1 Introduction
Herbaceous ecosystems represent more than 25% of the Earth's landscapes [1]. In Europe, despite the fact that their origins are still controversial [2–4], their persistence is due to grazing by domestic livestock since the Neolithic [5,6]. Grazing by large mammals has a direct effect on vegetation by modifying the structure and the composition of plant communities [7,8], and by limiting or excluding ligneous species establishment [9]. Moreover, moderate grazing is known to maintain greater plant species richness and the abandonment of this intermediate disturbance regime usually leads, sooner or later, to a decrease in plant species richness [10–12]. The effects of grazing on animal communities, which are associated with these rich herbaceous communities, are more difficult to apprehend, particularly for insects. Indeed, the impacts of grazing on insect communities are mainly indirect through the modifications of vegetation structure and species composition [13,14]. Studies carried out in the North of Europe have shown that species richness is maximized under intermediate grazing regimes [14,15], with variable responses depending on the trophic group considered [16].
In the Mediterranean Basin, the effect of grazing on vegetation is amplified by particular climatic and soil conditions (aridity, drought, oligotrophic soils) greatly reducing the successional dynamics of plant communities [17]. This leads to herbaceous semi-natural steppe-like vegetation, often rich in plant species [18]. Since steppe ecosystems tightly evolved with recurrent grazing regime over long periods of time (several centuries), they represent good models to study the role of this type of disturbance regime on their dynamics and biodiversity.
The plain of La Crau (Bouches-du-Rhône, southeastern France) is partly covered with a steppe ecosystem unique in France [19]. Its vegetation has been initially described by [20] as dry grasslands with Asphodelus ayardii (Asphodeletum fistulosi phytosociological community). These grasslands have been used as pastures for itinerant sheep grazing for several centuries, as shown by archaeological traces dating from the Neolithic, the Antiquity and the Middle Ages [21]. Grazing thus allowed the vegetation to remain prostrated and dominated by two species, Brachypodium retusum (Pers.) P. de Bauv. and Thymus vulgaris L. This vegetation included both calcicolous and silicolous species with locally rare species, such as Taeniatherum caput-medusae (L.) Nevski, Hyssopus officinalis L., Plantago holosteum Scop. or Bufonia tenuifolia L. [19].
The plain of La Crau is known to provide a suitable habitat for several protected or threatened animal species, such as the pin-tailed sandgrouse (Pterocles alchata L.), the little bustard (Tetrax tetrax (L.)), the lesser kestrel (Falco naumanni Fleischer) and the jewelled lizard (Timon lepidus (Daudin)). The plain shows relatively rich terrestrial arthropod communities as well, which are characteristic of open and arid biotopes of the Mediterranean region [22–24], with endemic species such as an apterous grasshopper (Prionotropis rhodanica Uvarov) [25–27].
Although this ecosystem is unique in France, the structure and ecological processes of La Crau plant communities are representative of those of other herbaceous communities, such as ‘Dehesas’ in Spain (3 million ha), ‘Montados’ in Portugal (700,000 ha) and the semi-arid steppes of Northern Africa and of the eastern Mediterranean [28]. In La Crau, 60,000 ha of steppe were greatly degraded and fragmented by human activities in the 20th century (cultivation, quarries, industry, etc.). About 11,000 ha of steppe currently remain. Numerous studies have quantified the impact of human disturbances on plant communities [29,30], birds [31,32], Prionotropis [25–27], and beetles [23,24]; however, few data are available on the dynamics of plant communities and their associated entomofauna once grazing is abandoned. The overall global warming hypothesis in the Mediterranean Basin projects a 2 °C increase in the average annual temperature and an extension of drought periods over the next 100 years. Such a change may lead to an extension of evergreen species at the expenses of the traditional grazing practices in the western Mediterranean Basin [33]. This may cause major changes in specific arthropod communities, and in beetle assemblages in particular [24].
Beetles are believed to contribute more than 40% to the world's number of described species of insects [34,35], with a major proportion of rare or endangered species [35–37]. They occupy almost all types of available habitats and are present at all levels of trophic chains [38–41]. In La Crau, the number of species of Coleoptera and their biomass (591.5 g ha−1 annual mean) exceed that of other groups of Arthropods [22].
Through a diachronic study carried out on exclosures at different times, the present work aims to (1) study the composition and structure of beetle assemblages on a temporal gradient of grazing abandonment going from four to 23 years, and to (2) address the results within the framework of the future conservation management plan for the plain.
2 Methods
2.1 The plain of La Crau
The former delta of the Durance River, the plain of La Crau (Bouches-du-Rhône, southeastern France) is found within a triangle formed by the cities of Salon-de-Provence, Arles and Fos-sur-Mer. The region has a Mediterranean climate, with long hot summers, mild winters (mean annual temperature: 15 °C), and main rainfall occurring in spring and autumn, reaching an average of 545 mm yr−1. The yearly average of sun exposure is over 3000 h and a NW–SE strong wind blows more than 334 days/year, 110 days/year over 50 km/h [19]. The substrate is a layer 5 to 40 m thick, with, at the top of this layer an impermeable conglomerate made of a calcareous matrix and a mixture of calcareous and siliceous stones that makes the water table inaccessible to the vegetation [19]. The ground is characterized by a cover of large stones deposited by the Durance River, which, by filling up its bed, progressively moved westward, while the water flow decreased between 600 000 and 30 000 BP. In the plain, this led to a reduction in the size of the deposited stones across a gradient going from the northeast to the southwest [19]. Stones are likely to create a microclimate by (i) protecting the soil from high temperature variations (60 °C on the soil surface and 30 °C under stones in summer [42]), (ii) reducing evapotranspiration [43], (iii) allowing water condensation [44], (iv) protecting plants from grazing [45], and (v) favouring perennial species [46]. Stones thus play an important role in structuring vegetation and the variation in stone cover in this grazed plain leads to various steppe types [19]. In areas where stones are dispersed, thus providing limited protection against grazing, vegetation is prostrate and dominated by annual species, there are few Brachypodium plants and numerous bare ground patches. Conversely, in areas where stones are dense, vegetation is taller, dominated by Brachypodium and there are few to no bare ground patches.
