1 Introduction
The swim-bladder nematode Anguillicola crassus (Kuwahara, Niimi and Itagaki 1974), is originally a parasite of the Japanese eel, Anguilla japonica Temminck and Schlegel. It was probably accidentally introduced into Europe in 1980 following import of infested Asian eels from Taiwan into Germany [1,2]. Later, A. crassus was transferred from its native host to the European eel, Anguilla anguilla L. and has been recorded in several European countries [3]. This parasite, qualified as a very successful colonizer, was reported, in American eel, Anguilla rostrata, in the eastern states of North America [4–6]. Thereafter, A. crassus has expanded its distribution to Africa. In fact, this nematode has been reported in Egypt [1] and in Morocco [7]. In Tunisia, the presence of A. crassus was first discovered in 1999 in the Ichkeul lagoon [8]. The presence of this parasite in Tunisia is undoubtedly the consequence of its accidental introduction during the import of freshwater fish, from Europe, when dams were stocked with fish. The nematode was introduced perhaps through the fortuitous import of live eels containing the adult nematode, or alternatively from intermediate or paratenic hosts carrying the infective parasites stages.
The focus of the present study is on the dynamic of parasitism throughout the year from one month to the next, and on the size of the hosts in the lagoons of Northeast Tunisia.
2 Materials and methods
The sampling of eels was conducted in the lagoons of Northeast Tunisia, namely Ichkeul, Bizerte, Ghar El Melh, and Tunis. The Ichkeul lagoon communicates with the sea via the Bizerte lagoon. The other lagoons open directly onto the sea via channels. The Bizerte lagoon is the deepest (12 m). The other lagoons are on average about 1.5 m in depth.
A total of 878 eels (66–79 specimens per month) (Fig. 1) were examined from December 2003 to November 2004. The materiel was studied as soon as possible after capture. The total length and weight of each eel were recorded. The length of eels varies from 20 to 70 cm. The eels were opened by a longitudinal incision along the belly. The intestinal tract was cut at each end and lifted out to reveal the swim bladder. The swim bladder was dissected out, placed in a Petri dish, split longitudinally and carefully examined under a binocular microscope. Nematodes collected were identified and counted before preservation in 70% ethanol. The nomenclature adopted in reporting the prevalence and the mean intensity values of Anguillicola crassus by months and the size of eels is that customarily used [9,10]. The non-parametric test of Kruskal Wallis was used in this study.
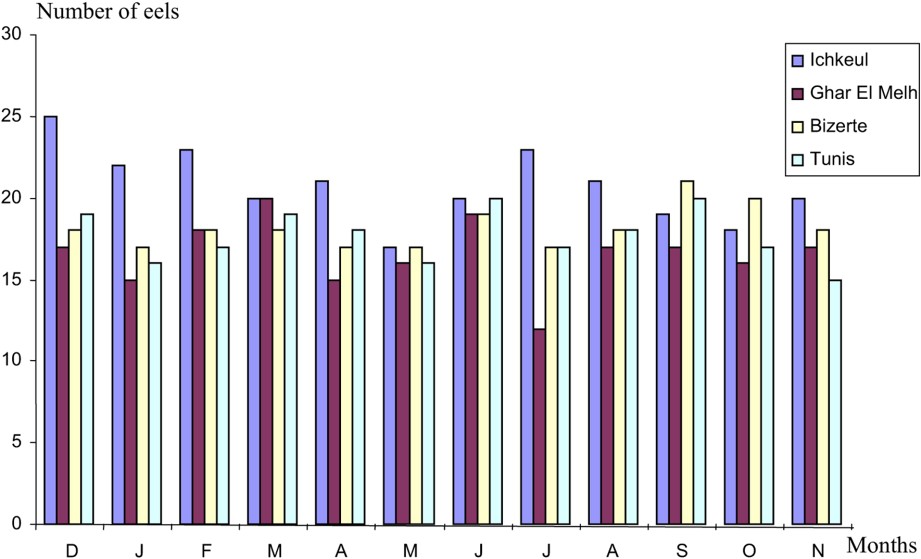
Number of eels as a function of the sampling localities.
