Epithelial stem cells are responsible for the continuous regeneration of tissues such as hair, skin, and gut. The stem cells reside in specific niches that regulate the self-renewal and differentiation of the adult stem cells [1–3]. The regenerative potential of mammalian teeth is generally limited, and in humans as well as in most other mammals teeth are replaced only once and their growth arrests after the eruption has completed. However, some mammals have continuously growing teeth. Rodents are characterized by sharp incisors that grow throughout life. A stem cell niche has been identified in mouse incisors at their proximal ends in the so-called cervical loops [4,5 (Fig. 1)]. The evidence includes data from DiI labelling studies, studies on cell cycle kinetics and localization of long-term BrdU label retaining cells [4,6]. This stem cell niche bears anatomical similarities to the niches in other epithelial organs, in particular in hairs and intestine.
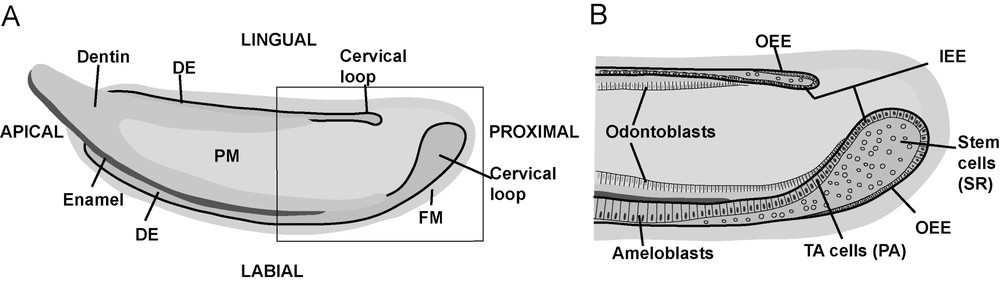
Schematic pictures of mouse incisor. (A) The incisor grows continuously from the proximal end towards the apical end, where the tooth is worn down as the asymmetry of enamel coverage maintains the cutting edges of the teeth sharp. (B) Detailed picture from the boxed region in (A). Epithelial stem cells are located in the stellate reticulum (SR) compartment. They proliferate, migrate apically, and differentiate into ameloblasts that deposit enamel matrix. Dental epithelium (DE), papilla mesenchyme (PM), follicle mesenchyme (FM), inner enamel epithelium (IEE), outer enamel epithelium (OEE), pre-ameloblasts (PA), transient amplifying cells (TA cells).
Compared to the niches in other organs, the incisor stem cell niche has a powerful property, which we have exploited in our studies: the stem cell compartments on the different sides of the tooth, namely the labial (anterior) and lingual (tongue-side) cervical loops vary greatly in size and proliferative capacity. This contributes to the characteristic asymmetric growth and enamel distribution, which together maintain the sharpness of the continuously growing incisor. In the labial cervical loop, the epithelial stem cells proliferate actively and migrate along the labial surface, differentiating into enamel-forming ameloblasts. In contrast, the lingual cervical loop contains fewer proliferating stem cells, and the lingual surface of the incisor lacks ameloblasts and enamel (Fig. 1). Comparisons of the stem cell niches in labial and lingual cervical loops allowed us to identify components of the genetic networks regulating the proliferation and differentiation of epithelial stem cells.
We have shown that epithelial stem cells' proliferation in the cervical loops is controlled by signals from the adjacent mesenchyme. The first mesenchymal signals that were associated with epithelial-cell division were FGF3 and FGF10 [4]. The FGF pathway was linked with Notch signalling in organ culture experiments, in which the dissected cervical loops of mouse embryos were exposed to beads releasing the FGF10 recombinant protein. The expression of the Notch signal modulator Lunatic Fringe was markedly upregulated in the epithelium [4]. Lunatic Fringe is intensely expressed in the basal epithelium of the cervical loop, whereas Notch1 is expressed in the stellate reticulum cell compartment, in the centre of the cervical loop epithelium. All current evidence supports the idea that the epithelial stem cells are located in the Notch1-positive stellate reticulum compartment (Fig. 1).
