Version française abrégée
Les études épidémiologiques sont informatives et concordantes quant aux facteurs faisant varier en fréquence les conduites addictives (sexe, âge, statut marital, trouble psychiatrique et surtout dépendance à une autre substance). Or, le rôle de ces facteurs, dont certains relèvent de l'environnement, est à confronter à l'importance d'une vulnérabilité génétique aux addictions.
Les études de jumeaux aident à quantifier le poids respectif de l'environnement et de l'hérédité. Elles retrouvent de manière homogène un poids significatif des gènes dans la vulnérabilité aux dépendances, quelle que soit la substance, voire l'objet de cette dépendance (substances ou comportement). Plus encore, ces études montrent qu'au sein de l'hérédité des addictions, une vulnérabilité génétique commune compte plus que la part spécifique de gène à chaque dépendance. Elles aident ainsi à préciser le phénotype héritable comme relevant de conduites addictives associées entre elles et à d'autres troubles comportementaux, comme les conduites agressives précoces et l'hyperactivité.
Pour autant, et ce malgré les arguments en faveur de l'intervention de gènes majeurs dans cette vulnérabilité, aucun allèle n'a pu être clairement impliqué. L'hétérogénéité du phénotype, les phénomènes de phénocopies et de pénétrance incomplète, comme les interactions épistatiques, pourraient expliquer ces difficultés. La prise en compte conjuguée des facteurs génétiques et environnementaux doit permettre de mieux déterminer les facteurs de vulnérabilité impliqués ainsi que la manière dont ils s'associent.
L'étude de l'interaction gène–environnement (
Certaines études récentes ont prouvé ce type d'interaction. Par exemple, une étude d'adoption montre que les conduites agressives de l'enfant adopté étaient associées en fréquence à une alcoolo-dépendance chez les parents biologiques uniquement en cas d'environnement familial perturbé dans la famille adoptive. Ce type d'interaction est également mis en évidence au niveau moléculaire. Dans une étude portant sur les singes rhésus, la séparation précoce du nouveau-né entraîne un risque de consommation d'alcool uniquement en présence de l'allèle court d'un polymorphisme du promoteur de la sérotonine. Chez l'homme, ce type d'interaction est montré par des protocoles de pharmacogénétique (variation de l'efficacité de la naltrexone dans la dépendance à l'alcool en fonction d'un polymorphisme d'un récepteur opioïde), ou dans la sévérité des symptômes de sevrage à l'alcool, voire des complications de sevrage en fonction du génotype du transporteur de la dopamine. Ces résultats chez l'homme sont d'autant plus pertinents qu'ils concernent tous deux des molécules centrales du système de récompense, voie biologique candidate principale dans la vulnérabilité aux dépendances en général.
La mesure précise de ce type d'interaction
1 Epidemiology of addictive disorders
Alcohol and illicit drug abuse are among the top 10 major risk factors in global burden of mortality and morbidity according to the assessments based on the DALY (Disability adjusted life years) [1]. Epidemiology of addictive disorders needs to analyse both clinical and general population samples to understand the complex relationships between drinking and drug use, drinking problems and drug-related problems, alcohol and drug abuse or dependence, and to measure consequences (mortality, somatic and psychiatric co-morbidity, social difficulties, antisocial and criminal behaviours...) of these addictions.
The estimations of abuse and dependence prevalence for any type of addictive substance are based on a limited number of studies. Five large epidemiologic researches based on the general population have examined the co-morbidity of alcohol- and drug-use-related disorders worldwide. The Epidemiologic Catchment Area (ECA) in the 1980s [2], the National Comorbidity Survey (NCS) [3], the National Longitudinal Alcohol Epidemiologic Survey (NLAES) [4] were all made in the USA. The 1990 Mental Health Supplement of the Ontario Canada Health Survey (MHS-OHS: [5]) and the 1997 Australian National Survey of Mental Health and Well Being (NSMHWB: [6]) were also derived from a large sample of the general population. Unfortunately, such a study was not performed in France, although the latter has one of the most important consumption of alcohol in the world. Indeed, according to one study focusing on consumption, the French general population (between 18 and 75 years old) had high prevalence rates of alcohol regular use (31%), tobacco (29%), cannabis (1.4%), and cocaine or heroin (0.4%) in 2002 [7]. The only information we have is derived from the ESCAPAD study, related to a one-month prevalence of drug use among young people, between 17 and 18 years old. The estimated prevalence was 1% for poppers, 0.7% for solvents, 0.9% for cocaine, 0.9% for amphetamines, 0.5% for LSD, 1.6% for ecstasy, 0.4% for heroin, and 0.3% for crack [8].
