Version française abrégée
Dans la famille des Orchidaceae, deux grands groupes morphologiques se distinguent en fonction de leur mode de croissance, monopodial ou sympodial. Toutefois, parmi le très grand nombre d'études chez cette famille, l'architecture végétale est en grande partie négligée et les descriptions détaillées du développement morphologique sont rares. Les orchidées sympodiales, dans leurs stades adultes, sont normalement composées d'une succession de pousses végétatives à croissance déterminée (appelés modules) portant des inflorescences latérales ou terminales. Il existe toutefois une grande diversité de sympodes. Par exemple, un type distinctif de croissance a été décrit par Barthélémy [9] chez Gongora quinquenervis Ruiz et Pav., où les plantes matures se composent d'une alternance régulière de modules végétatifs et génératifs.
Plusieurs auteurs ont rapporté des formes de croissance pouvant sans doute se rattacher à ce cas chez des espèces de la tribu des Collabieae. Des études moléculaires récentes ont montré qu'il y avait 2 principaux groupes chez cette tribu, les Phajinae et les Collabiinae. Les descriptions présentes dans la littérature laissent supposer qu'il existe également le même motif de croissance décrit par Barthélémy [9] chez des espèces de Collabiinae. Les descriptions superficielles disponibles ne permettent néanmoins pas une compréhension précise de la dynamique de croissance dans ce groupe. Dans ce contexte, nous avons eu la possibilité d'observer des individus de Chrysoglossum ornatum Blume dans la nature au Laos, où cette espèce n'avait jamais été identifiée. L'objectif de ce travail a été d'analyser son mode de croissance en détail, et de le comparer à celui des espèces du même genre.
Onze individus furent collectés dans deux localités du Laos. Trois proviennent des forêts tropicales humides de montagne de la Cordillère des Annamites (province du Bolikhamxay), les 7 autres, du plateau d'altitude des Bolovens (province de Champassak). Ces individus ont été cultivés dans les serres de l'Université Nationale du Laos, à Vientiane. Des observations complémentaires ont été menées dans l'herbier National de Hollande de Leiden, sur 8 échantillons d'herbier de C. ornatum, 1 de C. ensigerum W. Burgh and de Vogel, et 3 de C. reticulatum Carr. Tous ces individus ont été décrits selon les méthodes et concepts architecturaux présentés par Barthélémy et Caraglio [23].
Ces études ont mis en évidence une production alternée de modules végétatifs et génératifs dérivant les uns des autres chez les 3 espèces de Chrysoglossum étudiées. Les modules végétatifs présentent une partie proximale constituée d'un rhizome, à l'extrémité duquel se trouve un pseudobulbe surmonté par une feuille terminale. Un bourgeon latent situé à la base du pseudobulbe produit un nouveau module, végétatif ou génératif. Les modules génératifs sont quant à eux constitués d'un court rhizome, dont l'extrémité se rétrécit brusquement et prend une orientation orthotrope, pour former le pédoncule et le rachis inflorescentiel. C'est dans la partie distale du rhizome de ce module génératif qu'un bourgeon latent produit un nouveau module végétatif.
Ce modèle de développement correspond à celui décrit par Barthélémy [9]. On constate cependant des différences associées au mode de vie, épiphyte chez les Gongora et terrestre chez les Chrysoglossum. Ces informations apportent des éléments nouveaux sur l'architecture des Orchidées ainsi que sur la valeur potentielle de ces caractères dans le cadre d'analyses phylogénétiques. Des études complémentaires sont actuellement en cours sur d'autres espèces de la tribu des Collabieae.
1 Introduction
In the Orchidaceae, two major morphological groups are traditionally distinguished according to their growth pattern: monopodial versus sympodial. However, among the huge number of orchid studies, vegetative architecture is largely neglected and detailed descriptions of morphological development are scarce for this family ([1] and Pfitzer, 1889 cited in [2–8]).
