be extrapolated to humans, raising concern about the physiological role of visfatin in patients. The role of vaspin as an insulin-sensitizer is even more difficult to envision in humans as its protective role in the development of type 2 diabetes has only been reported in mice [47].
3.8 Deleterious secreted factors acting through systemic and local effects
Owing to its sizeable mass, adipose tissue contributes also to the massive production of various proteins implicated in the metabolic syndrome and adipose tissue expansion. Adipocytes from white adipose tissue (WAT) represent a major extra-hepatic source of angiotensinogen (AGT). WAT contains all the components of the renin-angiotensin system which gives rise to angiotensin II (AngII) [48]. Hypertension is known as a frequent complication of obesity and although the mechanisms by which fat excess leads to hypertension have remained largely unknown, increased adipose AGT production could contribute to the elevation of blood pressure in obese patients. Insights into the relationships existing between adipose AGT, fat mass and blood pressure have been obtained through the generation of transgenic mice which either over-express adipose AGT or in which AGT expression is restricted to adipose tissue. From AGT knockout mice, re-expression of AGT confined to adipose tissue leads to the presence of AGT in the blood stream and the restoration of normal blood pressure. Importantly, from wild-type mice, overexpression of AGT in adipose tissue leads to enhanced plasma AGT levels and increased blood pressure [49].
Quite recently has been developed another transgenic model in which 11-β-hydroxysteroid-dehydrogenase-type 1 is overexpressed in adipose tissue. Transgenic mice develop abdominal obesity and the main features of the metabolic syndrome. In particular, mesenteric AGT mRNA is increased, due likely to an increase in the activity of the glucocorticoid receptor as a consequence of increased corticosterone levels in situ. Blood pressure measurements indicate that the animals are hypertensive. Together, these observations implicate adipose AGT in the regulation of blood pressure and are entirely consistent with human data [50].
Among the cluster of metabolic dysfunctions, hemostatic abnormalities associated with increased PAI-1 plasma levels are also a characteristic feature. Like vaspin, PAI-1 is a member of the SERPIN family. It inhibits fibrinolysis but also exhibits complex interaction with cellular matrix (ECM) components. Importantly, it is associated with fibrosis and thrombosis in animals and humans. PAI-1 is known to be overexpressed in adipose tissue of obese mice and individuals [51]. Surgical removal of fat in obese subjects is associated with a decrease in plasma PAI-1 levels. In addition to a systemic effect in atherothrombosis, recent reports are in favour of local effects of secreted PAI-1 in adipose tissue growth. PAI-1 deficiency, or inhibition of PAI-1 by neutralizing antibodies acting exogenously, promote adipocyte differentiation [52]. This observation is consistent with the fact that PAI-1 overexpressing mice gain weight slower than their wild-type counterparts [53]. It is conceivable that local alteration of PAI-1 levels affects remodelling of ECM and adipose tissue mass by altering the number and/or size of adipocytes. In agreement with this hypothesis, metalloproteinases 2 and 9, two key enzymes implicated in the ECM remodelling, are secreted from rodent and human adipocytes and their inhibition with specific inhibitors markedly decrease adipocyte differentiation [54,55]. The role of PAI-1 in adipose tissue development remains, however, controversial, as other reports show that PAI-1 overexpressing mice do not exhibit fat mass alteration but rather a higher insulin sensitivity [56]. Together with leptin acting as a potent angiogenic factor, adipocytes are secreting other proteins which promote, at least in rodents, fat mass growth through adipocyte hyperplasia. Autotaxin is a lysophospholipase D released from adipocytes able to hydrolyze lysophosphatidylcholine (produced from preadipocytes) to lysophosphatidic acid (LPA) [57]. LPA is recognized by the endothelial differentiation gene-2 receptor which is expressed in preadipocytes and triggers their proliferation and spreading [58] (Fig. 1). Thus, in adipose tissue, a cross-talk between adipocytes and preadipocytes could be envisionned through the generation of LPA acting as a paracrine factor. Lastly, angiotensin II arising from angiotensinogen secreted from adipocytes is able, after binding to the AT2 receptor present in pre-adipocytes, to trigger prostacyclin release and adipocyte differentiation [59]. Microdialysis of periepidydimal fat pad performed in rats with AngII demonstrate a similar enhancement of fat cell formation in vivo, i.e. adipocyte hyperplasia [16]. Moreover, in mice which are both overexpressing AGT in adipose tissue and deficient in AT2 receptor, the results show that the up-regulation of fatty acid synthase does not take place, thus implicating the AT2 receptor in enhanced lipogenesis in wild-type mice [60,61].
A major recent breaktrough is the observation that macrophage accumulation in adipose tissue takes place in obese animals and individuals in proportion to adipocyte size, and that the majority of macrophages originate from bone marrow [5–9]. Monocyte chemoattractant protein-1 (MCP-1) secreted from preadipocytes and adipocytes [62,63] as well as colony stimulating factor-1 (CSF-1) [64] secreted from adipocytes are postulated to participate in the recruitment of monocyte/macrophage implicated in chronic low-inflammatory state observed in obesity (Fig. 1). Macrophages within adipose tissue are able to secrete cytokines (tumour necrosis factor-α, IL-6,...) that are thought to participate in the emergence and/or the aggravation of insulin-resistance and the metabolic syndrome (see other articles in this issue). For instance, TNF-α strongly up-regulates in adipocytes the expression of pentraxin 3 [65] and haptoglobin [66] which may play a role in hemostasis and angiogenesis, respectively.
4 Conclusions
Among the genes encoding secreted proteins, only a few dozen of them have been characterized so far and the functions of a only small proportion of these proteins are presently well documented. As adipocytes play a key role in the regulatory cascades involved in the metabolic syndrome and in the cross-talk with major organs (brain, liver, muscles), it seems reasonable to assume that novel and major discoveries should be made in a near future.