The most interesting study on this topic is the NESARC [9], which recently analysed a large (more than 43 000 subjects) representative sample of the US population, examining current prevalence and associations between alcohol and specific drug-use disorders.
The one-year prevalence rates of abuse or dependence are ranging from 0.02% (cocaine dependence) to 1.13% for cannabis abuse (Table 1). These frequencies are limited to syndromic addictions that were observed during the current year.
Annual prevalence of abuse or dependence (DSM-IV) in the USA (NESARC)
| Toxic | Abuse | Dependence | ||
| (%) | (d.s.) | % | (d.s.) | |
| Tranquillizers | 0.08 | (0.02) | 0.05 | (0.01) |
| Sedatives | 0.09 | (0.02) | 0.07 | (0.01) |
| Amphetamines | 0.09 | (0.02) | 0.07 | (0.02) |
| Hallucinogen | 0.12 | (0.02) | 0.02 | (0.01) |
| Cocaine | 0.13 | (0.02) | 0.13 | (0.02) |
| Opioids | 0.24 | (0.04) | 0.11 | (0.02) |
| Cannabis | 1.13 | (0.06) | 0.32 | (0.04) |
More interestingly, a large number of parameters were analysed in the groups of patients with, or without, any addictive disorder (Table 2). It can be observed that not only males are more exposed to any addictive disorders, but they are also more exposed to co-morbid addictive ones (alcohol and drug abuse or dependence). One of the interesting ideas of the NESARC study was to enrich a subgroup of subjects from ethnic minorities. With this approach, they showed that African persons are more exposed to any drug abuse or dependence, Asians are less exposed to alcohol abuse or dependence, and subjects with Hispanic origins are more frequently observed in the group of co-morbid addictive disorders. The NESARC study also confirmed that young subjects (18–29) are more at risk, as subjects who were never married. Personality disorder and past-year anxiety or mood disorders were associated with a dramatically increase of any abuse or dependence diagnosis, an important result regarding the efforts that were made in the NESARC study to correctly assess such co-morbidity, usually very difficult to disentangle. Another major replication of the NESARC investigation is the very high co-morbidity of addictive dependencies, presence of one dependence dramatically increasing the risk of dependence on another substance (Table 3), up to a 43-time increased risk (
Demographic characteristics and psychiatric co-morbidity of subjects, with versus without alcohol or drug abuse or dependence (DSM-IV) (NESARC)
| Characteristics | Abuse or dependence | ||||
| Alcohol | Drug | Alcohol or drug | None | ||
| Sex | Men | 69.4 (1.04) | 60.1 (2.97) | 73.9 (2.64) | 45.7 (0.32) |
| Women | 30.6 (1.04) | 39.9 (2.97) | 26.1 (2.64) | 54.3 (0.32) | |
| Ethnic group | Caucasian | 75.8 (1.85) | 68.5 (2.90) | 68.5 (3.06) | 70.6 (1.61) |
| African | 8.7 (0.67) | 15.8 (2.14) | 11.5 (1.83) | 11.2 (0.65) | |
| Asiatic | 2.3 (0.48) | 3.6 (1.61) | 2.6 (0.99) | 4.6 (0.56) | |
| Hispanic | 10.8 (1.59) | 8.9 (1.88) | 11.0 (1.82) | 11.7 (1.23) | |
| Age | 18–29 | 38.3 (1.18) | 47.8 (3.30) | 65.0 (3.02) | 19.7 (0.37) |
| 30–44 | 37.0 (1.09) | 33.8 (3.19) | 25.9 (2.63) | 30.4 (0.33) | |
| 45–64 | 21.6 (0.98) | 15.9 (2.31) | 9.1 (1.85) | 32.3 (0.32) | |
| >65 | 3.2 (0.34) | 2.6 (0.78) | 0.1 (0.07) | 17.6 (0.37) | |
| Marital status | Married | 47.7 (1.08) | 45.0 (3.14) | 20.2 (2.21) | 63.4 (0.50) |
| Divorced | 16.7 (0.85) | 12.8 (1.96) | 16.6 (2.18) | 17.6 (0.24) | |
| Never married | 42.2 (3.04) | 63.2 (2.80) | 19.0 (0.49) | 35.6 (1.21) | |
| Education | <College | 12.2 (0.98) | 18.3 (2.51) | 18.2 (2.67) | 15.9 (0.49) |