The monopodial growth form is mainly present in the lianoid taxa of the subfamily Vanilloideae and in the predominantly epiphytic species of the Vandeae tribe (subfamily Epidendroideae). The sympodial growth pattern is present in both terrestrial and epiphytic taxa in all five currently recognized subfamilies. Sympodial orchid plants, in the mature stage, normally consist of a succession of vegetative shoots with determinate growth (called modules) carrying either terminal or lateral inflorescences. There is, however, considerable diversity among the sympodials. For example, a distinctive type of growth pattern was described by Barthélémy [9] for Gongora quinquenervis Ruiz and Pav., where the mature plant consists of a regular alternation of vegetative modules and generative ones.
Several authors have reported similar growth patterns for species of the tribe Collabieae. In his revision of the genera Hancockia, Mischobulbum and Tainia, Turner [10] mentioned the presence of “sterile shoots alternating with the fertile shoots”. Van der Burgh and de Vogel [11] described in their revision of the genera Chrysoglossum, Collabium, Diglyphosa and Pilophyllum the presence of “two types of pseudobulbs” and stated “one or several of these leaf-bearing pseudobulbs alternate with one inflorescence-bearing pseudobulb”. Holttum [12] referring to the Nephelaphyllum tribe (i.e. including Diglyphosa, Chrysoglossum, Nephelaphyllum and Tainia) speaks of “shoots of the sympodium forming either slender pseudobulbs or erect inflorescences”. Pridgeon et al. [13] used the same expression as Van der Burgh and de Vogel [11], for their descriptions of pseudobulbs in the genus Chrysoglossum, while Comber [14] mentioned the presence of an “inflorescence arising from the top of a rudimentary growths placed on the rhizome between normal pseudobulbs”. Cribb and Whistler [15] spoke for the same genus of “terminal inflorescences borne on leafless rudimentary pseudobulbs alternating with vegetative shoots”. For other authors, similar interpretation may arise from published drawings [6,16,17]. Schlechter [18], while describing the subtribe Collabiinae, even distinguished this subtribe from the otherwise similar Phajinae on the basis of this character. He wrote (our translation): “the inflorescences arise on short leafless shoots that alternate with the always one-leaved vegetative shoots.” Since these inflorescences are clearly terminal, Schlechter, essentially adopting the earlier system of Pfitzer, placed the Collabiinae in the series (‘Reihe’) Acranthae, while he placed the Phajinae, with lateral inflorescences, in the series Pleuranthae. The most recent publication treating the Collabieae identified 18 genera [13], merging both subtribes Collabiinae and Phajinae. Molecular studies [19,20] showed that there are two clear groups within this tribe: Phaius / Calanthe / Acanthephippium and Collabium / Nephelaphyllum / Tainia / Ancistrochilus, which again correspond with the former subtribes Phajinae (also called Bletiinae) and Collabiinae respectively.
All these studies suggest that the particular growth pattern observed by Barthélémy [9] could be phylogenetically informative. Nevertheless, the superficial descriptions available do not allow a precise understanding of growth dynamics in this group and further descriptions and thorough architectural analyses are still needed in order to reach a sound morphological interpretation. In this context we were fortunate to be able to observe several individuals of Chrysoglossum ornatum Blume in the wild in Laos and thus to analyze its natural growth pattern in detail. The genus Chrysoglossum includes four species present in tropical Asia from Sikkim to New Caledonia. One species, Chrysoglossum ornatum Blume is widely distributed throughout the range of the genus, but has never been observed previously in Laos [21,22]. During this study, this species has been observed in two different localities in Laos thus allowing for a better understanding of its habitat and distribution, whereas a precise architectural analysis was made on several individuals, and complemented by the study of herbarium specimen of this and other species of this genus.
2 Materials and methods
Architectural concepts and methods used in the following descriptions are based on the publication of Barthélémy and Caraglio [23].
