1 Introduction
Schizophrenia is a complex and potentially severe mental illness, characterized by its heterogeneous clinical presentation [1]. Several authors attempted to isolate an essential trouble that may underlie its main symptoms. In an assay to facilitate diagnosis, Kurt Schneider [2] defined first-rank symptoms that he claimed were specific to schizophrenia. The first-rank symptoms relate to an abnormal recognition of one's own action, and an abnormal attribution of one's actions to others (see [3] for review). Patients describe their symptoms as experiences consecutive to the influence of alien forces or other people on their own thoughts (made thoughts, thought broadcasting, withdrawal, or insertion), actions (made actions) or emotions (made feelings). Other symptoms are described as very extraneous experiences, like auditory verbal hallucinations, e.g., sentences that seem to be pronounced around the subject.
This impaired attribution of action has been interpreted as an abnormal matching between information related to voluntarily generated actions and their sensory consequences [4]. This effect could be caused by a deterioration of the forward model [5,6] responsible for the prediction of the consequences of voluntary acts. Numerous studies have evaluated the capacity of action attribution of patients with schizophrenia [7–9]. In general, they used experimental paradigms based on the alteration of the sensory feedback associated with voluntary actions (hand gesture or language) to determine whether subjects were able to recognize their own actions. Globally, patients with schizophrenia show a reduced accuracy in discriminating between their own action and a foreign, or modified, action [10,11].
Neuroimaging studies in control subjects have shown that the activity of the inferior part of the right parietal lobe is modulated by the degree of discrepancy between an executed movement and its observed consequences: the larger the discrepancy, the greater the activation [12]. In schizophrenic patients, the activity in this same parietal area is poorly modulated by the degree of discrepancy [13]. These studies, although they provide valuable information about brain areas involved in action recognition, fail to describe the chronology of brain events. This aspect is particularly important, since an abnormal time course of events could account for the main symptoms that have been described in schizophrenic patients [14]. The Event-Related Potential (ERP) technique is an adequate method for the study of the temporal aspects of cognitive processes, due to its high temporal resolution. In the context of action recognition, it can provide a physiological marker associated with the processing of the sensory consequence of a voluntary movement.
We carried out an ERP experiment in 15 patients with schizophrenia and 15 normal subjects using a task designed to evaluate action recognition and attribution [9]. In this task, subjects moved a joystick while observing a virtual image of their hand holding a joystick. The movement of the virtual image could correspond, or not, to the real movement. We measured ERP associated with the display of the virtual image corresponding to the end-result of the movements made by the subjects.
2 Material and methods
2.1 Subjects
Fifteen right-handed patients (13 males, 2 females) diagnosed with schizophrenia according to DSM-IV were recruited from the Le-Vinatier Hospital in Lyons, France. They had a mean age of years. All were exempted of any neurological antecedent. Their clinical status was evaluated with the Scale for Assessment of Positive Symptoms SAPS [15] and the Scale for Assessment Negative Symptoms SANS [16]. The mean total SAPS and SANS scores () were respectively 30.9 (24.2) and 38.3 (21.2). The mean duration of illness was years. All patients were under stable second-generation antipsychotic treatment during the experiment.
Fifteen right-handed subjects (8 males and 7 females) with a mean age of age of 26.8 years±6.3 without psychiatric and neurological antecedents have been included. They formed the control group.
This experiment was approved by the local ethical committee.
2.2 Stimuli and task
A realistic image of a virtual right hand holding a joystick was generated on a computer using specific software. Simultaneously, the subject held a real joystick fixed on a table, 66 cm away from the screen. The real hand was hidden by a black cloth so that the subjects could only see the movement of the virtual hand on the screen. In normal trials, the virtual image exactly followed the hand movements made by the subjects. A variable angular bias could be introduced electronically between the movements of the real hand and those of the virtual hand.
The experiment included 160 trials. For every trial, the task consisted to perform a straight movement of the joystick in any direction (free choice of the subject) after the display of a yellow cross in the centre of the screen. Subjects were instructed to maintain the position at the end of the movement and to avoid returning to the starting position of the joystick. 500 ms after the end of the movement, the image of the virtual hand holding the joystick was displayed. This image remained for 2000 ms and was replaced by a screen with the question: “Did the picture that you just saw correspond to the end of the movement you made?”; the subject responded verbally by yes or no whether the image corresponded or not to the movement. The verbal response was recorded by the experimenter (see Fig. 1 for a description of the protocol). Trials involved either no bias (0 degree) or biases of 15, 30, or 60 degrees. Forty trials in each condition were used. The order of presentation was pseudo-random; the same bias was never repeated more than two times in a row.