2.2 Study sites and exclosures
In 2004 and 2005, four sites were studied, each having an exclosure of a different age (Fig. 1; Table 1): Merle, located in the North of the plain, at which the exclosure was set in 1982 (23 years ago) was characterised by a deeper soil and a moister weather than in the other sites [19]. The grazing pressure was lower than at the other sites as well; Peau-de-Meau and Termes-Blancs, found in the centre of the plain, with exclosures dating respectively from 1989 (16 years) and 2000 (5 years), were characterised by the same organization of sheep grazing and are located on the same type of soils and weather conditions [19]; Nègreiron, found in the South of the plain, had low stone ground cover, small stones, the highest grazing pressure and an exclosure set up in 2001 (4 years). The spatial distribution of the sites clearly showed various steppe vegetation types depending on climate, soil and grazing regimes that co-exist in the Crau area [30,42]. Indeed, there is a climatic gradient from the north (colder winters) to the south (more temperate winters) of the plain [19], as well as a stone size and density gradient (depending on the past hydraulic regimes of the Durance River) and a grazing gradient that depends on stone cover (plant species accessibility to sheep) and on climate (phenology). Nevertheless, despite these differences, all the sites were characterised by the same plant association (Asphodeletum fistulosi phytosociological community) and by sheep grazing that occurred since the Neolithic period; so, they can be considered as replicates of the treatment ‘presence or absence’ of grazing. The selected exclosures are large (0.2, 0.25, 3, and 3.7 ha) and were sampled in their centre, more than 10 m away from the fences in order to minimise edge effects [47–49] and the ‘fence effect’ [50]. These exclosures are larger than those used in other studies on the interaction between large mammals' grazing and arthropods [51–53].
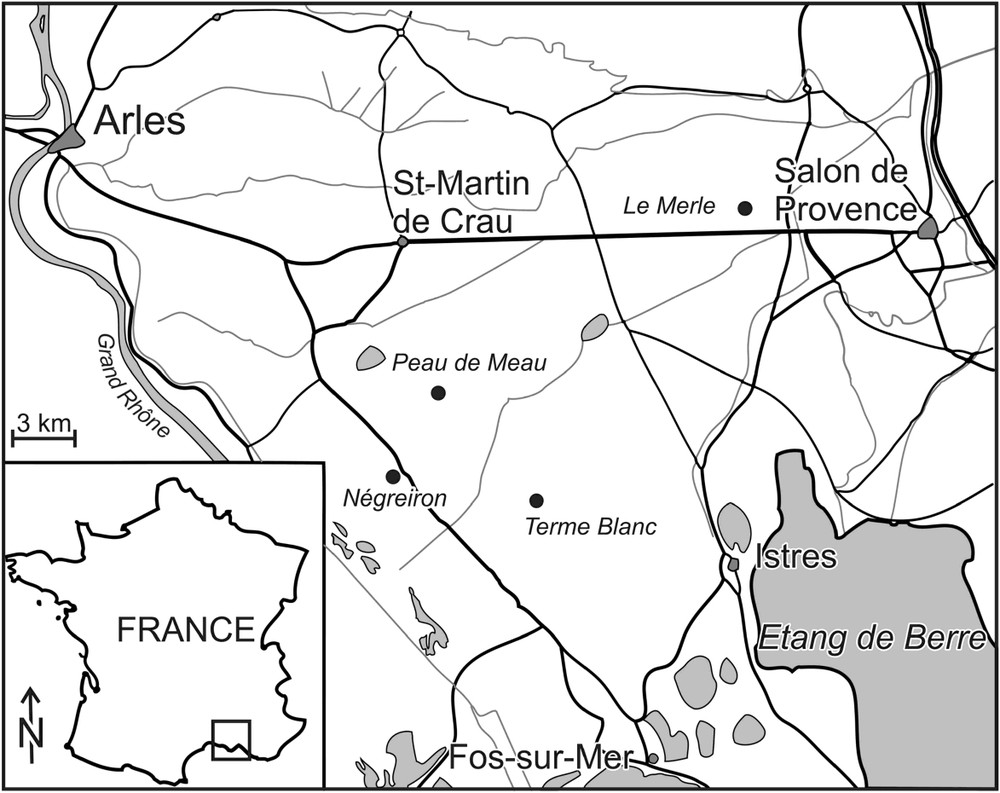
Site location in France and in the plain of La Crau.
Details on studied exclosures
| Location | Year | Area | Coordinates | Former studies |
| Merle | 1982 | 0.2 ha | 43°38′57.10″N | [54,55] |
| 5°00′48.81″E | ||||
| Nature Reserve of Peau-de-Meau | 1989 | 0.25 ha | 43°33′25.41″N | [56,57] |
| 4°49′50.37″E | ||||
| Termes-Blancs | 2000 | 3.7 ha | 43°32′04.70″N | [58] |
| 4°52′06.09″E | ||||
| Nègreiron | 2001 | 3 ha | 43°32′20.90″N | [58] |
| 4°49′05.66″E |
2.3 Sampling design
For each of the four sites, beetles were sampled inside (ungrazed) and outside (grazed) the exclosures. Within these two treatments, sampling was carried out far enough (more than 10 m) from the fences to avoid any bias [50]. The sampling design was established to the scale of the smallest exclosure (Merle). For each sampled zone (8), six sampling units (SU) were set on two transects (Fig. 2). One sampling unit is constituted by three pitfall traps (plastic containers 50 mm × 110 mm half-filled with conservative liquid glycol), each one set at the tip of a 1 m equilateral triangle. The sampling units were spatially arranged so that two traps of different sampling units were more than 10 m apart from each other [59]. In total, 144 traps were buried flush with the soil surface. Sampling was carried out during two consecutive years in 2004 and 2005 in order to include interannual climatic variations. Traps were checked and replaced twice a month continuously from the beginning of April to the end of June and from mid-August to the beginning of November. Sampling was not carried out in winter and summer as Coleoptera species richness is very low during these seasons in La Crau [22,23]. Imagoes of Coleoptera were sorted out to morphospecies [60] and then identified to species or the closest taxonomic level using the laboratory reference collection. Taxonomic nomenclature follows Fauna Europea [61]. Species were classified into four basic functional groups, namely phytophagous, predators, saprophagous (including necrophagous) and coprophagous [62], based only on the imago diet. Coprophagous group was not considered for multivariate analyses.
