1 Introduction
Desiccation-tolerant organisms (anhydrobiotes), such as pollen and seeds, are capable of surviving the complete removal of their cellular water. Apart from the ability to tolerate this desiccation, they are also able to survive in the dry state for extended periods of time. The lifespan of seeds can be remarkably long, ranging from decades to centuries [1–3] and even millennia [4]. In the 1980s, Burke [5] discussed the possibility that the cytoplasm of drying seeds could enter into a glassy state as a defense mechanism. A glass is defined as an amorphous metastable state that resembles a solid, brittle material, but retains the disorder and physical properties of the liquid state, as indicated in Fig. 1 [12]. In other words, a glass is a highly viscous solid liquid. Its high viscosity has been shown to slow down severely molecular diffusion and to decrease the probability of chemical reaction – for reviews see [7,8]. Burke suggested that in dry anhydrous organisms, glasses could be formed from cell solutes like sugars that were known in food science to provide protection from denaturation of large molecules and formation of molecular aggregates. Also, it was proposed that glasses might fill spaces in a tissue during dehydration and that their high viscosity may stop all chemical reactions requiring molecular diffusion [5]. Examples of well-known molecules that can undergo glass transitions can be found in food and polymer sciences. They include sugars, proteins, starch, complex food systems, and a wide range of polymers [6–8]. The glassy state is known to confer stability to many of these model systems. Thus, the glass concept turned out to be an interesting hypothesis to account for the survival in the dry state. Subsequently, efforts focused on detecting glasses in plant tissues and investigated its physiological importance in desiccation tolerance and storage longevity and on the assessment of physical properties, such as molecular mobility and density of the intracellular glassy matrix [9]. This review will discuss the physiological functions that these biological glasses exert in plant anhydrobiotes as well as the peculiar properties of intracellular glasses compared to model glasses. For information on the characteristics of glasses, we refer the reader to more detailed reviews on glasses in model systems [6–9].
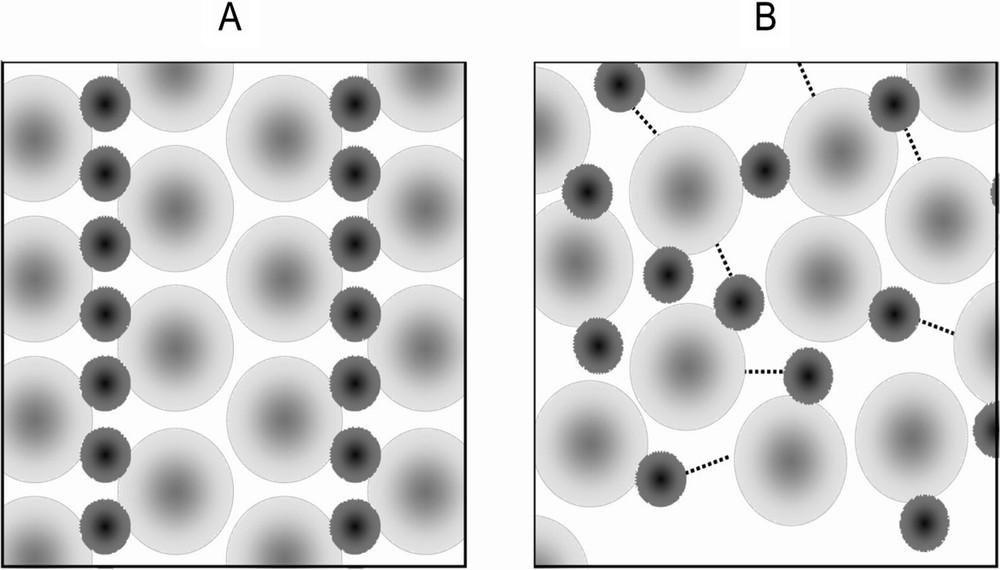
Schematic 2-dimensional diagram of a crystalline structure (A) and glassy state (B) composed of two types of molecules. The crystalline structure is regular and symmetrical, whereas there is no particular orientation to the molecules in the glass. The lines between the molecules represent hydrogen bonding or ionic interactions in the glassy state.
