1 Introduction
Water is one of the most important ecological factors determining crop growth and development; water deficit plays a very important role in inhibiting the yields of crops [1]. Water-limited crop production depends on the intensity and on the pattern of drought, which vary from year to year. In some temperate environments, however, there is a high probability that crop water deficit increase in severity as the season progresses, due to lack of rainfall and to the high evaporative demand [2]. An efficient use of limited water resources and better growth under limited water supply are desirable traits for crops in drought environments. Crop production and sustainable development are severely constrained by water limitations during the growing season [3]. In recent years, many studies about the effects of supplemental irrigation on yield performance and water use efficiency (WUE) have shown that proper supplemental irrigation can increase crop yield by significantly improving soil water conditions and their WUE [4]. Improving the efficiency of water use in agriculture is associated with increasing the fraction of the available water resources that is transpired, because of the unavoidable association between yield and water use [5,6].
Drought stress occurs when the available water in the soil is reduced and atmospheric conditions cause continuous loss of water by transpiration or evaporation. One way to ensure future food needs of the increasing world populations should involve a better use of water by the development of crop varieties that need lesser amounts of water and that are more tolerant to drought [7]. Water-stress tolerance is seen in all plant species, but its extent varies from species to species. Although the general effects of drought on plant growth in crop plants are fairly well known [8,9], the primary effects of water deficit on medicinal plants are not well understood [10].
A better understanding of the physiological strategy adopted by a drought-resistant variety to cope with water deficit requires thorough study of the relationship between WUE and transpiration. In crops, the detrimental effects of water deficits on the harvest index (HI) also minimize the impact of the water limitation on crop productivity and increase the efficiency of water use [4]. Therefore, transpiration efficiency (TE) and HI are three important avenues for the importance of agricultural productivity. Additionally, the aerial environment plays a role in determining the carbon-gain-to-water-use ratio, because the vapour pressure deficits between the leaf and the air determine the transpiration rate [11]. WUE is the ability of the crop to produce biomass per unit of water transpired, and HI is the fraction of total dry matter harvested as yield. The efficiency for producing dry matter per unit of absorbed water, and the ability to allocate an increased proportion of the biomass into grains are also important factors. In this scenario, WUE, transpiration efficiency, and HI can be regarded as important adaptive traits to drought environment.
Catharanthus roseus (L.) G. Don. is one of the most important medicinal plants and is also an ornamental bedding plant belonging to the family Apocyanaceae. Two distinct varieties based on the flower colour exist in C. roseus viz., the pink-flowered rosea and the white-flowered alba [12]. C. roseus plant got commercial importance due to the presence of medicinally important alkaloids and also by its ornamental value [13]. Previous works revealed the influences of triadimefon on the antioxidant metabolism and ajmalicine production [14], on the paclobutrazol-mediated growth regulation [15], on salinity problems [16] and salt-stress protection by paclobutrazol [17] in C. roseus. The present study was made to check the WUE under drought condition in C. roseus varieties (rosea and alba) by the gravimetric method.
2 Materials and methods
2.1 Seed Collection and pot culture
The seeds of both varieties of Catharanthus roseus (L.) G. Don. (Family: Apocynaceae) were collected in the Botanical Garden of the Department of Botany, Annamalai University, Tamil Nadu, India. The experiments were conducted at the botanical Garden and the Plant Stress Physiology Laboratory, Department of Botany, Annamalai University, Tamilnadu, India. The seeds were surface sterilized with a 0.2% mercuric chloride (HgCl2) solution for five minutes, with frequent shaking, and were thoroughly washed with tap water. The experiments were carried out in plastic pots by the gravimetric method. The pots were washed thoroughly with tap water and holes were made near the bottom of the containers. The weights of the empty pots were recorded, and they were filled with 10 kg of uniform soil mixture containing red soil, sand, and farmyard manure (FYM) in 1:1:1 ratio. The pot culture studies were conducted to measure the WUE and other related parameters more precisely under two different moisture levels. The pots were maintained under rainout shelter (ROS).