3 Results and discussion
The survey of the evolutionary dynamic of parasitism reveals that A. crassus is present throughout the year in the Ichkeul lagoon, which is characterized by a low salinity (3–16‰). The lagoon is fed by several wadis, where the intermediate host copepod, cyclopidae or ostracod [3,11–14], the paratenic hosts fish [13–15], Amphibians and larvae of aquatic insects [3] are undoubtedly present, favouring the progress of the life cycle of this parasite. The low prevalence values recorded in December (12%) increase slightly in February and January, reaching a maximum in March (35%). This is followed by a decrease during the summer, with a minimum in July (4.35%). This is followed by a gradual increase in autumn to reach 30% in November (Fig. 2). This monthly variation is statistically confirmed (H: 8.13, df: 3, p: 0.044). The amplitude of variability of the mean intensity is low. The majority of the values are between 1 and 1.5 parasites per host (Fig. 3).

Prevalence of A. crassus as a function of the months.
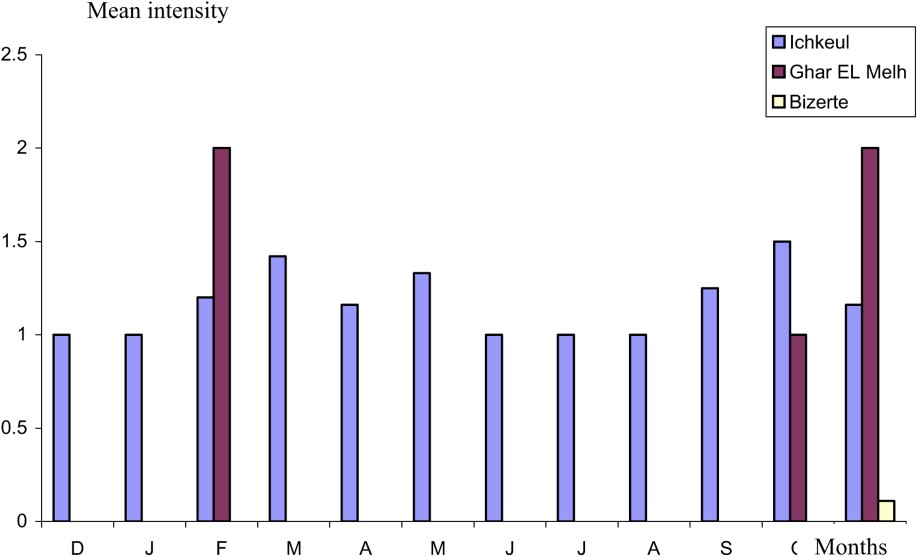
Mean intensity of A. crassus as a function of the months.
The monthly epidemiological variation may be related to the thermal changes. High summer (29–30 °C) and lower winter temperature (8–10 °C) in the Ichkeul lagoon would prevent the progress of the life cycle of this nematode. These results have been experimentally tested and verified [11]. In fact, the larval development of this parasite at the intermediate host cyclopidae is inhibited at a temperature varying from 1 to 13 °C. Under such conditions, the larvae did not grow in cyclopidae and died after about one month. However, temperature from 20 to 22 °C encourages rapid growth of larvae in this host's haemocoel. Other authors [16] have demonstrated that the increase of the temperature could be lethal for larvae of this parasite. Others [17] suggest that the seasonal decrease in the prevalence of A. crassus in summer may be attributed to the death of the more severely affected eels during the warmest seasons.
The seasonal fluctuation of the rate of parasitism may also be related to changes in the period of eel feeding. The eels undergo two critical periods during which they do not feed: once in the winter, when the temperature is lower than 10 °C, and again in the summer, when the temperature may rise above 30 °C [18]. Abstinence from food could limit the arrival of the infective parasite stage at the eel during these periods. Otherwise, it was revealed that the principal active phase of feeding extends from April to September, with a maximum between April and June [19]. This period represents the ideal phase for the success of the available larval stage transmission at the intermediate or paratenic hosts. Others authors [20] think that the low prevalence value in the summer may be explained by a change of diet; the epibenthic preys among which appear the first intermediate host of this parasite are replaced by the ichtyophagy, which increases in June and July. Knowledge of the life cycle of the parasites and in particular their transmission to the final host makes it possible to consider these parasites as a biological indicator providing information concerning the period of feeding and the nature of food of the hosts.