2 Drying results in intracellular glass formation
Intuitively, one can imagine that as the concentration of solutes increases, the viscosity of the solution increases, resulting in a decrease in molecular mobility of molecules. The same is true for the cytoplasm of seeds upon drying. To measure this viscosity or mobility in the cells electron paramagnetic resonance (EPR) spectroscopy proved to be an excellent technique to study molecular mobility in vivo in biological samples [10,11]. After incorporation of spin probe molecules into the tissues and removal of the extracellular signals by the addition of potassium ferricyanide, this technique can provide a spectrum of the spin probe from the cytoplasm. From the spectral line shapes, a measure of molecular mobility can be derived [11]. This rotational mobility can be taken as a measure for the intracellular viscosity of the environment in which the spin probe resides. An example of this kind of measurement is shown in Fig. 2. Using 3-carboxy-proxyl, a polar nitroxide spin probe with the size of approximately a glucose molecule, the rotational mobility in pea (Pisum sativum L.) axes was determined during drying at room temperature [11]. In the embryonic tissues below 0.8 g H2O/g DW, the cytoplasm becomes increasingly viscous. The removal of water induces a supersaturation of the cytosolic components leading to an increase in the cohesive forces between molecules and restriction of the molecular mobility within the cytoplasm. Furthermore, drying below 0.3 g H2O/g DW leads to a decrease in the molecular mobility in the cytoplasm of over five orders of magnitude (Fig. 2). Finally, at around 0.1 g H2O/g DW, the cytoplasm vitrifies and enters into the glassy state. Glass formation has been detected in seeds [13–16], pollen [17] and the resurrection plant Craterostigma plantagineum (J. Buitink, unpublished results). Apparently, glass formation is a characteristic that can be found in all desiccation-tolerant tissues. The water content at which the cytoplasm transforms into a glassy state during drying depends on the temperature. The lower the temperature of drying, the higher the cellular water content at which the cytoplasm becomes glassy. From the water content/ relationship, it can be calculated that a cytoplasmic glass is formed at ca. 25 °C when the embryonic tissues reaches an equilibrium relative humidity of 44–49% (Table 1). Interestingly, there is little variation between species. Practically, seeds stored at ambient conditions are very close but not yet in the glassy state (when it is assessed by differential calorimetry). However, the molecular mobility within the cytoplasm is already severely restricted (Fig. 2), bringing the metabolism to a halt and severely slowing down degradative reactions.
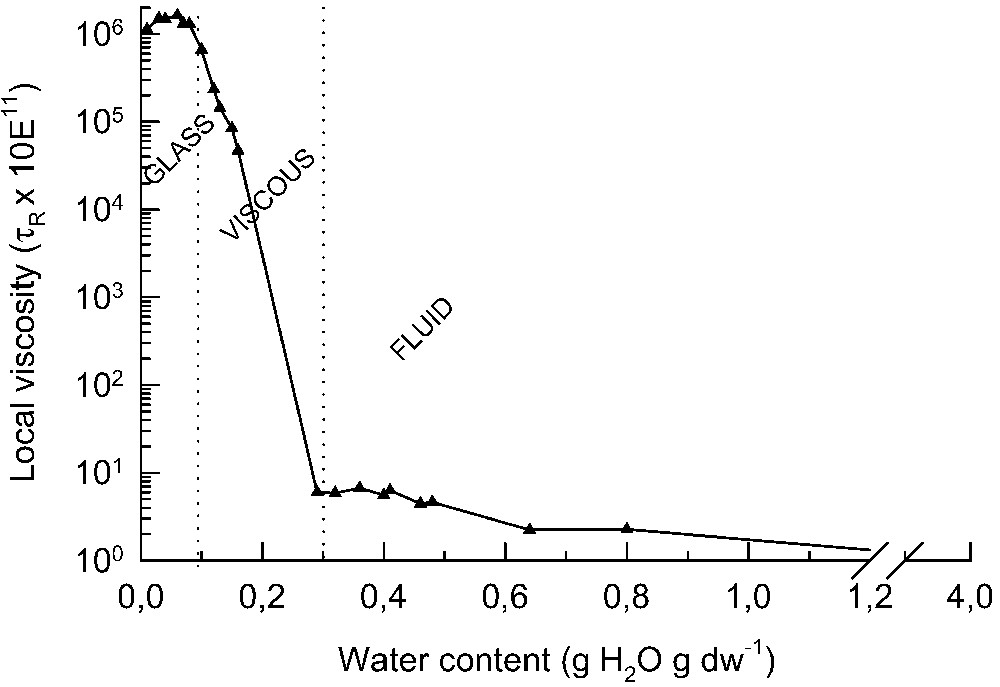
Increase in molecular viscosity in embryonic axes of Pisum sativum during drying at 20 °C. Local viscosity is expressed as the rotational correlation time of the polar spin probe 3-carboxy-proxyl that was introduced in the seed tissues, measured using EPR spectroscopy as described in Buitink et al. [11]. is related to the viscosity by the Stokes–Einstein equation [11]. The water content range corresponding to a fluid, liquid phase, viscous and glassy sate is indicated.