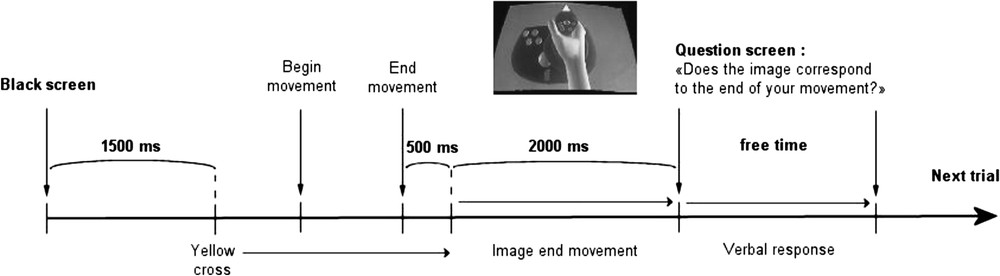
Temporal description of the different events succeeding in one trial of the task.
2.3 EEG recording
Scalp voltage was collected with 65 channels Geodesic Sensor Net through AC coupled high-input-impedance amplifiers (, Net Amps, Electrical Geodesics Inc., Eugene, OR, USA). The net had silver/silver chloride (Ag/AgCl) electrodes. Amplified analogue voltages (0.1–200 Hz bandwidth) were sampled at 500 Hz. The electrode impedance was kept below . An electrode placed in the neck and another one placed in the vertex served as ground and record reference, respectively.
Trials contaminated by eye blinks or eye movements were rejected offline from the analysis using an algorithm developed by Electrical Geodesic Inc, which detects fast and high voltage variations. The same algorithm, but with lower thresholds, was also used to remove artefacts. We used a criterion of rejection for all segments with a voltage higher than . All channels with more than 50 percent of artefacts were removed from the analysis. In total, 20% of the segments were eliminated in the patients group and 14% in the control group.
2.4 EEG analysis
EEG records were segmented aligned to the display of the image of the virtual hand showing the end-result of the movement. ERPs of each bias were calculated by averaging the segments. A baseline correction was applied by subtracting the average value of the segment before the display of the stimulus. The ERPs of the 65 channels were averaged in nine symmetrical regions on the scalp. For the analysis of the different ERP components, we averaged the waveform using three temporal windows: 100–300 ms, 300–500 ms, and 500–900 ms.
The statistical analysis was carried on with a four-level repeated measures ANOVA Group (patients, controls) × bias (four modes: 0, 15, 30, and 60 degrees) × Ant-Post (anterior, central, posterior) × Laterality (left, middle, right) for each temporal window. The Huynh–Feldt correction was applied for the repeated measures of the ANOVA analysis.
3 Results
3.1 Behavioural data
The percentage of yes responses for each value of bias is presented in Fig. 2. In each group of subjects, the percentages of yes responses decreased when the angular bias was increased. However, the two groups differed in the value of the bias for which the subjects responded at a chance level (50% yes responses). In the control group, the chance level approximately corresponded to a bias of 15°, whereas in the patients group, the chance level was attained for larger biases (beyond 30°).
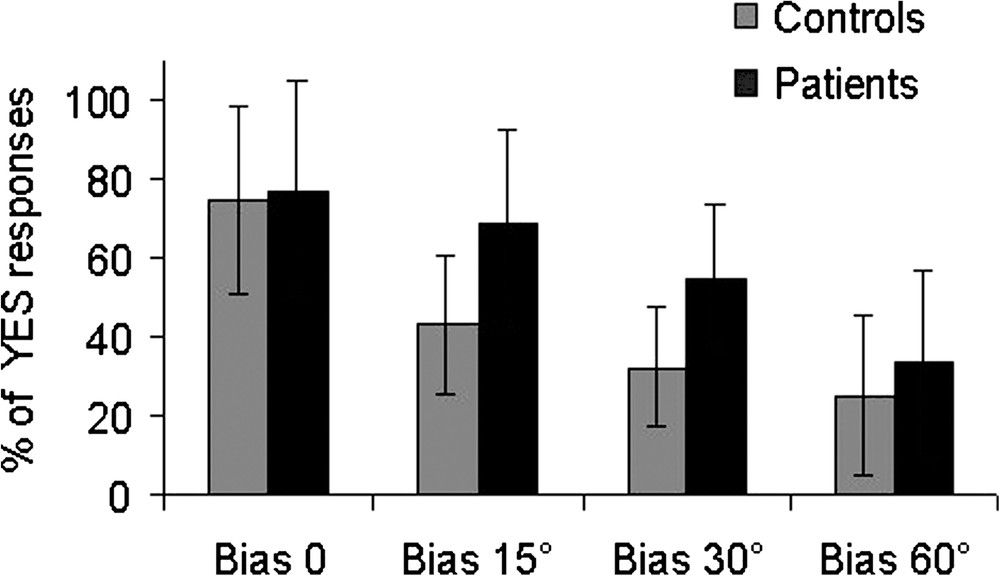
Percentage of yes responses for each value of the angular bias in the patient and the control groups.