| College | 27.9 (1.09) | 38.1 (3.29) | 33.3 (2.77) | 29.3 (0.56) | |
| >College | 59.8 (1.32) | 43.6 (2.93) | 48.4 (3.01) | 54.8 (0.63) | |
| Income (US $) | 0–20 k$ | 39.3 (1.22) | 66.1 (3.31) | 65.4 (2.89) | 47.5 (0.59) |
| 20–35 k$ | 25.8 (1.06) | 18.7 (2.69) | 23.1 (2.45) | 22.4 (0.36) | |
| 35–70 k$ | 25.5 (1.00) | 11.3 (2.13) | 9.7 (1.61) | 21.9 (0.40) | |
| >70 k$ | 9.4 (0.71) | 4.0 (1.68) | 1.8 (0.72) | 8.2 (0.38) | |
| Living | Town | 79.6 (1.83) | 84.3 (2.60) | 78.4 (3.09) | 80.3 (1.62) |
| Rural | 20.4 (1.83) | 15.7 (2.60) | 21.6 (3.09) | 19.7 (1.62) | |
| Personality | Pathological | 25.3 (1.00) | 44.0 (3.45) | 50.8 (3.05) | 13.2 (0.33) |
| No | 74.7 (1.00) | 56.0 (3.45) | 49.2 (3.05) | 86.8 (0.33) | |
| History of independent mood disorder during the current year | |||||
| Yes | 16.4 (0.81) | 27.5 (2.58) | 35.3 (3.32) | 8.1 (0.21) | |
| No | 83.6 (0.81) | 72.5 (2.58) | 64.7 (3.32) | 91.9 (0.21) | |
| History of independent anxious disorder during the current year | |||||
| Yes | 15.6 (0.86) | 24.0 (2.66) | 26.5 (2.82) | 10.4 (0.32) | |
| No | 84.4 (0.86) | 76.0 (2.66) | 73.5 (2.82) | 89.6 (0.32) |
Increase of risk (adjusted odds ratio with 95% confidence interval) of alcohol abuse or dependence, for subjects with another dependence (controlling for the impact of socio-demographic factors, associated personality disorder, and independent mood and anxiety disorders)
| Toxic | Abuse | Dependence |
| Sedatives | 5.3 (2.44–11.31) | 1.8 (0.65–4.93) |
| Tranquillizers | 7.1 (2.52–20.17) | 4.0 (1.30–12.16) |
| Cannabis | 6.2 (4.80–7.90) | 7.3 (3.92–13.72) |
| All | 5.7 (4.49–7.30) | 9.9 (6.47–15.01) |
| Opioids | 6.1 (3.76–9.91) | 12.9 (6.18–26.89) |
| Amphetamines | 20.3 (6.18–66.94) | 5.2 (2.14–12.59) |
| Cocaine | 10.5 (4.85–22.56) | 43.0 (17.83–103.49) |
The role of environmental risk factors is not in contradiction with a genetic approach of addictive disorders, as vulnerability genes might lead to the disorder through these factors. For example, if genes coding for male hormones are involved in opiate dependence, the gender is a risk factor through genetics. An alternative explanation is that environmental factors might decrease the threshold, allowing the genetic vulnerability to be expressed. For example, if stressful life events are associated with an increased risk of relapse in alcohol dependence, the role of the gene coding for the serotonin transporter (the target of antidepressive drug) might be involved, and only observable in patients exposed to such revealing conditions.
2 Genetics of addictive disorders
The heritability concept relates to the percentage of the total variance of a disorder that is explained by genes. It is usually computed on the basis of twin studies that help to disentangle the respective role of the specific environment (the part of the environment that is not shared by the two twins in the same family, whether they are monozygotic or dizygotic), the shared or family environment (by definition comparable between two twins raised in the same families, at the same period of time... roughly comparable for monozygotic and dizygotic twins), and addictive genetic effects (i.e., heritability, which is nearly the only source of difference between the two types of twins). Indeed, monozygotic twins share 100% of their genes (as it is the same oocyte, fertilised by the same spermatozoid, that led to only one embryo that is accidentally divided in two after fertilization) and dizygotic twins share only 50% of their genes (as they come from two different oocytes, fertilised by two different spermatozoids, as all sibs).