The analysis of the incisor phenotypes in several mutant mouse lines has revealed a complex integrated signal network regulating stem cell proliferation (Fig. 3). This network consists of FGFs, BMPs, Activin, and Follistatin, which are all locally expressed within the incisor stem cell niche. Fgf3 is asymmetrically expressed and restricted only to the mesenchyme underlying the labial cervical loop. Activin βA is also asymmetrically expressed in the dental mesenchyme with more intense signals at the labial aspect than the lingual aspect, whereas Bmp4 and Fgf10 are expressed in the mesenchyme on both labial and lingual sides with similar intensities. Follistatin, on the other hand, is mainly expressed in the epithelium and restricted to the lingual side [7–9]. The Fgf10 null mutants die at birth, and the culture of their incisors in organ cultures showed that the cervical loops were not maintained in the mutant teeth [6]. Comparisons of the incisor phenotypes of Fgf3 null mutant mice and the Fgf3;Fgf10 compound mutants indicated that Fgf3 and Fgf10 interact to maintain the stem cell pool that provides the continuous supply of ameloblast precursors [7,8].
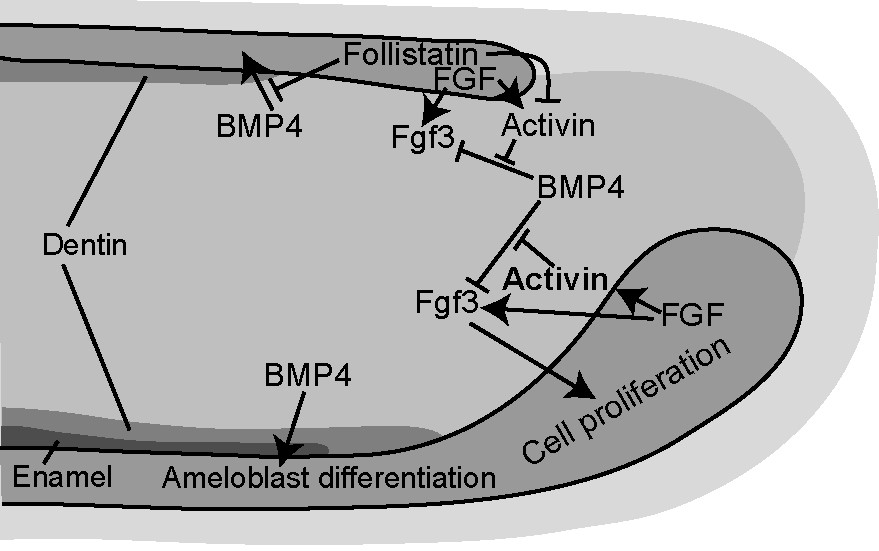
Schematic picture of the signal network regulating epithelial stem cell proliferation and differentiation in the incisor. BMP4 induces differentiation of ameloblasts, but inhibits proliferation of the stem cells by negatively regulating the expression of Fgf3. Activin counteracts this effect and stimulates Fgf3 expression and stem cell proliferation. Labial-lingual asymmetry is established in the incisor by higher Activin expression labially and by lingual expression of Follistatin, which acts by inhibiting Activin function in the stem cells and BMP function in the differentiating ameloblasts, respectively.
Two mutant mice lines, transgenic mice overexpressing Follistatin in the epithelium (under the keratin14 promoter), and Follistatin knockout mice have been informative in the studies on incisor stem cell proliferation and differentiation [8,9]. Follistatin is a well-known antagonist of TGFβ signalling, and it inhibits particularly the functions of Activin and BMPs [10]. In the Follistatin overexpressing mice, incisor growth was inhibited and no enamel formed. The labial cervical loop, was hypoplastic, resembling the lingual cervical loop, and ameloblasts failed to differentiate. In the Follistatin knockout mice, which die at birth, the analysis of the embryonic incisors indicated that the growth of the lingual cervical loop was stimulated and it began to resemble the labial cervical loop. The stellate reticulum compartment was large and the entire cervical loop epithelium showed increased BrdU incorporation. In addition, ameloblasts differentiated also on the lingual side and started to deposit enamel matrix. Fgf3 expression in the mesenchyme correlated with proliferation of the nearby epithelium. Fgf3 transcripts were absent in the K14-Follistatin incisors, whereas they were ectopically expressed in labial cervical loop mesenchyme in the Follistatin knockout incisors. Hence, the asymmetry of the incisor was lost in both Follistatin gain-of-function and loss-of-function mice, indicating an important role for Follistatin in the regulation of incisor asymmetry.
Organ culture studies on the effects of the various signals in isolated cervical loop tissues further clarified the functions of the different signal pathways and their integrations. Beads releasing BMP4 induced the differentiation of the epithelial cells into ameloblasts (Fig. 2). These and other observations indicated that Follistatin acts as a BMP antagonist during the differentiation stage in the lingual dental epithelium and prevents enamel formation, thereby contributing to the characteristic asymmetry of the incisor [9].