2.2 Plant cultivation and stress induction
Six seeds were planted in each pot and watered to FC to facilitate germination. On the 15th day after sowing (DAS), the seedlings were thinned to two healthy plants per pot. Plants were watered daily until the 30th DAS. On the 30th DAS, all pots were saturated with water and any excess was allowed to drain. Treatments were imposed from the 30th to the 70th DAS. All pots were watered to initial weight with ground water on every other day to maintain 60 and 100% FC. The exposed soil surface was covered with pieces of polythene to minimize soil evaporation. Water loss was determined by weighing the pots daily using an electronic weighing device. One pot from each replication was kept with soil and plastic mulch, but without plants, for monitoring soil evaporation in the absence of the plants. The experiment was terminated on the 70th DAS. The principle of the determination of the water-use efficiency by this technique consists in assessing the increase in biomass during a particular growth stage (30th to 70th DAS) and the cumulative water transpired (CWT) during this period as per Udayakumar et al. [18].
2.3 Analysis of various parameters
The observations recorded during the pot-culture experiment were CWT, leaf area duration (LAD), mean transpiration rate (MTR), net assimilation rate (NAR), and WUE. The biomass accumulated during the treatment period (30th and 70th DAS) was computed as the difference in the initial and final dry matter and expressed as gm plan−1. LAD was measured as days, where is the initial leaf area and is the leaf area at the end of the treatment period. The amount of water added daily to each pot after weighing to bring back to 100% FC was summated individually for each pot during the treatment period. The transpiration rate over the entire experimental period was measured as MTR. MTR was obtained by computing the CWT-to-LAD ratio and expressed as ml of water dm−2 leaf area day−1. Measurement of WUE by gravimetric approach involves the measurement of the dry matter accumulated over a specific period of time and the total water transpired by the plant during the same period. NAR was determined as the ratio of total dry matter (TDM) during the treatment period to LAD and expressed in dm−2 day−1. Individual plants were separated into stems, leaves and roots. The plants parts were dried at 70 °C for 2 days, then weighed. Dry matter (DM), NAR, CWT, MTR, LAD, WUE (ratio of the dry matter to the total water applied), and HI (ratio of grain yield to dry matter) were determined.
2.4 Statistical analysis
Statistical analysis was performed using the one-way analysis of variance (ANOVA) followed by Duncan's Multiple Range Test (DMRT). The values are mean ± SD for seven samples in each group. P values ⩽0.05 were considered as significant.
3 Results and discussion
Drought stress (60% FC) decreased the LAD on 70 DAS when compared to control (100% FC) in both varieties (Figs. 1 and 2). Among these varieties, alba had a reduced LAD. LAD was reduced under drought condition in higher plants [19–21]. The CWT level decreased in both varieties under drought stress (Fig. 3). Among the varieties, rosea showed a lower level of transpiration rate. A higher level of transpiration rate occurred in alba. These results are similar to those reported for other plant species, like cowpea cultivars, and in many tree species found to have a higher mean stomata frequency on the lower side than on the upper side of their leaves, which leads to reduced transpiration [22,23].