A statistical analysis was carried on with a two-level repeated-measure ANOVA group (patients, controls) × bias (0°, 15°, 30°, 60°). The analysis of variance revealed a group effect (, ), a bias effect (, ), but no significant interaction group × bias (, ). However, a direct analysis (1 factor ANOVA) clearly revealed that the proportion of yes responses significantly differed between the two groups for the 15° bias () and the 30° bias ().
3.2 ERP data
Figs. 3B–C and 4B–C show the pattern of ERPs obtained in control and patient groups in the posterior–middle and the central–middle regions of the scalp, respectively. Although the global pattern of the responses appears to be relatively similar in the two groups, significant differences between groups were revealed by the statistical analysis of the waveform of responses to the different values of the bias, according to the three time windows.
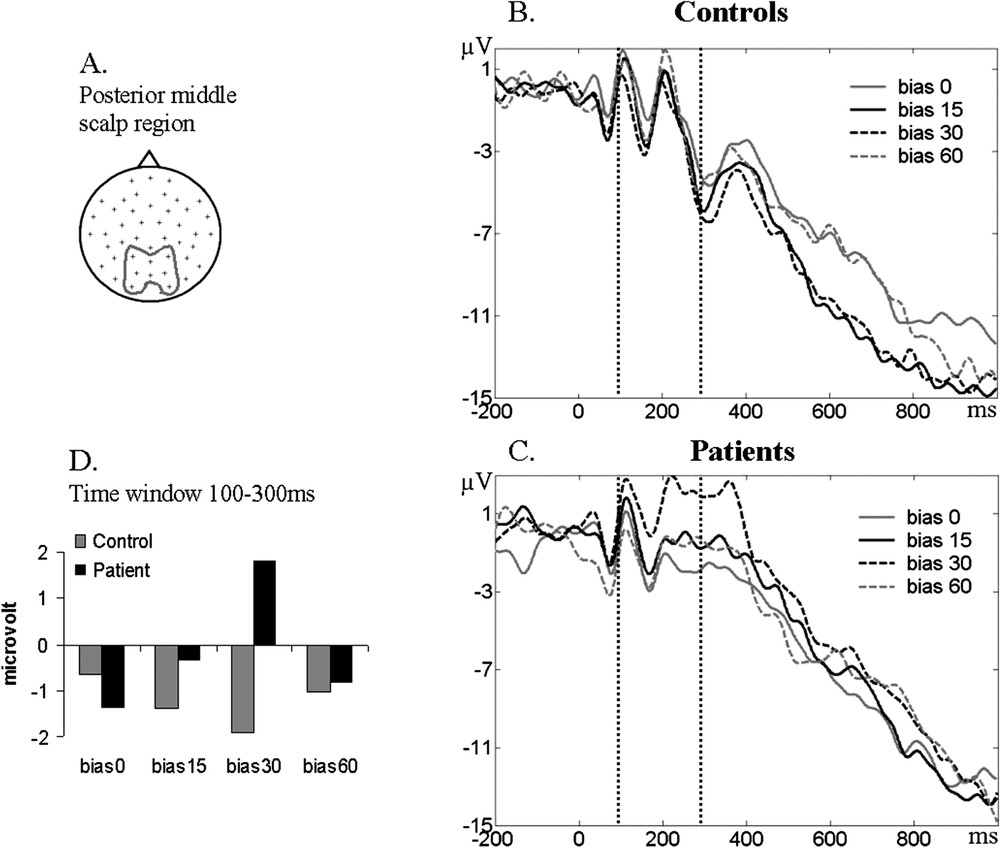
Example of the ERP activity in the posterior–middle region of the scalp. (A) Scalp location of the averaged electrodes. (B–C) ERP waveform for each bias in the control and patients group; 0 corresponds to the visual feedback display. (D) Histogram with the values of the ERP amplitude averaged in the 100–300-ms time window for each bias in each group.