In the Bizerte and Ghar El Melh lagoons, where the salinity is close to that of the seawater (33–34‰), the nematode is present only during one to three months, with low epidemiological values (Figs. 2 and 3). In the Ghar El Melh lagoon, A. crassus was harvested in February, October and November, whereas, in the Bizerte lagoon, the harvesting was limited to November. The cycle of the parasite takes place only in water of low salinity [21]. Subsequently, the presence of eels infested in these biotopes can be explained by their ulterior stay in the relatively fresh waters. The Bizerte lagoon is, indeed, linked to the Ichkeul lagoon by the channel of Tinja. The lagoon of Ghar El Melh receives freshwater during a short period of the year from wadis (El Kherba, El Nechma, and Cherchara), the brook waters, the source of El Ayoun and the channel of El Mebtouha. Investigation in the Tunis lagoon, which is not supplied by freshwater, and is hence characterized by high salinity (37.7–38.1‰), has not revealed yet the presence of A. crassus.
The study of the parasitism evolution from a consideration of the size of the host shows that the highest prevalence values are recorded in young eels (Fig. 4). The value decreases with the increase of the size of the host. However, the values of mean intensity reveal a linear relationship between eel size and the number of parasites (Fig. 5). The same result has been reported from the Rhone River Delta [22]. This result might be due to the eel diet. In fact, the selection of food by eel is influenced by the size of the prey. Thus, young eels consume shellfish rather than fish [23]. Eels are believed to become more piscivorous as their size increases [24]. Subsequently, we think, in agreement with other authors [3], that crustaceans as an intermediate host serve as the source of infection for smaller eels, whilst larger eels are mainly infected by preying on paratenic hosts, such as fish, which form an important part of their diet. The low mean intensity values in large eels might be explained by inhibition of juveniles migrating into the swim-bladder lumen when adults are already present there [25].
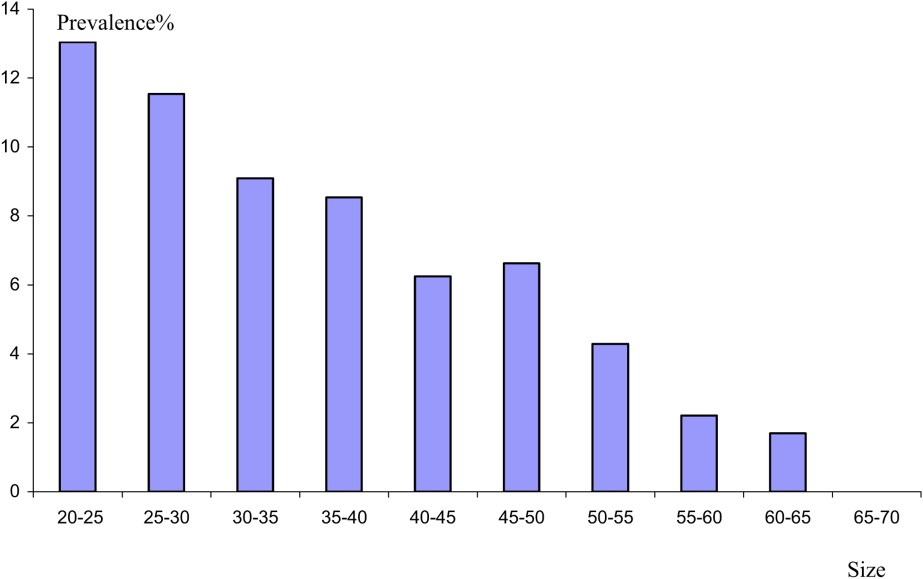
Prevalence of A. crassus as a function of the size.