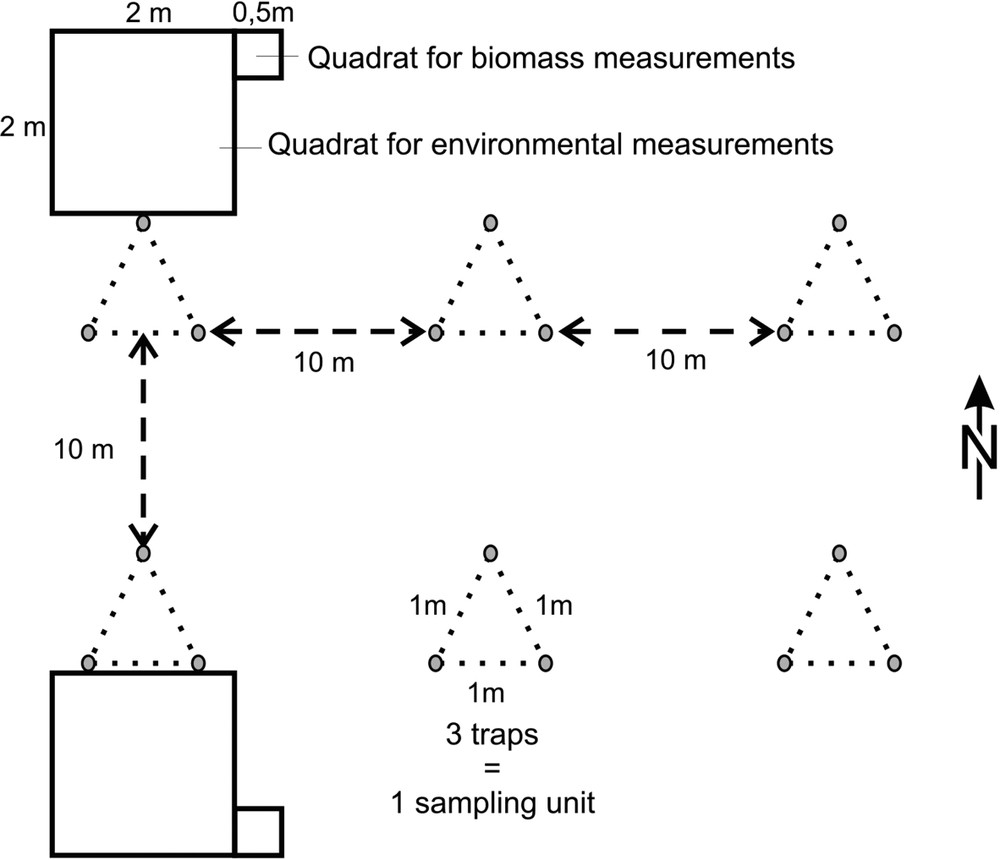
Sampling design in each of the four exclosures and in their associated closed by grazed areas.
Environmental measurements were carried out in May 2005 near each sampling unit in a quadrat (Fig. 2) divided into 25 sub-quadrates of 0.16 m2 using the frequency method [63] (vegetation species richness, T. vulgaris and B. retusum percentage cover, stone percentage cover, bare ground percentage cover, vegetation percentage cover for three groups of plant heights: , 5 to 20 cm, ) (see [63]). Vegetation biomass was assessed near each sampling unit by cutting all plant material at 3-cm height and above in a quadrat. Biomass was dried (70 °C) until weight was constant and weighed.
2.4 Statistical analyses
All traps sampled at the same spot all along the study period were summed, thus excluding seasonal and annual variations. All three traps sampled in each sampling unit were also summed [23]. The resulting data matrix thus includes 48 SU ( sampled zones).
Correspondence analyses (CA) were performed in order to discriminate the sites and the changes induced by grazing abandonment (Canoco 4.5). These analyses were first processed with all species but coprophagous, and then considering individually phytophagous, predators, and saprophagous species. The ‘down-weighting of rare species’ option was applied to reduce the inertia of the less abundant taxa [64]. A canonical correspondence analysis (CCA) was performed on the all species but coprophagous matrix (Canoco 4.5), in order to compute species data with environment variables, also applying the ‘down-weighting of rare species’ option. In order to compare each grazed area with the corresponding ungrazed area of each site, Mann–Whitney U tests (Statistica 6.0) were carried out on (i) abundance, species richness and evenness data, (ii) abundance and species richness within each trophic group, (iii) abundance of the 30 most frequent species, and (iv) vegetation richness and evenness. The diversity was assessed with evenness (E), calculated from Shannon index on the data of each sampling unit, using the formula [65].
In order to quantify changes induced on species composition, Sørensen (presence/absence data) and Steinhaus (species abundance) similarity indexes were calculated for all sampled zones [66].
Abundance, species richness and abundance of the three most frequent species were compared with environmental data using Spearman rank tests (Statistica 6.0).
3 Results
3.1 Beetle assemblages
In total, 7533 individuals, belonging to 42 families and 221 species, were captured (Table 2; supplementary material). The dominant species were Poecilus sericeus Fisher von Apfelbeck (Carabidae, 2402 individuals, 31.9% of total abundance), Asida sericea (Olivier) (Tenebrionidae, 864 individuals, 11.5%) and Coniocleonus nigrosuturatus (Goeze) (Curculionidae, 506 individuals, 6.7%). The other 218 species contributed to less than 50% of total abundance, of which 70 species were represented by only one individual (singletons).
Total number of species collected in the grazed (G) and ungrazed (X) areas
| Zone | Grazed areas | Exclosures | Total | |
| Species | Common exclusive | 110 | 221 | |
| 50 | 61 | |||
| Total | 160 | 171 |
The CA considering all species but coprophagous (i.e. [48 SU × 208 species]) discriminated the four sites and the two treatments (grazed/ungrazed) (Fig. 3). Axis 1 (21.7%), opposing the samples of Nègreiron, Termes-Blancs and Peau-de-Meau with those of Merle. Axis 2 (12.9%) discriminated the grazed areas from surrounding ungrazed areas. Moreover, the longer since grazing abandonment, the further the ungrazed areas were from their respective grazed area on the scatterplot.