Eleven individuals were collected in Laos for this study. All were kept in the living collection at the National University of Laos. Their culture medium was composed of 1/6 of charcoal, 1/6 small stones, 1/3 of pine bark and 1/3 of sphagnum. They were kept in a shady area, on a table located 50 cm above a tray containing water in order to maintain a constant humidity of their culture medium. They were investigated at the time of their collecting, and regularly observed ever since.
3 of these 11 individuals were found in tropical humid mountain forest, at 1070 m above sea level, in the north of the Annamite mountain chain. The first two specimens (L1, Fig. 1F and L2) have flowered in May 2007, the third one is a sterile individual (L3, Fig. 1A).
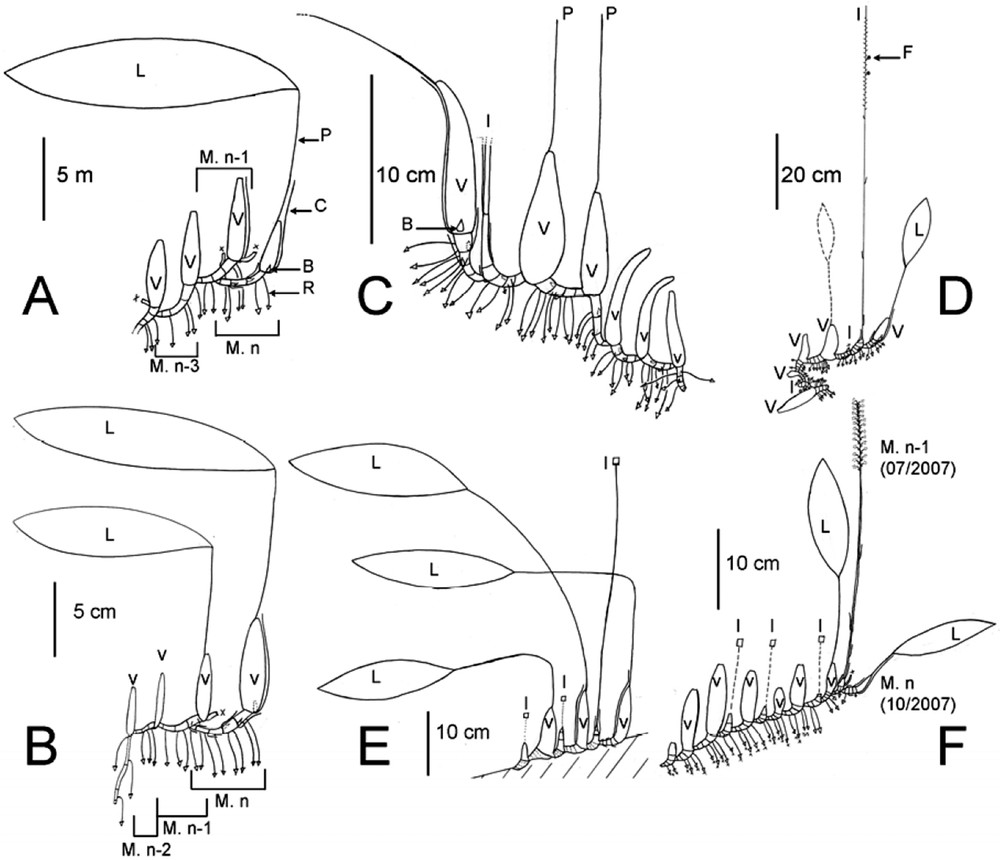
Diagrammatic representation of architectural development of representative specimens analyzed. Legend: B, Bud; C, Cataphyll; F, Fruit; I, Inflorescence or generative module; L, Leaf; M, Module; P, Petiole; R, Root; V, Vegetative module; ●, Flower bud; ○, Flower; ○ (with dots), Dead flower; ⊗, Fruit; □, Inflorescence; ▿, Root. A: Individual L3. Young vegetative plant. Module formed by a rhizome segment which bears roots (R.). Some cataphylls (C.) are represented, at the base of which there is a resting bud. The pseudobulb (Psb.) of the module n has a terminal leaf with a long petiole (P.). Two branches from the module n − 1 were dead, thus an axillary resting bud at the third internode of the rhizome of this module has provide the module n. B: Individual L5. Young vegetative plant. The oldest module (n − 3) has an orthotropic long thin rhizome. C: Individual L8. Adult plant with a succession of vegetative modules (V.). The youngest module has growth from the base of the fourth internode of the generative module (Inflorescence: I.). D: Individual L10. Adult plant with a succession of vegetative modules (V.). The module n − 3 carries an old dead leaf (in dots). At the base of its pseudobulb, a first generative module with its inflorescence (I.) grew, but could not finish its development. A second generative module with fruits grew from the distal part of the first one. The vegetative module n arose from the base of this infructescence (M. n − 1). E: Individual L11. Adult plant showing a succession of alternating vegetative and generative modules. F: Individual L1. Adult plant showing an irregular succession of vegetative and generative modules.