Water content and corresponding minimum equilibrium relative humidity to be reached to form a cytoplasmic glass at 25 ± 2 °C for various crops containing different amount of storage oil
| Species | Oil content (% DW) | Water content (% FW) | Relative humidity (%) |
| Glycine max | 19.6 | 9.0 | 47 |
| “ “ cv. Dunfield | 20.9 | 9.0 | 49 |
| “ “ cv. Peking | 17.6 | 9.0 | 46 |
| Pisum sativum | 1.2 | 10.3 | 44 |
| Phaseolus vulgaris | 1.0 | 10.3 | 44 |
| Zea mays (embryo) | 8.0 | 10.2 | 48 |
Although glass formation in seeds drastically decreases molecular mobility, the molecules in a glass are not completely restricted in their movement. In time, diffusion will be possible, albeit at a rate presumably considerably slower than that in hydrated cytoplasm. This explains why seeds still age, because deteriorative processes such as lipid oxidation can take place, though at a very slow rate. Another example of this is the natural after-ripening process taking place in dry seeds after harvest, during which seeds escape dormancy (reviewed in [18]). Indeed, Walters [19] demonstrated using theoretical considerations coupled to measurements of relaxation times that mobility is not restricted until at least 70 °C below the glass transition temperature, which corresponds to for dry seeds. It is evident however, that the processes taking place in dry seeds, i.e. with a water content below 0.10 g H20 g/g DW (corrected for lipid content), do not involve the true metabolism involving energy production via electron transport chains. Even so, the reduced mobility in the cytoplasm after drying is responsible for the extreme longevity that seeds can achieve in the dry state, as described here below.
3 Importance of the glassy state for desiccation tolerance
Intracellular glasses were originally suggested to confer desiccation tolerance. Williams and Leopold [20] showed that the (glass transition temperature) of desiccation-sensitive pea embryonic axes obtained after long imbibition was much lower than that of axes imbibed for a short time and that still remained desiccation-tolerant. In desiccation-sensitive tissues, loss of membrane integrity upon drying is a common symptom of injury. However, glasses can also be found in desiccation-sensitive tissues, indicating that the lack of desiccation tolerance was not related to an absence of a glassy state [17]. In somatic embryos of carrot (Daucus carota), rapid drying leads to low survival as opposed to slow drying [21]. However, the overall protein secondary structure of slowly and rapidly dried embryos resembled one another to a large extent. This absence of protein denaturation can only be explained by the formation of a glass in both drying regimes, which prevented changes in protein conformation. It is important to realize that the water content at which glass formation occurs during drying at room temperature in seeds is around 0.1 to 0.12 g H2O/g DW (Fig. 2). The water contents at which most seeds of desiccation-sensitive (so-called recalcitrant) species die occur before the formation of the glassy state [22]. Apparently, dehydration-induced damage in these seeds occurs at water contents far above those at which protection of the glassy state can be effective. Thus, glass formation is not a mechanism that initially confers the tolerance to desiccation during drying, but the formation of intracellular glasses is indispensable to survive the dry state.