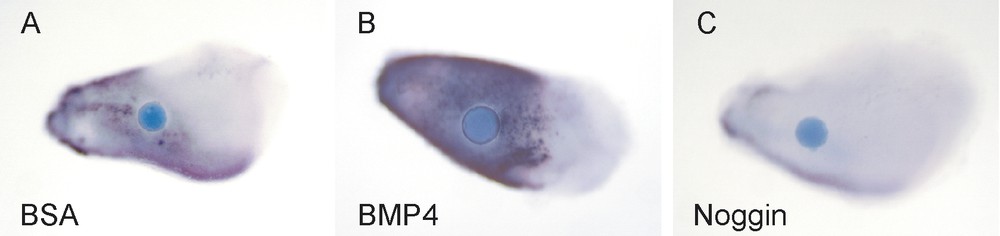
BMP4 induces differentiation of the dental epithelial cells into ameloblasts. Beads releasing the indicated proteins were placed on incisor explants and the expression of the enamel protein Ameloblastin was analysed after 24 h. (A) Some endogenous Ameloblastin expression is seen in the incisor explants (BSA (bovine serum albumin) control bead). (B) The BMP4 bead has induced intense expression of Ameloblastin. (C) Ameloblastin expression is downregulated by the BMP inhibitor Noggin.
Interestingly, at the proliferation stage, Follistatin was shown to antagonize Activin in the lingual epithelium, where it limited the number of lingual stem cells and contributed to the asymmetric growth of the incisor. Activin was shown to be a major stimulator of epithelial stem cell proliferation and, as it was more intensely expressed on the labial than on the lingual side dental mesenchyme, this conceivably also contributed to the asymmetric growth of the incisor. In vitro experiments where BMP4 releasing beads were inserted on cervical loop tissue indicated that BMP4 dramatically inhibited the expression of Fgf3, whereas Activin-releasing beads induced ectopic expression of Fgf3 in the lingual mesenchyme. When Activin-releasing beads together with BMP4 beads were inserted on the cervical loop explants, the repressive effect of BMP4 on Fgf3 expression was inhibited. Finally, when the cervical loop explants were grown for four days in the presence of Activin in the culture medium, a dramatic stimulation of epithelial proliferation was seen, and the loops on both labial and lingual sides had developed extra buds. It was concluded that Activin counteracts the inhibitory effect of BMP4 on Fgf3 expression and thereby restricts Fgf3 expression to labial dental mesenchyme, and that this results in increased epithelial cell proliferation and a large labial cervical loop with abundant stem cells (Fig. 3).
The results of these studies indicated that BMP4 has opposite roles on the proliferation and differentiation of the dental epithelial stem cells: it inhibits the proliferation of stem cells, whereas it stimulates their differentiation into ameloblasts (Fig. 3). These conclusions were supported by the phenotype of the incisors of transgenic mice overexpressing the BMP inhibitor Noggin in the epithelium (K14-Noggin mice). Their incisors are large and dramatically overgrown, and they lack enamel [11].
Several observations indicated that the primary target tissue of mesenchymal Activin and BMP4 is the cervical loop epithelium. However, their effects on the proliferation of this epithelium apparently took place secondarily via the regulation of mesenchymal Fgf3, which stimulates epithelial proliferation. The direct targets of BMP and Activin in the epithelium as well as the reciprocal epithelial signals regulating Fgf3 in mesenchyme remain to be identified in future studies.
Conclusions
The work presented here shows how the spatially restricted and balanced effects of specific components of a signalling network can regulate stem cell proliferation and differentiation. The findings indicate that the continuous growth and asymmetry of the incisor, as well as the formation of enamel, can be significantly affected by modulating a complex network of FGF, BMP, and Activin signals that regulates dental epithelial stem cells.
Continuously growing teeth are common in many animals and there are variations in the extent of enamel coverage of the teeth. For example, even primates possess ever-growing incisors, as seen in the Aye-aye lemurs [12], and elephant incisors (tusks) have no enamel. Some rodents such as voles have continuously growing molars in addition to the ever-growing incisors. Work from our laboratory had previously shown that the cervical loops of continuously growing molars were anatomically similar to those of incisors, and that components of the same molecular signal network were in place [13]. Hence, it is possible that the evolutionary variation in the growth capacity of teeth and the extent of enamel deposition has resulted from fine-tuning of the complex signal network that regulates the maintenance, proliferation, and differentiation of epithelial stem cells. In addition, subtle variations in this or related regulatory networks may explain the different regenerative capacities of various organs and animal species.