The other eight specimens were collected and observed during a field trip in October 2007 in a rainforest of the Champassak province (Paksong district), in the southern part of the Bolovens plateau, at 1245 m asl. As a large population of this species was present there, observations were also made on three other individuals that were not collected. The identification of the plants in this population, which were not seen in flower, is based on vegetative characteristics and fruit morphology, compared with the collected specimens. They are labelled L4 to L11 in this study.
Observations in the field and in the greenhouse of the Faculty of Sciences, of the National University of Laos in Vientiane, were completed by the study of 1 specimen of C. ornatum (L12) in the greenhouse of the Hortus Botanicus of Leiden. This specimen (nr. 20020234), was collected on the island of Java and is cultivated in Leiden since 2002. It has flowered in March 2003, but not afterwards. It is cultivated in a medium consisting of a mixture of bark, sphagnum and synthetic foam.
Eight herbarium samples of C. ornatum, 1 of C. ensigerum (Fig. 4C), and 3 of C. reticulatum were also studied. These samples were analyzed during a visit in January 2008 at the National Herbarium of the Netherlands, in Leiden. The specimens studied are listed below:

Diagrammatic representation of the architectural development of the species Gongora quinquenervis, Chrysoglossum reticulatum, Chrysoglossum ensigerum. A: Adult plant of G. quinquenervis showing a succession of vegetative and generative module (from Barthélémy, 1987). B: Diagrammatic representation of the architectural development of Chrysoglossum reticulatum. C: Diagrammatic representation of the architectural development of Chrysoglossum ensigerum.
Chrysoglossum ornatum Blume:
- – H.Ch.o1: Korthals 897, from Sumatra.
- – H.Ch.o2: van Leeuwen-Reijnvaan 2548, from Java, collected on 19/04/1917 at 1000 m asl.
- – H.Ch.o3: Kleinhoonte 180, from Java, collected on 22/04/1932 at 2100 m asl.
- – H.Ch.o4: S.H. Koorders 39060, from Java, collected on 25/05/1901 at 800 m asl.
- – H.Ch.o5: Backer 6219, from Java.
- – H.Ch.o6: Junghuhn, s.n., from Java.
- – H.Ch.o7: Zollinger, s.n., from Java, collected on 19/04/1989.
- – H.Ch.o8: W. Meijer 9539, from Malaysia, collected on 24/04/1975 at 1000 m asl.
Chrysoglossum ensigerum W. Burgh and Vogel:
- – H.Ch.e1: W.J.J.O de Wilde and B.E.E de Wilde-Duyfjes 13350, from Sumatra, specimen collected on 26/06/1972 in a mossy montane forest, at 1800 m asl.
Chrysoglossum reticulatum Carr:
- – H.Ch.r1: J.H. Beaman 8953, from Malaysia, collected on 16/03/1984 at 1500 m asl.