CA on the [48 SU × 208 species] matrix applying the down-weighting of rare species option. (a) Sampling units (Neg = Nègreiron, TB = Termes-Blancs, PdM = Peau-de-Meau and Mer = Merle; ‘X’ = exclosure). Ellipses gather sampling units from a same sampling zone (white for grazed areas and shaded for exclosures). (b) Species. Only species contributing to more than 1% of the total inertia are shown (32 species: Acpi = Acinopus picipes; Anin = Anotylus inustus; Antr = Anthicus tristis; Apeu = Aphtona euphorbiae; Asse = Asida sericea; Atsp = Atheta sp.; Bime = Bioplanes meridionalis; Brbi = Bruchidius bimaculatus; Brfo = Bruchidius foveolatus; Caco = Carabus coriaceus; Chdo = Charopus docilis; Cima = Cicindela maroccana; Coni = Coniocleonus nigrosuturatus; Enpi = Enicopus pilosus; Ente = Endomia tenuicollis; Hyda = Hypera dauci; Losu = Longitarsus succineus; Mefu = Melanophtalma fuscipennis; Milu = Microlestes luctuosus; Ocob = Ocypus obscuroaenus schatzmayeri; Ocop = Ocypus ophtalmicus; Oecr = Oedemera crassipes; Opsu = Ophonus subquadratus; Pose = Poecilus sericeus; Prob = Protaecia oblonga; Psci = Pseudocleonus cinereus; Ptse = Ptomaphagus sericatus; Rhci = Rhizotrogus cicatricosus; Seim = Sepedophilus immaculatus; Spru = Sphaeroderma rubidium; Tani = Tachyporus nitidulus).
In grazed areas, the highest number of individuals was found in Nègreiron (1289 individuals) and the highest species richness was found in Merle (104 species; Table 3). Grazing abandonment significantly led to (Fig. 4):
- – an increase in species richness and evenness and no changes in abundance in Nègreiron (4 years);
- – an increase in abundance, no changes in species richness and a decrease in evenness in Termes-Blancs (5 years);
- – a decrease in abundance, no changes in species richness and an increase in evenness in Peau-de-Meau (16 years);
- – no change in abundance, species richness or evenness in Merle (23 years).
Total abundance and species richness at each site, in the grazed (G) and ungrazed (X) areas
| Nègreiron | Termes-Blancs | Peau-de-Meau | Merle | Total | |||||
| G | X | G | X | G | X | G | X | ||
| Abundance | 1289 | 1109 | 792 | 1424 | 823 | 572 | 778 | 746 | 7533 |
| Species richness | 71 | 85 | 50 | 53 | 75 | 78 | 104 | 98 | 221 |

Mean (a) abundance, (b) species richness, and (c) evenness for each sampling unit at each site (4 yrs = Nègreiron, 5 yrs = Termes-Blancs, 16 yrs = Peau-de-Meau, and 23 yrs = Merle) and for each grazed (G) of ungrazed (X) area (±SE). Mann–Whitney U tests (n=6): *p<0.05; **p<0.01.
Sørensen similarity indexes (presence/absence) calculated between the assemblages in grazed areas and in ungrazed areas were 0.59, 0.54, 0.63 and 0.47 in Nègreiron, Termes-Blancs, Peau-de-Meau and Merle, respectively. Steinhaus similarity indexes (abundance) were respectively 0.60, 0.54, 0.33 and 0.27 (Fig. 5). The composition of the assemblage was therefore similar in Nègreiron after four years of grazing abandonment, and differed progressively across times. The different trends observed in Fig. 5 between the evolution of the Sørensen and of the Steinhaus indexes in Peau-de-Meau and Merle (16 years and 23 years) showed that some species can be found both in the grazed area and in the exclosure, but with a different abundance distribution.
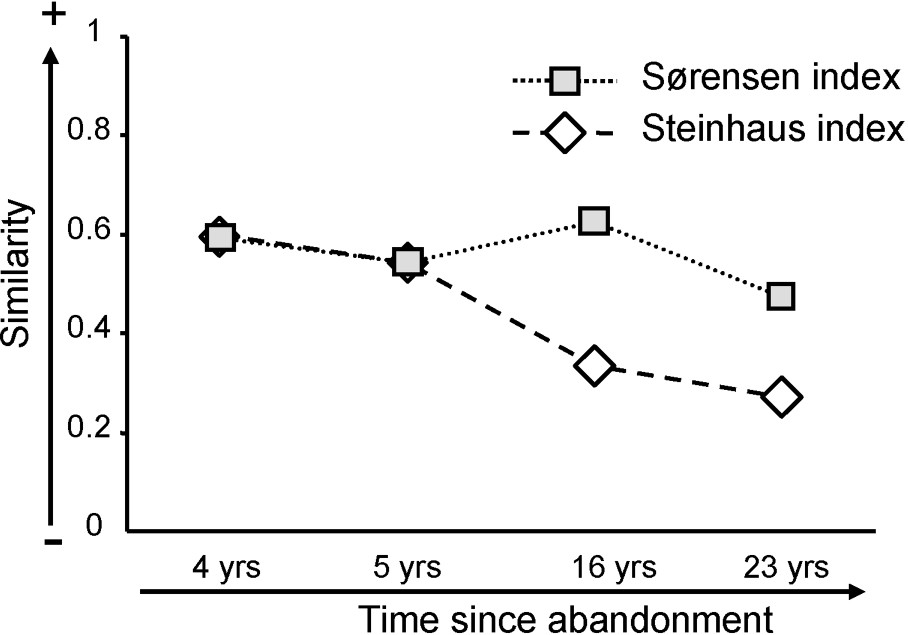
Evolution of Sørensen (presence/absence) and Steinhaus (abundance) similarity indexes between beetle assemblages in both grazed and ungrazed areas for the four sites, which are related to time of grazing abandonment (non-proportional temporal scale).
Abundances of two species, Asida sericea and Coniocleonus nigrosuturatus, significantly decreased in all the exclosures. Conversely, abundance of Microlestes luctuosus (Carabidae) and Sepedophilus immaculatus (Staphylinidae) significantly increased in all the exclosures older than three to four years (in Termes-Blancs). A similar trend was observed for a larger Staphylinidae, Ocypus ophtalmicus, with a significant increase in Termes-Blancs only (Fig. 6).

Abundances of Asida sericea, Coniocleonus nigrosuturatus, Microlestes luctuosus, Sepedophilus immaculatus, Ocypus ophtalmicus, and Poecilus sericeus at the four sites (4 yrs = Nègreiron, 5 yrs = Termes-Blancs, 16 yrs = Peau-de-Meau and 23 yrs = Merle) in the grazed (G) and ungrazed (X) areas (±SE). Mann–Whitney U tests between the grazed and ungrazed areas (n=6): *p<0.05; **p<0.01.
The most abundant species, Poecilus sericeus, was not found in Merle. In Termes-Blancs, it was particularly abundant in the exclosure (1070 individuals inside vs. 440 in the grazed area), whereas in Peau-de-Meau, it was less abundant in the exclosures (Fig. 6).
3.2 Trophic groups
After grazing abandonment, the increase in species richness in Nègreiron was due to an increase in the number of predator and saprophagous species (Fig. 7). In Termes-Blancs, the decline in phytophagous species in exclosure was compensated by an increase in predator and saprophagous species. For the other two sites, the contribution of species to each trophic group was relatively stable.