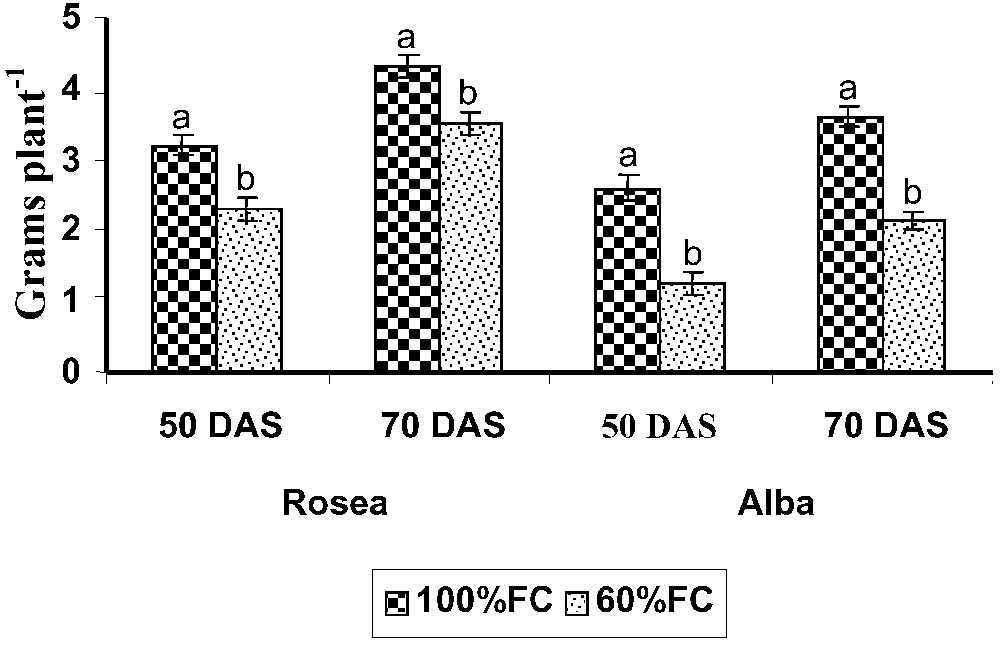
Effect of drought-stress-induced changes in whole-plant dry weight of rosea and alba varieties of Catharanthus roseus. Values are given as mean ± SD of seven samples in each group. Bar values that are not sharing a common superscript (a, b) differ significantly at p⩽0.05 (DMRT).
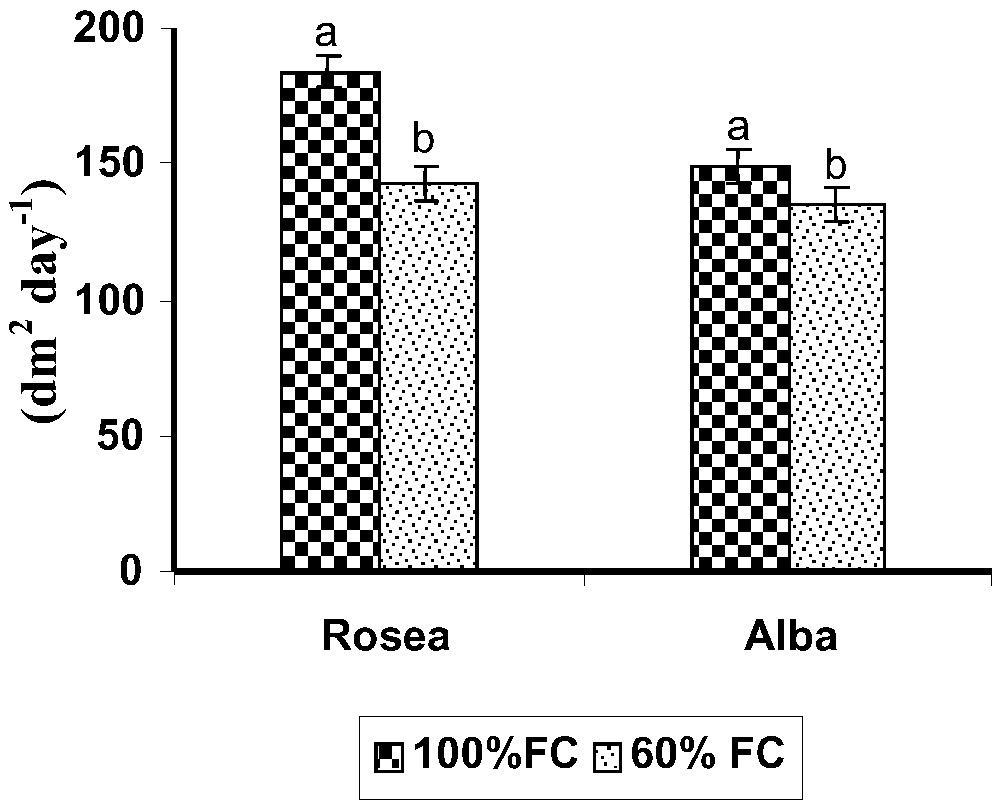
Leaf area duration (dm2 day−1) during the pot culture experimental growth period in two moisture regimes in Catharanthus roseus varieties on 70 days. Values are given as mean ± SD of seven samples in each group. Bar values that are not sharing a common superscript (a, b) differ significantly at p⩽0.05 (DMRT).