- – H.Ch.r2. Nooteboom 968, from Malaysia, collected on 22/02/1969 in montane forest at 1500 m asl.
- – H.Ch.r3: J.J. Vermeulen and H. Duistermaat 676, from Malaysia, collected in December 1986, in a montane forest on sandstone.
In the context of our broader studies on the vegetative architecture of the Collabiinae we intend to examine material from other key herbaria for Southeast Asia, e.g., the National Museum of Natural History of Paris, in the near future. For the present article, however, we considered as sufficient the sample studied.
3 Results
All individuals of C. ornatum collected in the field were terrestrial, growing in the understory of non-disturbed tropical mountain forest in a moist, humus-rich soil.
3.1 The sterile individuals
The 4 sterile individuals L3 (Fig. 1A), L4, L5 (Fig. 1B) and L6 consisted of a succession of 3–4 vegetative modules of increasing size from oldest to youngest. Part of each of these modules was a green proximal rhizome segment (1.1–3.3 cm long; from 2–6 mm diam. in its proximal part to 4–12 mm in its distal one). This segment was prostrate, and consisted of 4 internodes of increasing length and diameter, bearing cataphylls inserted distichously. Each rhizome segment is terminated by an erect leaf-bearing pseudobulb.
On each of these vegetative modules, we observed that each internode had an increasing length (from 1–5 to 3–19 mm long and 3–7 to 4–12 mm diam.). Internodes each carry a cataphyll (from 2–6 to 12–32 mm long), that protects a resting bud on the underside of the rhizome. These resting buds usually do not become active, except in cases of damage of the bud located on the distal part of the rhizome (as seen in L3, L5 and L6). The first internode has no root. The following ones carry 1–2 roots (2–9 cm long; 1.5–2.5 mm diam.), which are filiform, non-branched, of whitish colour, and do not penetrate deeply into the ground.
Vegetative modules are terminated by a purplish green orthotropic pseudobulb, consisting of one internode (2–6.4 cm high; 4–17 mm diam.), without root, and with a long sheathing cataphyll (2.4–7 cm long). This cataphyll protects an axillary bud and covers the petiole of the terminal leaf. The leaf is green, lanceolate (), with a non-sheathing long petiole (5.2–14.5 cm long), channelled in its proximal part. The petiole is not articulated with the pseudobulb. Modules n and , with n being the most recent module, still carry a leaf in L5 (Fig. 1B), while the only vegetative module carrying a leaf in L3 and L4 is the module n, whereas all the older vegetative modules have lost their leaf (Fig. 1A).
3.2 The fertile individuals
3.2.1 Vegetative modules
Modules of fertile individuals L7, L8 (Figs. 1C and 2C), L9 increase in size from the younger to the older one, with dimensions slightly larger than the largest vegetative modules previously described on juveniles. This series of a vegetative modules then stops with the emergence of a generative one. Structure and size of vegetative modules among which sexuality appear are as follows:

Representative photographs of some of the specimens studied. A: General view of the individual L10, after its collection in the field. The succession of a generative module, with a long erected inflorescence (I.), and a vegetative module with a large leaf (L.) is easily observed. B: View of the succession of the four youngest modules on the individual L10. The vegetative module has a large pseudobulb (Psb.), while the generative ones carry inflorescences. The inflorescence of the generative module n − 2 has aborted before its development. This module n − 2 is thus followed by other generative module. C: Succession of vegetative and generative module on individuals L8. D: Overview of the individual L11, in the field. E: Close-up showing alternating vegetative and generative modules on individual L11. F: Growth of a vegetative module at the base of a generative one, in November 2007, on the individual L1.
Rhizome segment (22–38 mm long; 5–8 mm diam. in its proximal part and 10–18 mm in its distal one) of each module consists of 4 internodes. The internodes (4–13 mm long; 5–18 mm diam.) carry a cataphyll (8–50 mm long). Two to four roots per internodes spread under a thin layer of humus. They possess a constant diameter throughout their length, which can be up to 20 cm. As in the sterile individuals they are present from the second to the fourth internode.