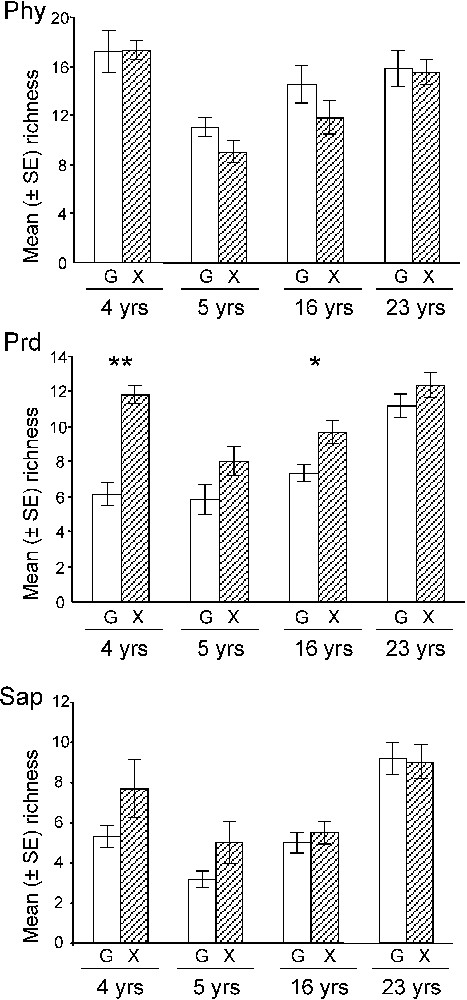
Mean species richness of (a) phytophagous, (b) predators, and (c) saprophagous for each sampling unit at each site (4 yrs = Nègreiron, 5 yrs = Termes-Blancs, 16 yrs = Peau-de-Meau, and 23 yrs = Merle) and for each grazed (G) of ungrazed (X) area (±SE). Mann–Whitney U tests (n=6): *p<0.05; **p<0.01.
Axis 1–2 of the CA on the phytophagous species (29.2%) discriminated the four grazed areas and their respective exclosure. Axis 1–2 of the CA on the predator species (47.2%) and the saprophagous species (33.6%) did not discriminate grazed areas of Nègreiron, Termes-Blancs and Peau-de-Meau, but discriminated the four exclosure areas (Fig. 8) on axis 1.
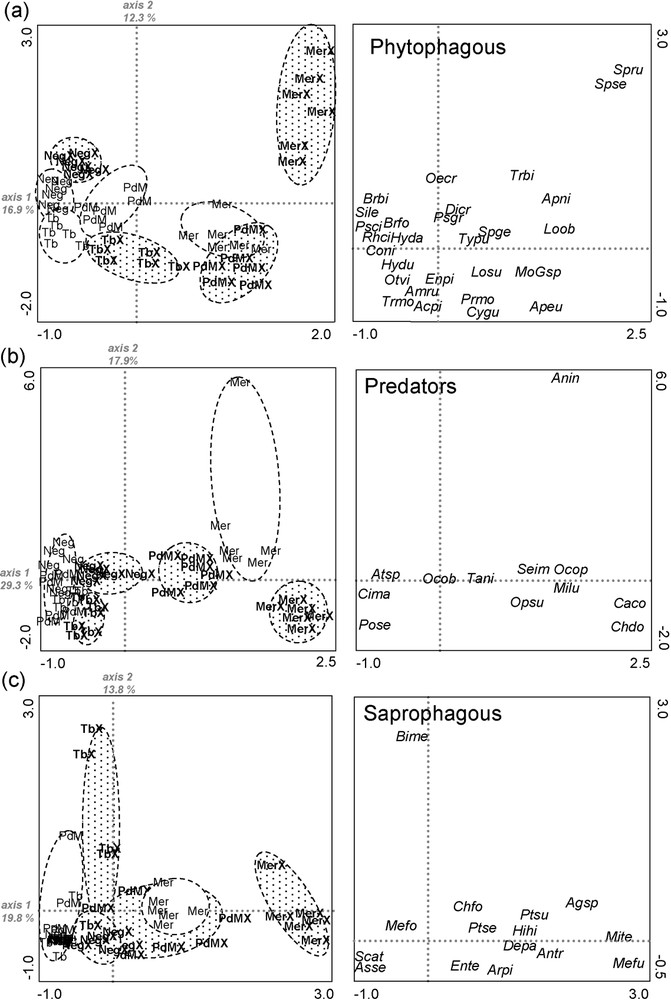
CAs on the (a) phytophagous matrix [48 SU × 101 species], (b) predator matrix [48 SU × 63 species] and (c) saprophagous matrix [48 SU × 44 species] applying the down-weighting of rare species option. Left: sampling units (Neg = Nègreiron, TB = Termes-Blancs, PdM = Peau-de-Meau and Mer = Merle; ‘X’ = exclosure). Ellipses gather sampling units from a same sampling zone (white for grazed areas and shaded for exclosures). Right: species. Only species contributing more than 1% of the total inertia are shown. Phytophagous: Amru = Amphimallon ruficorne; Apeu = Aphtona euphorbiae; Apni = Aphtona nigriceps; Brbi = Bruchidius bimaculatus; Brfo = Bruchidius foveolatus; Coni = Coniocleonus nigrosuturatus; Dicr = Dibolia cryptocephala; Enpi = Enicopus pilosus; Hyda = Hypera dauci; Hydu = Hycleus duodecimpunctatus; Loob = Longitarsus obliteratoides; Losu = Longitarsus succineus; MoGsp = Mordellidae G. sp.; Oecr = Oedemera crassipes; Otvi = Otiorhynchus vitellus; Prmo = Protaetia morio; Psci = Pseudocleonus cinereus; Psgr = Pseudocleonus grammicus; Rhci = Rhizotrogus cicatricuosus; Sile = Sitona lepidus; Spge = Sphenoptera gemmata; Spru = Sphaeroderma rubidium; Spse = Spermophagus sericeus; Trbi = Trachyphloeus bifoveolatus; Trmo = Trachyphloeus monspeliensis; Typu = Tychius pusilus. Predators: Anin = Anotylus inustus; Atsp = Atheta sp.; Caco = Carabus coriaceus; Chdo = Charopus docilis; Cima = Cicindela maroccana; Milu = Microlestes luctuosus; Ocob = Ocypus obscuroaenus schatzmayeri; Ocop = Ocypus ophtalmicus; Opsu = Ophonus subquadratus; Pose = Poecilus sericeus; Seim = Sepedophilus immaculatus; Tani = Tachyporus nitidulus. Saprophagous: Agsp = Agathidium haemorrhoum; Antr = Anthicus tristis; Arpi = Artholips picea; Asse = Asida sericea; Bime = Bioplanes meridionalis; Chfo = Cholovocera formicaria; Depa = Dermestes pardalis; Ente = Endomia tenuicollis; Hihi = Hirticomus hispidulus; Mefo = Merophysia formicaria; Mefu = Melanophtalma fuscipennis; Mite = Microhoria terminata; Scat = Scaurus atratus; Ptse = Ptomaphagus sericatus; Ptsu = Ptomaphagus subvillosus.