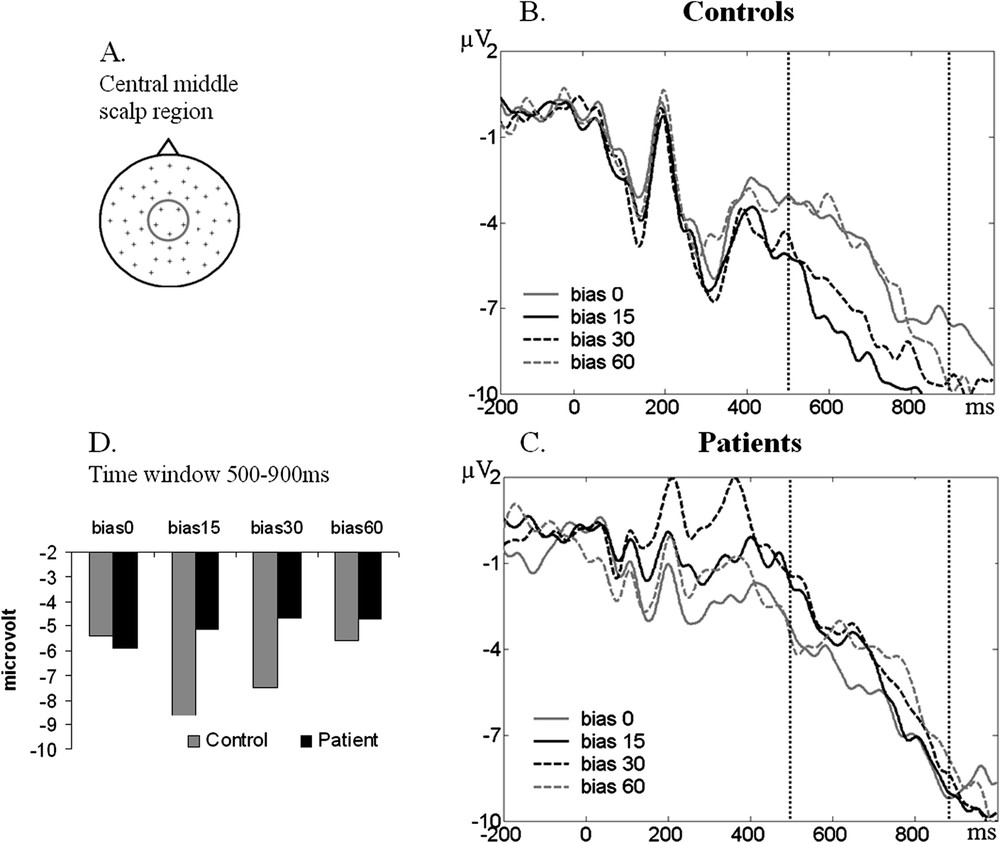
Example of the ERP activity in the central–middle region of the scalp. (A) Scalp location of the averaged electrodes. (B–C) ERP waveform for each bias in the control and patients group. (D) Histogram with the values of the ERP amplitude averaged in the 500–900-ms time window for each bias in each group.
The statistical analysis of the average ERP voltage in the 100–300-ms time window (Fig. 3D) revealed an interaction group × bias (, ) and an interaction group × bias × antero-posterior (, ). The post-hoc analysis showed ERP waveform differences in the patient group, between the 0° and the 30° biases (), between the 15° and the 30° biases (), and between the 30° and the 60° biases (), especially in the posterior region of the scalp. No such differences were found for this time window in the control group.
Similar results were found in the 300–500-ms time window. The same interaction group × bias × antero-posterior was present (, ). Again, the post-hoc analysis showed ERP waveform differences in the patient group between 0°–30° biases (), but this time in the central region of the scalp.
Different results were found in statistical analysis of ERP waveforms in the late time window (500–900 ms) (Fig. 4D). The ANOVA also revealed an interaction group × bias × antero-posterior (, ), but the post-hoc analysis revealed different effects. Post-hoc comparisons showed differences in the control group only, between the 0° and the 15° biases (), the 15° and the 60° biases () and the 30° and the 60° biases () in the central region of the scalp.
4 Discussion
In this study, we measured the brain activity (using the ERP technique) associated with the recognition of one's own movements in patients with schizophrenia. The subjects had to identify (by a yes/no response) whether the end-result they saw corresponded to the movement they had made. A stepwise distortion of the visual feedback of their movement was produced by introducing angular biases. The proportion of 50% of yes responses represents the level of discrepancy at which the subject could not determine whether the end-result corresponded, or not, to his/her movement, and responded at chance. In terms of the ability to attribute correctly a movement to its author, the 50% value may therefore be considered as the threshold that separates a self-attributed movement from a movement perceived as originating from another agent. According to the behavioural data recorded in the present experiment, this threshold value was found to be higher in patients (beyond the 30° bias) than in control subjects (around the 15° bias). This result confirms previous results by Franck et al. [9].