4 Role of the glassy state for seed longevity
One of the most studied functions of glasses is preservation of the structural and functional integrity of macromolecules [7,8]. Glasses are known to decrease detrimental reactions, such as the rate of browning reactions [23], to increase the stability of enzymes [24] and to prevent conformational changes of proteins [25]. The stabilizing effect of glasses on macromolecular and structural components during storage makes it likely that glasses play an essential role in the longevity of seeds and pollen. Indeed, the secondary structure of proteins in dry seeds of several species appears to be very stable and remains preserved after several decades of open storage. For example, no protein aggregation and denaturation was found after 28 years of dry storage of wheat (Triticum spp.) seeds, despite loss in viability [26]. Aging rates of plant anhydrobiotes are strongly dependent on the water content and temperature of storage, conditions that determine glass formation [27]. Furthermore, a link with the intracellular glass properties under the same storage conditions has been well established [28–30]. Typically, seed and pollen deterioration is accelerated when the tissues are not in a glassy state [28,31]. Conditions that bring the anhydrobiotes above their (such as those used in the so-called accelerating aging) result in an increase in the activation energy of the aging rate within the same order of magnitude as that of rotational mobility in the cytoplasm: namely a factor of 2 to 3 [11,28]. Additional convincing evidence to suggest that aging rates are affected by the viscosity of the intracellular glass came from the linear relationship between aging rate and molecular mobility found for many different tissues over a wide range of temperatures and water contents [29]. All these arguments suggest that aging rates and, consequently, lifespan of germplasm are influenced by the molecular and cellular stability of the cytoplasm, signifying the pivotal function of intracellular glasses in conferring such stability during storage. Furthermore, these results reinforce the hypothesis that cytoplasmic molecular mobility can indeed be used to describe and even predict longevity. The linear relationship between the logarithms of molecular mobility and longevity or aging rates enabled the extrapolation of aging rates or longevity to sub-zero temperatures by simply measuring the mobility at the required temperatures, assuming that the relationship between aging rate and rotational motion remains linear at low temperatures. Our predictions on the basis of molecular mobility support the contention of the existence of optimum water content for storage that shifts to higher values with decreasing storage temperatures [28,32]. Furthermore, if the predictions of longevity based on the molecular mobility model are correct, then longevity at sub-zero temperatures is higher than estimated by the viability equation at elevated water contents. Currently, seed storage protocols recommend storage at 5% moisture and . The predictions of longevity based on the molecular mobility model suggest that longevity at could be increased considerably by storing seeds at water content higher than 7%, suggesting that care has to be taken not to overdry seeds before storing them at low temperatures [2]. An analysis of the relaxation times occurring in seeds using differential scanning calorimetry (DSC) indicates that the temperature at which molecular motion is severely limited is 70 °C below the glass transition temperature [19], suggesting that indefinite shelf-life may not be possible using current cryogenic temperatures for the storage of hydrated tissues.
5 Intracellular glasses have peculiar properties that confer extreme stability to the seeds
The consequences of being in or near the glassy state gained further physiological significance with a study on the molecular mobility in desiccation-tolerant tissues indicating that the cytoplasm remains quite immobile even when the glass has melted, as measured by DSC [33]. In model systems, it has been observed that melting of the glass increases the molecular mobility. However, only at a slightly higher temperature, the collapse temperature (), the viscous matrix completely melts and mobility increases 5 orders of magnitude [34]. The occurrence of has been coupled to the collapse temperature of sugar glasses (; Fig. 3), a phenomenon that is attributed to a reduction in viscosity such that a flow on a practical time scale is observed. Although the viscosity increases around , it is not until the temperature is reached that the viscosity abruptly drops (Fig. 3). This is contrary to the assumption that the viscosity increases abruptly at the . The in seeds could not be determined because of the loss of the spin probe signal at high temperatures (>90 °C). However, considering that no kinetic change occurred in the rotational mobility when heating the seeds to 45 °C above their (Fig. 3), one can derive that the of seeds occurs at temperatures higher than 45–50 °C above . A high implies high stability as a result of high viscosity (>108 Pa s) far above . This high in biological tissues might have important implications for germplasm survival in its natural environment. Under ambient conditions (say 20 °C and 50% RH), seed glassy matrix is close to its . This means that any fluctuation of the environment that results in an increase in RH or temperature will bring the tissue above its . However, the unique properties of the intracellular glass protect the tissue from dramatic changes caused by such environmental fluctuations. If the intracellular glass was composed of sucrose alone, a small increase in RH or temperature would bring the glass above its (Fig. 3), resulting in phase separation (crystallization, aggregation) and loss of macromolecule function or integrity (see [7] for review). Therefore, the characteristically high of intracellular glasses could serve as a physiological and ecological advantage.