Many twin studies were performed on addictive disorders. In alcohol consumption, the heritability is relatively high (
Interestingly, the role of shared (familial) environment is usually not significantly different from zero, at least for hallucinogens, opiates, and sedatives [12]; the involved risk factors could therefore mainly concern genes and specific environment. The heritability of addictive disorders is largely variable from one study to another, which is not surprising as patients are collected in very different ways, using different instruments and collected from different settings. The Virginie cohort from Kendler and some of the Australian studies relate to the general population, and a large part of other studies are based on treated population. Roughly, the heritability of addictive disorders is higher for abuse than for usage and for dependence than for abuse, ranging between 21% and 72%. The heritability scores of substance abuse or dependence have surprisingly a low level of covariance with the heritability score observed in substance consumption, i.e., the genes involved in consumption are not systematically involved in abuse or dependence, and vice versa.
Some twin studies focused on this problem, assessing the cross-twin cross-trait heritability to pinpoint the shared heritability between two different addictive disorders. Tsuang et al. [13], for example, showed that a large majority of the genes involved are common to the different disorders, with the role of genes for one specific disorder being 0% for LSD, 15% for sedatives, 25% for stimulants, and 33% for cannabis [13]. In another study, the co-morbidity between pathological gambling and alcohol dependence is mainly depending on the same genes, the heritability ranging between 12% and 75% [14,15]. Not all studies are that clear for the existence of a common genetic basis in the different addictive disorders [16]. Nevertheless, when researches are focusing not only to initiation, maintenance or level of consumption, but rather on dependence symptoms, then the involved genes might be much more frequently common than different, explaining in part, for example, the high co-morbidity between cigarette and alcohol dependence [17].
Few studies have been performed on behavioural addictive disorders, such as pathological gambling. In the same line with what is observed for chemical addictions, the more frequent an addictive behaviour is performed with deleterious consequences, the higher is the heritability (
It is not easy to delimit what syndrome belongs to the addictive disorder spectrum. Indeed, Cloninger [18] showed that alcohol dependence is not really inherited, but rather that a subgroup of patients is especially concerned by genetic vulnerability, with young age at onset, severe dependence, male gender, and antisocial behaviour, this group of patients explaining a large proportion of the alcohol-dependence heritability. Such type-II male-limited alcoholism has in fact an 88% heritability, while type-I alcoholism only has a heritability of 21%.
3 The definition of
The fact that so important evidence favours the hypothesis of a major role of genes in the development of any abuse or dependence is counterbalanced by the absence of vulnerability genes that has yet been definitely involved. Indeed, the only genes that have a clear-cut role in alcohol or tobacco dependence are more devoted to protection than to vulnerability [19], and are related to the metabolism of the drug rather than to temperament, psychiatric vulnerability, or social behaviour. Many reasons could be raised to explain such discrepancy:
- • the absence of valid criteria for the concepts of abuse or dependence is an important limitation, as the criteria used for the diagnosis are chosen for their clinical relevance, not according to genetic studies;
- • the presence of phenotypical heterogeneity is also a source of problems, as not all cases of addictive disorder for different types of drug or behaviour share the same mechanisms. Accordingly, analysing samples of patients with different type of dependence, based on different risk factors (see Table 2) may add confusion rather than increase the power of the statistical analyses;
- • the ‘phenocopy’ and ‘incomplete penetrance’ phenomenon could act as a break upon the discovery of vulnerability genes too. Some patients may indeed have a dependence for reasons that are independent of genetic vulnerability, (i.e., phenocopy) and not all cases of genetically vulnerable patients will have the disorder at least once in their life (i.e., incomplete penetrance);
- • epistatic interaction (i.e., different combination of genes might explain the disorder rather than isolated gene) is also rarely taken into account in genetic analyses of addictive disorders.
A highlighting way of facing these problems is mixing the approaches devoted to environmental factors with those devoted to genetic analyses. Such a gene × environment interaction (usually entitled “
For example, being male, Caucasian, between 18 and 29 years old, never married, with a low level of education, with a personality disorder and with lifetime mood and/or anxiety disorder is associated with a dramatically increased risk of addictive disorder. Such group of patients has more chance to share common vulnerability genes, as they represent a more homogenous sample. Specific risk factors may also help to pinpoint some candidate genes. For example, as mood disorder is associated with an increased risk of alcohol or drug abuse/dependence, patients with both alcohol dependence and independent major depressive disorder could have more chance to have vulnerability genes involved in these two disorders, such as those related to serotonin receptors, transport, and metabolism.