Pseudobulbs on these individuals measure 4.8–10.3 cm high and 16–28 mm in diameter, with a sheathing cataphyll at their base (7–10 cm long), and a terminal petiolate leaf . Petiole is 15–20 cm long.
3.2.2 Generative modules
Generative modules are located at the axil of the last cataphyll, just at the base of the pseudobulb of a vegetative module (Fig. 2C). Their proximal part consists of a short rhizome segment (16–22 mm long) of 4 (3–5) internodes, with distichous cataphylls, their distal part of a long erect peduncle and rachis (Figs. 2A, 2B, 2C).
The first two internodes (4–6 mm long each) of these generative modules increase in length. Normally the first one does not have a root (one exception in L10, Fig. 1D, which has 2 roots), and is 8–9 mm diam. The second internode carries 1–2 roots (rarely 3) and is 9–11 mm diam. The final two of the proximal internodes are of equal length and diameter to the preceding one, and carry a resting bud on their underside. The fourth internode is abruptly narrowed in its distal part, which takes an orthotropic orientation. It is followed by 3 to 4 long internodes with a diameter of 3–5 mm, constituting the peduncle of the inflorescence (19–57 cm long). Each internode of the peduncle has a sheathing cataphyll measuring 35–45 mm in length. It is followed by the rachis (12–30 cm long), carrying 15 to 40 flowers opening in succession from the bottom to the top. Only 4 to 5 flowers are opened at the same time.
Usually, the bud at the base of the fourth internode of the generative module will produce the new vegetative module (Figs. 1F and 2E). More rarely, it can give a new generative module (L10, Figs. 1A, 1D and 2B) if the first one has aborted. If a trauma affects the development of this bud, a resting bud on the previous internode will produce the next module (L1 on Fig. 1F and L2).
Individuals L1 (Fig. 1F) and L2 have flowered in cultivation at the end of June 2007, 3 months after their collection. Their inflorescences stayed active until the middle of July. Vegetative modules at the base of generative one appeared in October 2007. These new vegetative modules continued their development until December. The entire erect part of the generative modules has gradually dried out during the end of August, and then quickly disappeared.
Individuals that have acquired their sexual maturity since several years presented a series of vegetative and generative modules with varying patterns. Some have a strict sequence of one vegetative and one generative module, while others expressed a more irregular rhythm.
3.3 Herbarium samples
3.3.1 Chrysoglossum ornatum
Specimens H.Ch.o1, H.Ch.o4 and H.Ch.o7 were too poor to provide additional elements for this study. Specimens H.Ch.o2, H.Ch.o6 and H.Ch.o8 showed respectively 1, 2 and 4 successive vegetative leafy modules followed by a generative one. Their structure and organization were identical with those observed in Laos.
Individuals H.Ch.o3 and H.Ch.o5 present a succession of vegetative modules arising from the base of a generative one. The specimen H.Ch.o3 shows a succession of a generative modules followed by 2 vegetative leafy modules. The second vegetative leafy module carries a generative one. Individual H.Ch.o5 presents the following long module succession: 1 generative module (g. m.), 1 vegetative module (v. m.), 1 g. m., 1 v. m., 1 flowering g. m.
3.4 The other species of Chrysoglossum
3.4.1 Chrysoglossum reticulatum
All specimens were fertile. H.Ch.r3 shows a succession of 3 vegetative modules followed by a generative one. Structure of vegetative modules is identical to the previous descriptions in C. ornatum: they are formed by a rhizome segment of 4 short internodes, followed by an erected pseudobulb carrying a single terminal leaf. The generative module consists of a rhizome segment of 4 internodes; the fourth one takes an orthotropic direction. The rhizome is prolonged by the stalk of the inflorescence of 3 long internodes with sheathing cataphylls. The rachis carries 15 floral bracts with only the last 5 flowers present and open. Specimen H.Ch.r2 shows a generative module followed by 7 successive vegetative modules, the last one emitting at its base an immature generative module. The oldest vegetative module has a significantly lower size than all other vegetative modules. The 3 following vegetative modules are of equal size and the last 3 leafy ones are slightly larger. The specimen H.Ch.r1 shows the following successive modules: 1 v. m., 1 g. m., 3 v. m., 1 g. m., 1 v. m., 1 g. m.