Cumulative water transpired (kg plant−1) during the pot culture experimental growth period in two moisture regimes in Catharanthus roseus varieties during 70 days. Values are given as mean ± SD of seven samples in each group. Bar values that are not sharing a common superscript (a, b) differ significantly at p⩽0.05 (DMRT).
Water deficit at 60% FC decreased the MTR in both varieties when compared to control (Fig. 4). Among these varieties, rosea showed a very low level of mean transpiration. Under drought conditions, the mean transpiration rate decreased in both varieties studied when compared to control. Among the varieties, rosea showed very low and high level of MTR under drought conditions. The mean transpiration rate was found to be decreased in Vigna unguiculata [24]. The fact that transpiration at the end of the stress period was not significantly affected by water stress despite the reduction of stomatal conductance is a surprising result. This could be due to the fact that a reduced stomatal conductance also increases leaf temperatures and leaf-to-air vapour pressure differences, which both counteract the decrease in leaf conductance [25]. This counteracting effect will be highest when leaf boundary conductance is low, ambient temperature is high and air humidity is low [26]; when these conditions are even only partly met, transpiration efficiency (TE) rather than transpiration is more likely to reflect WUE (Fig. 5), as shown in spring wheat cultivars grown under water-stress conditions. On the other hand, transpiration was quantified in saturating CO2 conditions, which may mitigate the effect of water stress on stomatal resistance [27].

Mean transpiration rate (water loss leaf area−1) during the pot culture experimental growth period in two moisture regimes in Catharanthus roseus varieties during 70 days. Values are given as mean ± SD of seven samples in each group. Bar values that are not sharing a common superscript (a, b) differ significantly at p⩽0.05 (DMRT).

Water use efficiency (g DW kg−1 H2O) during the pot culture experimental growth period in two moisture regimes in Catharanthus roseus varieties during 70 days. Values are given as mean ± SD of seven samples in each group. Bar values that are not sharing a common superscript (a, b) differ significantly at p⩽0.05 (DMRT).
Drought stress caused decreased dry weight in both varieties of C. roseus. The rosea variety was the highest responder to water stress when compared to control on 70 DAS. A decrease in the plant biomass and the dry weight was found in many previous works in various plants [28]. The decrease in total dry weight may be due to the considerable decrease in plant growth, photosynthesis and canopy structure, as indicated by leaf senescence during drought stress [29–32].
The fact that the two varieties investigated in the present work exhibited some contrasting physiological properties may help to evaluate the relative importance of these properties in terms of whole plant response. The two varieties were studied in terms of water consumption at end of the experiment. A significant varietal difference was observed under different moisture levels. The genotypic difference may play an important role in the WUE variation found in the two varieties [6,33]. Many previous investigations reported increased water use efficiency in different plant species [2,34,35]. The NAR decreased in all the drought-stressed C. roseus varieties (Fig. 6). Among the varieties, rosea showed the highest NAR. The NAR was relatively lower in drought-stress conditions in cluster bean [36]. The decrease in NAR strongly indicated a stomatal closure factor for reduction in the presence of an increased level of stress [37]. Water stress leads to a decline in net photosynthesis and an alteration of the chloroplast capacity [5,38]. The HI (Fig. 7) decreased on the 70th DAS in all the bhendi varieties when compared to control (100% FC). The reduction in HI was reported in many previous investigations on drought-stress influence [24,39,40]. Drought-stressed plants significantly reduced their fruits' dry matter, and this is possibly due to a source limitation resulting from large carbon demands under water-stress-induced limitations on photosynthesis [41].