The way to assess
Assessing G×E interaction based on the presence versus absence of each risk factor (genetic and environmental)
| Vulnerability gene (G) | Environmental factor (E) | |
| Absent | Present | |
| Absent | RR=1 | RR[E] |
| Present | RR[G] | RR[G×E] |
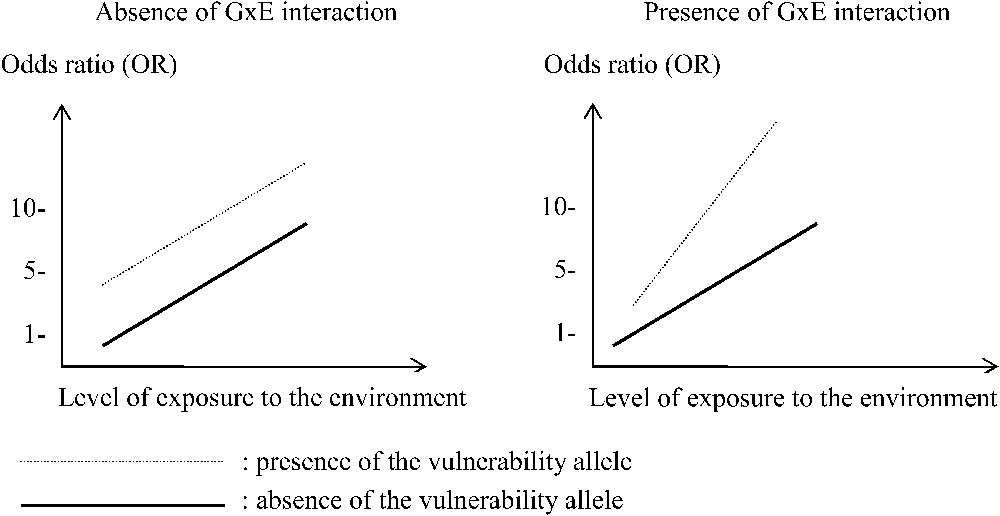
Two types of interaction between a gene (with or without the muted allele) and one environmental factor (with a quantitative variable exposure to an environmental risk factor) in order to explain an increased global risk of a disorder.
Other types of advantages can be raised for the
4 Evidence and examples of
Before the first molecular genetic analyses had been performed in different addictive disorders, evidence was raised in favour of a
One particularity of
A more direct assessment of
Another aspect of
As the mesolimbic dopaminergic system plays a key role in reward pathway, the genes involved in central dopamine functions are frequently studied in alcohol dependence. The gene encoding the dopamine transporter (DAT) could be a good candidate gene because this membrane-bound protein is essential for the homeostatic regulation of dopaminergic neurotransmission [29]. Given the heterogeneous results of the case-control association studies, particularly those on alcohol-dependent patients, it may be interesting to restrict this kind of study to more homogeneous subgroups of patients, taken into account the environmental effects. A key period to assess dependence is the withdrawal period, as acutely stopping alcohol consumption is an environmental factor that may highlight genetic vulnerability to severe dependence. A significant association between the 9-copy repeat allele (A9 allele) of a variable-number tandem repeat in the 3′ untranslated region of the DAT gene and two withdrawal complications, namely delirium tremens and alcohol withdrawal seizure were reported [30,31]. In addition, Schmidt et al. [32] found that withdrawal symptoms were more severe in alcohol-dependent patients carrying the A9 allele than among patients without this variant. Wernicke et al. also [33] tested two other polymorphisms in the
5 Conclusions
Assessing the role of the environment factors that are involved in addictive disorders while taking into account the existence of a genetic vulnerability is logical, this being true the other way round. On the other hand, adding another factor, and taking into account the interaction between these two factors also have some limitations. Assessing
Nevertheless, focusing on this
The shift from purely environmental studies to genetic research in addictive disorder is still recent, with promising but up to now disappointing results. Such cleavage probably has an internal coherence (not using the same tools), but probably has to be overtaken to get closer to what is happening in real life, i.e. the way we cope with addictive substances is a mix-up of many environmental effects, the genes that we are made of, and a large interaction between these two phenomena.