3.4.2 Chrysoglossum ensigerum
The specimen H.Ch.e1 of C. ensigerum shows a succession of 2 vegetative modules followed by a generative one. The vegetative modules of this individual consist of a rhizome segment of 3 to 4 swollen and short internodes, followed by a long internode forming a pseudobulb. The rhizome of the generative module is also short, while the peduncle and the rachis length are respectively 32 and 23 cm.
4 Discussion and conclusion
4.1 Distribution and habitat
The large range of distribution of C. ornatum from Nepal to New Caledonia [11], and more specifically in continental Southeast Asia (Cambodia, Thailand, Vietnam), already suggested that it would occur in Laos. Thus our researches have confirmed its presence for the first time in Laos. Given that this species is also present in southern China, it can be assumed that it may be found in the north of Laos, and also in Myanmar. The environments in which this species has been collected in Laos are in line with those in which it has previously been collected. Its presence thus seems to confirm that it is a good indicator of non-disturbed tropical forest environments. Three individuals have been seen on steep mountain slopes in the province of Bolikhamxay. A much larger population of several dozen individuals has been observed on the Bolovens plateau. One may perhaps conjecture that this species is better adapted for development on horizontal ground, because of the shallow depth of its root system that does not allow for much mechanical support on steep slopes.
The flowering period observed in Laos corresponds to the flowering period given by Van der Burgh and de Vogel [11] for this species in Thailand (Jan.–July). Observations in the greenhouse show a rapid disappearance of unfertilized inflorescences, analogous with the rapid drying of swollen part of plants of tropical woodland observed by Blanc [26]. This contributes to limit the loss of water by evapo-transpiration in a plant part that has lost its reproductive function.
The study of herbarium samples of this species reveals that even phenological shifts can appear from one region to another; however the architectural structure for this species seems stable between samples studied from Java, Sumatra, Peninsular Malaysia and Laos.
4.2 The establishment growth
Young individuals have a small number of roots (Figs. 1A and 1B). Their sympodial units are of increasing size (length and diameter) and their number of roots by module increases gradually. This process is typical of the “establishment growth” observed in several Monocotyledons [24].
When a young plant is too deeply buried in the ground, a sudden increase of the length of the rhizome with an orthotropic orientation (Fig. 1B) enables it to reach the surface of the ground and to continue photosynthesis. This corresponds to the regulatory response to the immediate environment described by Bell and Tomlinson [25] on rhizomatous plants.
4.3 First occurrence of flowering
When a plant reaches its generative stage, all the elements of the sympodial units have their maximum development (Fig. 3A). The pseudobulb is the most important reserve organ.