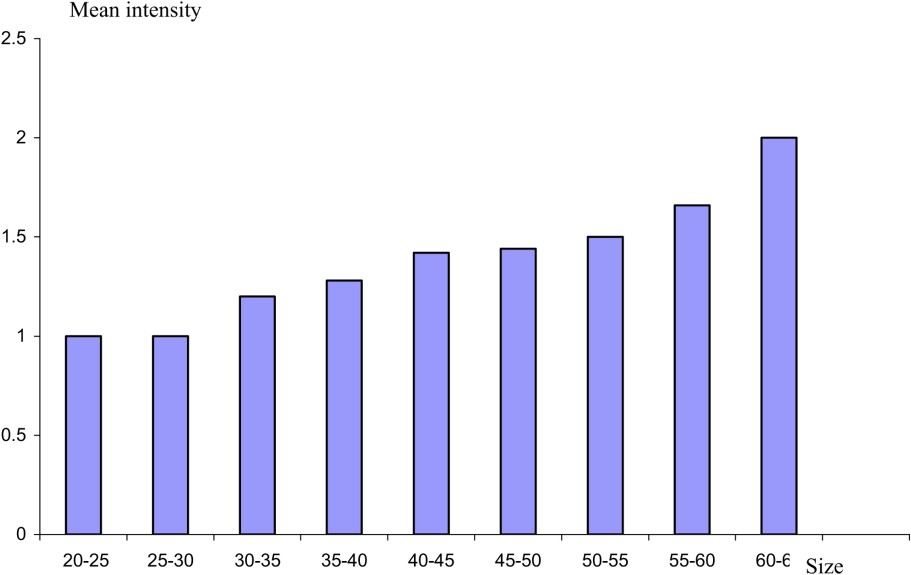
Mean intensity of A. crassus as a function of the size.
The ability of larvae recorded in water of high salinity perhaps contributes to the dissemination and transmission of this parasite in brackish water. This transmission constitutes a threat for eel populations. Because this parasite is blood sucking, it causes degenerative, inflammatory reactions and proliferative changes to the swim bladder. Repeated larval invasion of the swim bladder wall results in haemorrhage and injury to the connective tissue. Also, blood feeding by adults can cause mechanical injury to the epithelium [3,25]. Epithelial cells lining the swim-bladder wall undergo hypertrophy, hyperplasia and dysplasia. Thus the inner surface of the swim bladder becomes inflamed and folded, further reducing the volume of the lumen and provoking a loss of gas. The parasite action will impair swim-bladder function and therefore reduce the eel's swimming capacity at depth, which can compromise seriously the ability for transatlantic migration at the time of the return toward the Sargasso punter area [26,27]. Also, A. crassus has caused some cases of mortalities in intensive eel production [3,28]. All of the losses are evaluated to 250 tons of eels in Lake Balaton [3].
4 Conclusion
This study reveals that A. crassus is present in the Ichkeul lagoon throughout the year. However, it also highlights the importance of sampling by month. Seasonal differences in prevalence are pronounced. By contrast, variations in mean intensity values are slight.
This nematode expanded its distribution toward Bizerte and Ghar El Melh lagoons. However, A. crassus is harvested from Ghar El Melh lagoon only in February, October and November. This parasite is limited to November in the Bizerte lagoon.
Variation in epidemiological value with size reveals that A. crassus becomes less common as the length of eel increases.
Comparatively with the global epidemiological values (prevalence: 7.26%, mean intensity: 1.06) of A. crassus recorded in the Ichkeul lagoon [8], we note that the values obtained through our investigations (prevalence: 18.53, mean intensity: 1.17) are distinctly higher. These results agree with the infestation levels growth recorded in different European countries [29]. The continued increase of the A. crassus parasitism in the final host may be explained by the relative simplicity of its life cycle, and by its adaptability to a wide range of common intermediate hosts. Para-accumulation of juveniles in paratenic hosts is probably responsible for the rapid increase in epidemiological values [3]. The high infection levels may be also explained by the non-effective defence mechanisms of the definitive host against this allochtonous parasite. Indeed, the study of the larvae evolution in Japanese and European eels showed that 60% of larvae recovered from A. japonica were found dead, encapsulated in the swim bladder wall, while no dead larvae were found in A. anguilla. Additionally, the development of worms was shown to be significantly slower in A. japonica compared with A. anguilla [30]. The adaptation for resistance is undoubtedly acquired after a long host-parasite co-evolutionary period [3].