3.3 Vegetation
The total number of plant species found was 131, with 119 in the grazed areas and 100 in the exclosures. Eighty-eight species were common to both exclosures and grazed areas. Eleven species were exclusively found in exclosures and 31 in the grazed areas.
Grazing abandonment already led to a significant decrease in vegetation richness after five years of abandonment and to a decrease in vegetation evenness after 16 years (Fig. 9) [63].
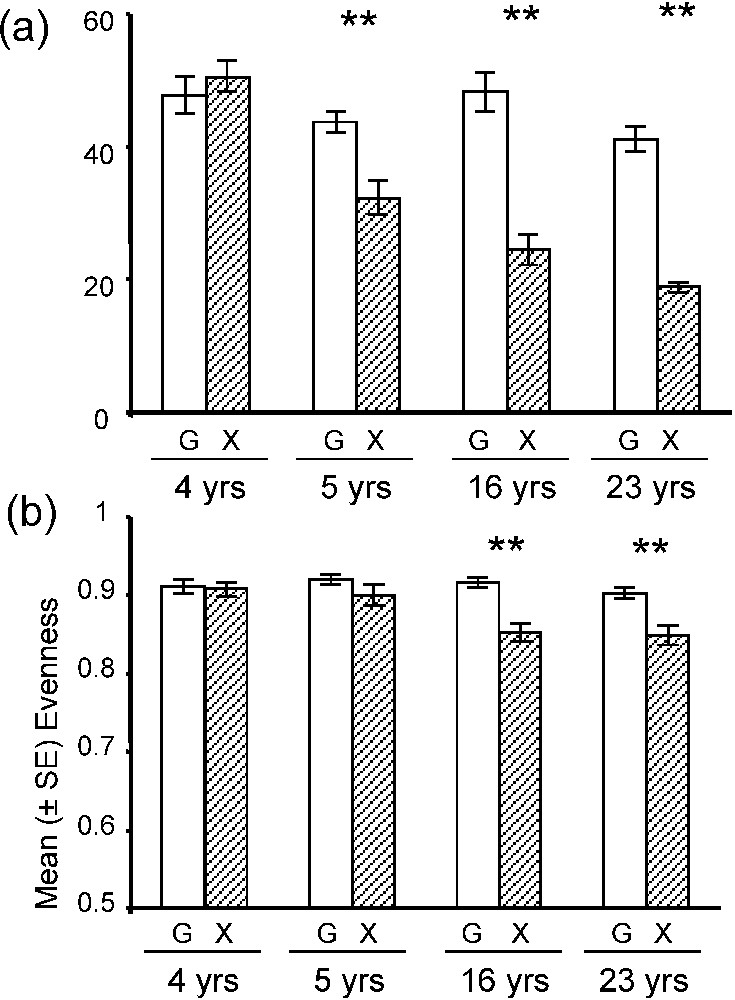
Mean (a) species richness and (b) evenness of plant communities for 4 m2 (4 yrs = Nègreiron, 5 yrs = Termes-Blancs, 16 yrs = Peau-de-Meau and 23 yrs = Merle) and for each grazed (G) of ungrazed (X) area (±SE). Mann–Whitney U tests (n=6): *p<0.05; **p<0.01.
3.4 Environmental data
The CCA (67.3%) discriminated three data sets (Fig. 10). The first was from Nègreiron in the grazed and ungrazed areas (Neg and NegX) and was correlated with plant species richness, bare ground cover and very short vegetation () and with species such as Asida sericea (Asse), Coniocleonus nigrosuturatus (Coni), Pseudocleonus cinereus (Psci), Protaecia oblonga (Prob), Hypera dauci (Hyda) and Oedemera crassipes (Oecr). The second data set was from Peau-de-Meau in the grazed area (PdM) and from Termes-Blancs in the grazed and ungrazed areas (Tb and TbX). This data set was correlated with stone cover, 5–20 cm vegetation height and species, such as Poecilus sericeus (Pose), Bioplanes meridionalis (Bime), Acinopus picipes (Acpi) and Cicindela maroccana (Cima). The third one was from Peau-de-Meau in the ungrazed area (PdMX) and from Merle in the grazed and ungrazed areas (Mer and MerX) and was correlated with cover of vegetation > 20 cm, Thymus cover and biomass, and with species, such as Sepedophilus immaculatus (Seim), Spheroderma rubidium (Spru) or Microlestes luctuosus (Milu).

CCA on the [48 SU × 208 species] matrix compared with environmental data (for 4 m2: Richveg = plant species richness; Biom = vegetation dry biomass, in grams; bare ground% cover; Thy = Thymus% cover; Brachy = Brachypodium% cover; Stone = stone% cover; −5 cm = % cover of vegetation lower than 5 cm; 20–5 cm = % cover of vegetation between 5 and 20 cm; +20 cm = % cover of vegetation higher than 20 cm). (a) Sampling units (Neg = Nègreiron, TB = Termes-Blancs, PdM = Peau-de-Meau and Mer = Merle; ‘X’ = exclosure), (b) species. Only species contributing to more than 1% of the total inertia are shown (32 species: Acpi = Acinopus picipes; Anin = Anotylus inustus; Antr = Anthicus tristis; Apeu = Aphtona euphorbiae; Asse = Asida sericea; Atsp = Atheta sp.; Bime = Bioplanes meridionalis; Brbi = Bruchidius bimaculatus; Brfo = Bruchidius foveolatus; Caco = Carabus coriaceus; Chdo = Charopus docilis; Cima = Cicindela maroccana; Coni = Coniocleonus nigrosuturatus; Enpi = Enicopus pilosus; Ente = Endomia tenuicollis; Hyda = Hypera dauci; Losu = Longitarsus succineus; Mefu = Melanophtalma fuscipennis; Milu = Microlestes luctuosus; Ocob = Ocypus obscuroaenus schatzmayeri; Ocop = Ocypus ophtalmicus; Oecr = Oedemera crassipes; Opsu = Ophonus subquadratus; Pose = Poecilus sericeus; Prob = Protaecia oblonga; Psci = Pseudocleonus cinereus; Ptse = Ptomaphagus sericatus; Rhci = Rhizotrogus cicatricosus; Seim = Sepedophilus immaculatus; Spru = Sphaeroderma rubidium; Tani = Tachyporus nitidulus).