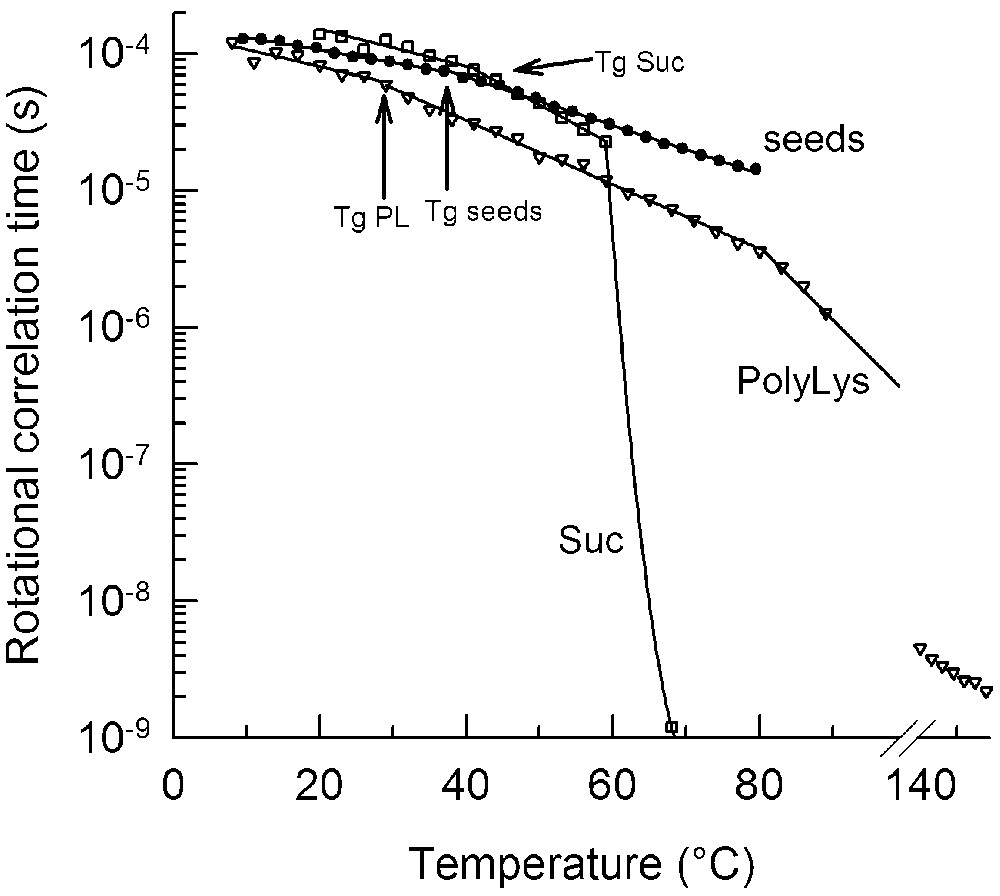
Temperature dependence of the rotational correlation time () of the spin probe 3-carboxy-proxyl in glasses of sucrose (Suc, open squares), poly-L-lysine (PL or PolyLys, open triangles) and embryonic axes of bean (closed circles). The was determined by saturation-transfer EPR. The temperature of the glass melting () measured by DSC is indicated by an arrow for each curve. Bean axes and poly-lysine samples were equilibrated to 33% RH, and sucrose to 3% RH.
6 Composition of intracellular glasses
For a long period of time sugars were considered to play an important role in the composition of the glassy state in seeds. Indeed, sugars are present in large amounts in desiccation-tolerant tissues and are known to be excellent glass formers. The correlation between oligosaccharides and longevity [35,36] and the knowledge that oligosaccharides increase in model systems [7,8] added to the hypothesis that sugars are important for in vivo glass formation. However, there is increasing evidence that does not support the contention that sugars play a dominant role in intracellular glass formation. Sun and Leopold [37] found that the magnitude of the glassy signal and decreased during accelerated aging of soybean (Glycine max) seeds. However, no differences were observed in the sugar content during the same period of time. In spite of different sugar compositions in different tissues, their state diagrams are similar [7,38]. Also, the state diagrams of immature and mature soybean axes are similar, despite the accumulation of oligosaccharides during maturation. Another indication that sugars alone are not responsible for the formation of the vitreous state in seeds came from Sun et al. [38] who showed that the state diagram of maize (Zea mays) embryos is different from that of a representative carbohydrate-mix. An extensive calorimetric study on the glass transition in bean (Phaseolus vulgaris) axes revealed the complexity of intracellular glasses [16]. Implementation of DSC data from bean in a theoretical model that predicts the effects of glass components on has suggested that intracellular glasses could be composed of a highly complex oligomeric sugar matrix, such as, for instance, malto-dextrin [16]. Buitink et al. [39] observed that a change in sugar composition upon priming (an invigoration treatment of seeds based upon their controlled imbibition and ultimately improving their vigor, see [40] in this issue) did not change or the molecular mobility in the intracellular glass. Comparison of the biophysical properties of intracellular glasses measured in bean embryos with those of model systems of different sugars shows that in intracellular glasses the mobility is slower and the density higher compared to the situation in a glass made of a mixture of stachyose and sucrose, the most abundant sugars in bean seeds. Density was measured using Fourier transform infrared (FTIR) spectroscopy, which allows for measurements on the extent of molecular interactions through hydrogen bonding and thus the molecular packing of the glassy matrix components [41,42]. All these data suggest that, besides sugars, other molecules play a pivotal role in intracellular glass formation. In this respect, the role of proteins in intracellular glass formation has received recent attention. Wolkers et al. [21,41] have found that the molecular density (i.e. hydrogen bonding strength) of dry seeds of Arabidopsis thaliana was quite different from that of a sugar glass, but much more comparable to that of a protein-sugar glass. Investigations on the glass properties in biological systems using EPR spectroscopy also point to a role for proteins in intracellular glass formation [34]. The temperature dependence of the molecular mobility in intracellular glasses is much more comparable with that in protein glasses than that in sugar glasses (see Fig. 3). However, the rotational motion in these protein glasses