The main result of the present study was that the ERP waveform recorded during identification of the end-result of a movement was modified according to the threshold values in each group of subjects. This modification, however, was observed in different time windows for each group. Let us first examine the pattern of ERPs in control and patient groups for the late time window (500–900 ms). In subjects of the control group, a clear and significant difference was observed between the ERPs obtained during the ‘unambiguous’ trials, i.e., those trials with no bias (0°) or with a large bias (60°), where the vast majority of responses were either yes or no, and the ‘ambiguous’ trials (15°), where responses were at chance. The late ERP component corresponding to these ‘ambiguous’ trials showed increased amplitude in comparison with the ‘unambiguous’ ones. The late ERP components are usually associated with the manipulation of the cognitive parameters of the stimulus (for example, introducing syntactic errors in a sentence [17]). It may be suggested that, in the present situation, the difficulty to evaluate the difference between the visual feedback and the movement during ‘ambiguous’ trials yielded, in the control group, a deeper cognitive analysis of the sensory information, which resulted in an increase of the ERP activity. This was not present in the patient group. Note that the absence of modulation of the late ERP components by cognitive manipulations is a common finding in schizophrenic patients (e.g., unlike normal controls, they do not show a N400 component for unexpected words or mismatch negativity for unexpected auditory stimuli [18–20]).
The ERP waveform of the patients also showed a modulation according to the degree of bias, but in a time window (around 200 ms) earlier than that of the control group. The ERP amplitude was larger for the 30° bias, in comparison to the others. As in the control group, this corresponds to the ‘ambiguous’ trials, those for which the patients responded at chance. In the ERP literature, the early components reflect processing of stimulus characteristics, rather than its cognitive content. For example, the N170 component (latency 170 ms) is associated with the processing of face images [21]. Similarly, the burst of EEG oscillations at the gamma frequency appearing 280 ms after the display of a stimulus is associated with the elaboration of a final coherent representation of the stimulus [22], and the integrated representation can be manipulated by cognitive processes only after this step [23].
The fact that in the schizophrenic patients, the ERP change triggered by the sensorimotor conflict was observed in a time window that is generally considered as corresponding to the sensory stage of processing, contrary to the control subjects who showed a change in their later, ‘cognitive’ window, is a novel and interesting finding. This result suggests an impaired processing of the basic aspects of a visual stimulus, i.e., those aspects that are critical for shape recognition. Having not properly analyzed the visual display of their hand position, they would not notice the discrepancy between this position and their movement until the mismatch becomes very large. This interpretation raises the question of a possible role of the antipsychotic drugs that the patients received at the time of the experiment, in perturbing their visual sensory processing. This role is unlikely, however, if one considers the fact that the difficulty of schizophrenic patients in recognizing their own movements is present (and even worse) at the acute stage of their disease, i.e., before they have received any treatment. It seems more reasonable to assume that the deficit in action recognition observed in our patients, and the concomitant changes in their ERP responses, is a genuine aspect of their pathology.
The significance of the present results showing an altered ERP response to a sensorimotor conflict in schizophrenic patients is still a matter of speculation. Sensorimotor conflicts like the one used in the present study are known to modulate the activity of the posterior parietal cortex [12,24]. As we saw in the Introduction, according to Farrer et al. [12], posterior parietal activity covariates with the degree of conflict, being higher when the degree of conflict increases. Interestingly, this modulation tends to disappear in schizophrenic patients [13]. The relation between these two facts (the change in parietal cortex activity and the change in ERP responses during a sensorimotor conflict) can be interpreted in two ways. According to one interpretation, the lack of modulation of parietal cortex activity can be seen as the consequence of the impaired early visual processing. Alternatively, the change in parietal cortex activity can be seen as the primary factor, the impaired ERP response being a consequence of a perturbed top–down modulation of visual cortex by parietal cortex. In support to the second interpretation is the general idea of a disruption of frontal cortex activity in schizophrenia (see review in [25]), with the correlative disinhibition of more posterior cortical areas, including posterior parietal cortex. Whichever is the correct one, the two interpretations would equally well account for the patients difficulty in recognizing their own movements.
Acknowledgements
This work was supported by the CNRS and the ‘Université Claude-Bernard’, Lyons, France. Funding was also provided by the European Union (Fifth Framework Program, Proposal No. QLRT-2001–00746). We thank Prof. Michel Marie-Cardine for his crucial help for the evaluation of the patients.