Net assimilation rate (μg cm−2 day−1) during the pot culture experimental growth period in two moisture regimes in Catharanthus roseus varieties during 70 days. Values are given as mean ± SD of seven samples in each group. Bar values that are not sharing a common superscript (a, b) differ significantly at p⩽0.05 (DMRT).
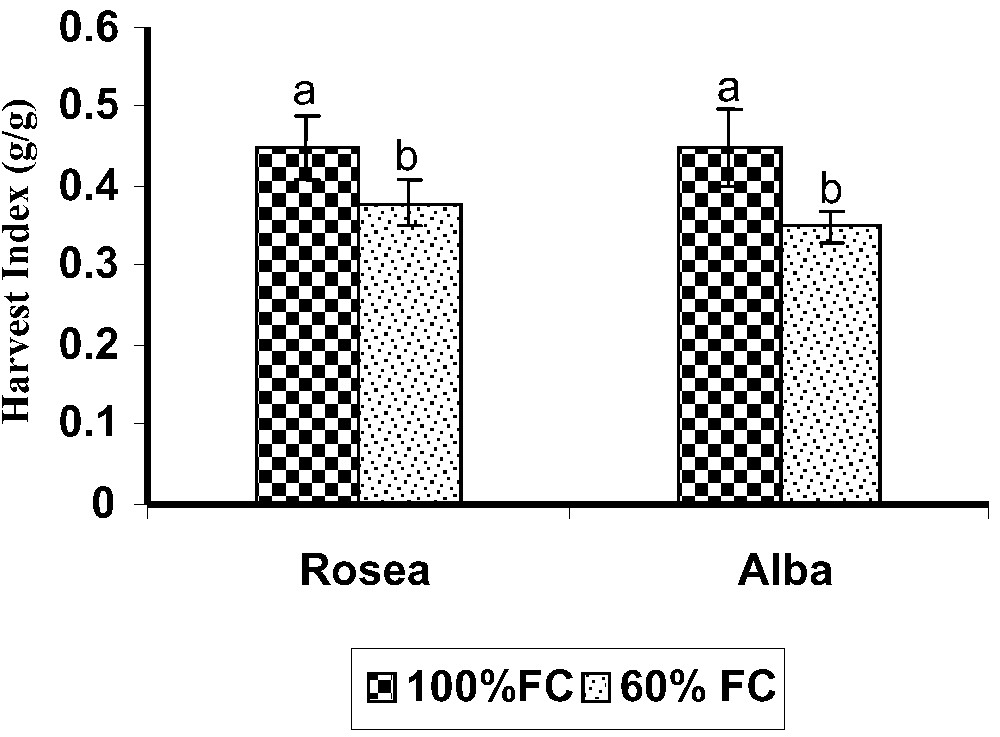
Harvest index (g/g) during the pot culture experimental growth period in two moisture regimes in Catharanthus roseus varieties on 70 days. Values are given as mean ± SD of seven samples in each group. Bar values that are not sharing a common superscript (a, b) differ significantly at p⩽0.05 (DMRT).
4 Conclusion
The LAD, CWT, NAR, MTR and HI decreased under a drought stress of 60% FC in both varieties. Among the varieties, the highest WUE was recorded in rosea, and the lowest WUE was found in the alba variety. From the results of this investigation, it can be concluded that the rosea variety of Catharanthus roseus is well suited for commercial cultivation in water-deficit areas; hence its higher alkaloid and antioxidant potentials were already proved [14]. However, the data presented here reflect the importance of a physiological analysis of plant response to water deficit stress, which must accompany field experiments and evaluation. Further investigations are required to ascertain this conclusion.