Finally, beetle abundance was positively correlated with plant species richness and very short vegetation (< 5 cm), while beetle species richness was positively correlated with the vegetation cover of > 20 cm and negatively correlated with stone cover. Abundances of Asida sericea and Coniocleonus nigrosuturatus were both positively correlated with plant species richness and very short vegetation (< 5 cm) (Table 4).
R and p of Spearman rank tests between environmental data (for 4 m2: vegetation species richness; <5 cm = % cover of vegetation lower than 5 cm; 5–20 cm = % cover of vegetation between 5 and 20 cm; >20 cm = % cover of vegetation higher than 20 cm; Biomass = vegetation dry biomass in g; Bare ground = bare ground percentage cover; Stone = stone percentage cover) and abundance, species richness and abundance of Asida sericea, Coniocleonus nigrosuturatus, and Poecilus sericeus (as this species was not found on Merle, this site was not taken into account for tests on this species); N=48 (N=36 for P. sericeus)
| Abundance | Species richness | Coniocleonus nigrosuturatus | Asida sericea | Poecilus sericeus | ||||||
| R | p | R | p | R | p | R | p | R | p | |
| Vegetation species richness | 0.33 | ⁎ | 0.00 | ns | 0.77 | ⁎⁎⁎ | 0.79 | ⁎⁎⁎ | 0.00 | ns |
| <5 cm | 0.40 | ⁎⁎ | −0.03 | ns | 0.73 | ⁎⁎⁎ | 0.68 | ⁎⁎⁎ | 0.14 | ns |
| 5–20 cm | 0.05 | ns | −0.12 | ns | −0.01 | ns | −0.06 | ns | 0.15 | ns |
| >20 cm | −0.12 | ns | 0.38 | ⁎⁎ | −0.61 | ⁎⁎⁎ | −0.51 | ⁎⁎⁎ | −0.39 | ⁎ |
| Bare ground | 0.22 | ns | 0.09 | ns | 0.79 | ⁎⁎⁎ | 0.73 | ⁎⁎⁎ | −0.12 | ns |
| Stones | −0.21 | ns | −0.39 | ⁎⁎ | −0.04 | ns | 0.05 | ns | 0.51 | ⁎⁎ |
| Biomass | −0.12 | ns | −0.03 | ns | −0.58 | ⁎⁎⁎ | −0.62 | ⁎⁎⁎ | 0.09 | ns |
⁎⁎⁎ ;
⁎⁎ ;
⁎ ; ns = no significant.
4 Discussion
4.1 Coleoptera assemblages in the grazed steppe of La Crau
One hundred and sixty species of Coleoptera were identified on the grazed steppe (221 in total including species found in exclosures). Previous studies [22] identified 50 species only, most of which were also found in our samples. The species richness may appear high, but was relatively low considering the extensive sampling that was carried out (2736 pitfall traps over six months on two consecutive years). This confirms the results of a previous study that showed that steppe communities were less species rich than adjacent formerly cultivated fields [24]. Despite their high vegetation species richness [19], the grazed steppes of La Crau are relatively poor in beetle species and have unbalanced populations due to two dominant beetle species (Poecilus sericeus and Asida sericea). This unevenness is often characteristic of oligotrophic habitats in harsh ecological conditions (e.g., moisture or climatic stress) in which only opportunistic species are favoured [65]. An analogy could be made with the vegetation of the steppe dominated by two species as well: Brachypodium retusum and Thymus vulgaris.
Drought stress is probably one of the reasons why beetle species richness was low. By actively searching beetles in the vernal pools of La Crau for a limited sampling period and without including all families, Atgay [67] found 191 Coleoptera species, out of which 89 were Carabidae. Our results showed that the Carabidae family does not contribute much to the overall species richness of the steppe (25 species), while it generally contributes a lot to the species richness of ground beetle Coleoptera assemblages on sites that does not experience such a harsh stress by drought such as grasslands of northern Europe [68–71], or the surrounding wetlands of La Crau [67].
Different assemblage structure and composition were found at the four sites, on a NE–SW gradient. A similar site effect was shown on the vegetation for these sites [63]. The Merle community was different from those of the three other sites because of different constraints: Poecilus sericeus was absent and abundances of Asida sericea and Coniocleonus nigrosuturatus were low. The Merle community was also richer and more balanced because of less stress (more moisture) and disturbances (lower grazing pressure).
4.2 Consequences of grazing abandonment on assemblages
After five years (Termes-Blancs) of grazing abandonment, plant species richness decreased and kept on decreasing with time since abandonment. Conversely, there were no significant differences in species richness of beetle assemblages between grazed and ungrazed areas, even after 23 years of grazing abandonment. We observed a significant increase in species richness four years after abandonment (Nègreiron), where grazing pressure is the highest. When grazing pressure is too strong, the beetle species richness usually decreases [72,73], whereas grazing pressure release leads to an increase in species richness [68,74], as observed at the Nègreiron site. This increase in species richness is likely to be due to new species colonizing the ungrazed site: predator and saprophagous species. The latter increased in the exclosures because of an increase in necromass (plant litter) when grazers were removed. Predators (Carabidae and Staphylinidae) are more sensitive to micro-changes of moisture and temperature induced by changes in the vegetation structure [75,76].
After 15 to 16 years of grazing abandonment, given that plant species richness decreased drastically, it was very surprising that beetle species richness did not vary, particularly that of phytophagous species. Indeed, a general positive correlation is usually observed between plant species richness and phytophagous species [77]. However, plant composition may sometimes maintain high beetle diversity [23]. In the Merle exclosure, one plant species took advantage of grazing abandonment: Galactites tomentosa Moench that was found 33 times more for 4 m2 in the exclosure than outside [63]. Some species were only found in the exclosure, and their presence can be directly linked to this particular plant species: Agapanthia cardui (Cerambycidae), Cassida deflorata (Chrysomelidae), Hadroplontus trimaculatus and Lixus filiformis (Curculionidae). Moreover, the flowers of the plants growing in the exclosures are protected from grazing and thus induce the colonization of floricolous species, such as Tropinota squalida or Meligethes sp. Despite the fact that great plant species richness can contribute to beetle species richness, the decrease in plant species richness in ungrazed areas was compensated in our case by changes in composition, structure and phenology (flowering) of plants that induced a great number of attractive habitats for many phytophagous beetle species [13].