is almost twice as fast as in intracellular glasses (Fig. 3) [9]. Because high molecular mobility in model glasses is generally associated with a loose hydrogen bonding network, it is suggested that the molecular packing of protein glasses is less dense than for intracellular glasses. Thus, additional molecules likely contribute to the increase of the local density in intracellular glasses. In this respect, it is interesting to note that inorganic salts (e.g., MgCl2) and small polyhydroxy compounds (e.g., glycerol) can interact together on drying to form a self-polymerizing protectant with co-ordinate polymer chains of high [43]. Whether similar molecules in the cytoplasm interact to form such self-polymerizing protectants is an intriguing question that remains to be answered.
7 Role of LEA proteins in intracellular glass formation
Proteins that are thought to be implicated in intracellular glasses are those from the LEA (late embryogenesis abundant) class, a family of highly hydrophilic proteins that are heat-soluble [21,36,44]. Considering that during maturation, seeds and pollen accumulate both non-reducing sugars and LEA proteins [45], it is thought that both types of molecules interact together in the formation of a glassy state. That these molecules interact was demonstrated in a study on the hydration characteristics of heat-soluble proteins extracted from mature wheat embryos. It was shown that after protein purification, the extract still contained sugars at about 1:1 (w:w) ratio. Only about half of the sugars could be removed by exhaustive dialysis and the rest appeared to be tightly associated with the proteins [46]. Wolkers et al. [44] showed the hydrogen bonding strength of a sucrose/LEA mixture is higher than that of a sucrose glass alone. More research is needed to confirm the implication of LEA proteins in biological glasses or elucidate whether other types of proteins play a role in the glass formation. Yet, the advantage of a combination of sugars and LEA proteins in the glass mixture would be that both types of molecules exhibit several and distinct functions, all being important for the preservation of structural functionality. Considering that LEAs are a heterogeneous class of proteins, the question as to whether there are specific LEA proteins involved in determining glass properties in dry anhydrobiotes remains to be addressed. Using a proteomic approach, a subset of LEA proteins have been identified in relation to survival in the dry state [47], making them possible candidates for the stabilization of the glassy state. Besides a putative role in the dry state, LEA proteins are also known to confer tolerance to osmotic and cold stress. The protective mechanisms behind this action are described in details in a recent review on LEA proteins [48].
8 Concluding remarks
The concept of a glassy state applied to seeds has greatly improved our understanding of anhydrobiosis. It provides a global explanation as to why metabolism is arrested in the dry state, why seeds gain so much stability after drying and deteriorate fast when moisture and temperature increase during storage. Also the characterization of the dry cytoplasm at the molecular level near and below has led to refine longevity predictions for storage conditions that do not permit experimentations. The concept of cytoplasmic glasses provides a theoretical framework from a biophysical point of view that can address questions about the mechanisms of seed deterioration and the nature of diffusion kinetics that can be expected for water molecules and oxygen. Since biological glasses appear to be different from simple sugar matrices, understanding their composition and their characteristics may lead to innovative lyopreservation methods for the food and pharmaceutical industry.
Acknowledgements
This work was supported in part by grants from the Ministère de l'Agriculture et de la Pêche and from the region Pays de la Loire (COSAVE).