4.3 Consequences of grazing abandonment on populations
Grazing abandonment does not seem to lead to the complete disappearance of the most abundant beetle species nor to the appearance of new highly frequent ones, even 23 years after abandonment. As shown by the differences between Sørensen (presence/absence) and Steinhaus (abundance) similarity indexes, most species are found in both the grazed and ungrazed areas, but with different abundance distributions, two of them being typical steppe species in La Crau: Asida sericea and Coniocleonus nigrosuturatus [23]. These two species were dominant in the community of the grazed area in Nègreiron and their abundance drastically decreased in the exclosure after only fours years of abandonment. This was the case at all sites, for all times since grazing abandonment. Even after 23 years of abandonment, one individual of Asida and three of Coniocleonus were still found on the Merle exclosure. The abundances of these two species were positively correlated with very short vegetation, bare ground percentage cover and plant species richness. However, this relationship is likely to be biased by the positive relationship between these three environmental variables [63].
It is obvious that these two species can be considered as indicators of overgrazed and open habitats. Thérond [78,79] had also observed these two species sharing a same habitat (both “often found under the plant Limonium sp.”). They however have a very different biology: Asida sericea is typically found in open dry [80] and always sandy habitats [78], and is a saprophagous and occasionally omnivorous species like most Tenebrionidae [81]. Its abundance in Nègreiron can be explained by a site effect, i.e. a higher percentage of fine sand in the soil in this area [63], but also by the fact that it is an opportunistic species that is able to make the most of the low resources induced by overgrazing. Coniocleonus nigrosuturatus is usually found on sunny and hot soils; it is a phytophagous species, but its behaviour and host plant(s) are actually unknown [82]. Its abundance in Nègreiron may therefore be explained (i) by a great abundance of its host plants on this site that might show a great adaptation to overgrazing or (ii) by a polyphagous ability that allows it to make the most of all available resources.
Therefore, their similar response to grazing abandonment is probably due to changes on different factors that induced the same consequence: for Asida, a decrease in the percentage of fine sand in the soil in exclosures [63]; for Coniocleonus, a decrease in the abundance of its host plants; for both species, an increase in resource heterogeneity [72].
The abundance of some other phytophagous species also decreased in exclosures, but to a lesser extent (Curculionidae particularly: Cycloderes guinardi, Hypera dauci, Pseudocleonus cinereus, Trachyphloeus monspeliensis), and it is expected to keep on decreasing with increasing time since grazing abandonment. Their abundance can be predicted to be following the same trend as their host plants like Plantago sp. or Erodium sp. [82–84], which tended to decrease in the exclosures [63]. Similarly, the abundance of some species tended to increase in exclosures, like Sphaeroderma rubidum, which is found on prickly Asteraceae like Galactites [85]. In the same way, floricolous species, such as Oedemera crassipes, Tropinota squalida or Charopus docilis, took benefit from the blossom of the vegetation, which is more abundant in exclosures [63].
Finally, the increase in abundance of predator species, such as Microlestes luctuosus, or Staphylinidae Ocypus ophtalmicus, Tachyporus nididulus and Sepedophilus immaculatus, may be due to the better accumulation of litter in exclosures, which induces changes in the microclimatic conditions, favourable to small detritivorous such as collembolas.
5 Conclusions: consequences for management
The contrasted responses of beetle assemblages and plant communities to grazing abandonment showed that a sound understanding of the impacts of management on biodiversity needs a comprehensive study of the different biological compartments. Changes occurring on plant communities include loss of species richness, species diversity and modification of plant composition after five years. After 23 years, grazing abandonment led to a great loss of species richness and diversity, however still reversible. Conversely, grazing abandonment did not seem to induce marked changes for beetles at first (four years) and simply led to a reduced abundance in a few species. With time, a progressive and relatively moderate change in species composition is observed along with the modification of community structure. After 23 years of grazing abandonment, there was no significant loss of species richness. This temporal shift in responses of vegetation and arthropods in the evolution of their communities has also been observed by Alard et al. [86] in calcareous grasslands of the Seine Valley between vegetation and soil mesofauna. Our results confirmed that a grazing pressure release could induce a significant increase in beetle species richness [68,72,74]. This increase can be observed only on a short-term basis (four years) and only in overgrazed sites. This result corroborated those related to restoration experiment where species richness increased in small four-year-old exclosures (4.5 m2) on the steppe and on former cultivated fields [87]. Therefore, a management plan including reduced grazing rates in La Crau would be interesting to increase beetle diversity.
In addition, among all the collected species (supplementary material), 30 can be considered as having a particular biological value and interesting for beetle conservation in La Crau because they are rare, their distribution area is limited, they are endemic or their ecology is poorly known [82–84,88–93]. These species were found in both exclosures and grazed areas at densities often too low to document whether grazing is important or not for their conservation. Sheep flocks are very important to maintain coprophagous species, which reasonably disappear in exclosures. The rarefaction of traditional sheep-breeding practices, associated with the use of antiparasite treatments, toxic for wildlife, on sheep flocks, threatens numerous coprophagous beetle species [94,95].
Broadly, it is important to highlight how slow plant successional processes are after grazing abandonment in the plain of La Crau: in 23 years, ligneous species have not colonized the exclosure, while it only takes 10 to 15 years in other Mediterranean ecosystems [11]. It is however difficult to tell if these processes are slow for beetle assemblages as well. Indeed, some species population responses are fast, while community evolves progressively with most of the changes occurring in species abundance only. It seems necessary to keep on studying communities in the exclosures to better understand these processes and to observe if, with time, more marked changes can be observed, notably a decrease in beetle species richness, like it happened for plant species richness.
Acknowledgements
We thank the CEEP–Écomusée de Crau and M. Jean Boutin for site access, Guillaume Aubin, Olivier Blight, Elsa Bonnaud, Pascal Campagne, Estelle Dumas, Déborah Pardo and Diane Zarzoso-Lacoste, for their help with field work, Michel Cornet for the identification of micro-Staphylinidae.