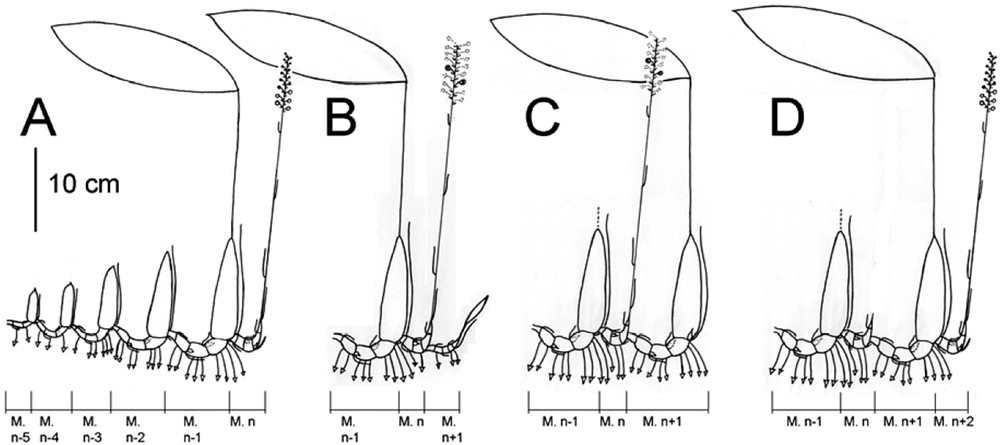
Diagrammatic representation of the architectural development of Chrysoglossum ornatum Blume. A: Establishment growth, with successive sympodial units that are increasingly large with their organs increasingly developed. B: Origin of a vegetative module n + 1, at the base of the generative one n. C: Growth and elongation of the new vegetative module n + 1 with roots, and formation of a resting bud. D: Development of a new generative module n + 2 on the pseudobulb of the vegetative module n + 1.
The leaf longevity must be variable under different conditions. In cultivation, plants can lose all their leaves. In the field, most of the plants collected in Laos had between 1 and 3 leaves. Leaves remain persistent for several months after their establishment, but it is difficult to know how long precisely. This should depend on the age of plants, and their environment. It seems that mature plant can flower annually, with only a single generative module each year. So the study of the individual L11 (Figs. 1E, 2D and 2E), and H.Ch.o8 enabled us to conclude that leaves can survive at least 3 years if one vegetative module is produced each year.
The development of generative modules began in May among specimens L1 and L2; their flowers were open in June 2007. On all living specimens, pseudobulbs that produced a generative module were more than 48 mm high. It seems that no sympodial unit can produce a generative module under this dimension. Generative modules display, even after the disappearance of their erect part, a strong differentiation with vegetative modules: their internodes number is usually lower, they have a smaller diameter and a lower number of roots. This makes it possible to easily identify what were the roles of successive modules in individuals in which a large number of modules are still present, even without old leaves or inflorescences (Figs. 1C, 1D, 1F).
Some individuals have a long series of vegetative modules during their establishment growth before the first occurrence of a generative one. Once they have acquired their sexual maturity, we observed an alternation of 2 to 3 vegetative modules between 2 generative modules (Figs. 1D, 1F). Generative modules have less roots than vegetative one, thus each individual must find a balance between investment in its production of generative and vegetative modules. This is important in order to always have a sufficient number of vegetative modules to ensure an adequate nutrient supply by a well developed root system. As successive vegetative modules are increasingly large, and have an increasing number of roots, it can therefore be assumed that a mature plant has less needs to produce a long series of these modules. The length of this series is determined by a point of balance where plants may produce alternately a vegetative module and a generative one each year (Figs. 1E, 3B, 3C, 3D).
4.4 Others species of Chrysoglossum
Herbarium samples of C. reticulatum present the same growth pattern as C. ornatum, with a new vegetative module at the base of a generative one. Individuals analyzed have between 3 and 7 vegetative modules followed by a single generative module (Fig. 4B). Van der Burgh and de Vogel [11] mention that they have seen between 1 and 3 successive vegetative modules on mature individuals. It can be concluded that the variability of the number of vegetative modules between two generative ones, which is from 1 to 7 is more pronounced than in C. ornatum. Further studies, however, should make it possible to know if important structural variability exists between these 2 species.
The study of a single specimen of C. ensigerum (Fig. 4C) does not give a relevant conclusion on its growth pattern and dynamic, however, the inflorescence position corresponds to that also observed in specimens of C. ornatum. This species seems to be able to present the same vegetative architecture as C. ornatum and C. reticulatum, but with smaller vegetative organs.
Bibliographic informations found on C. assamicum indicate that it shows the same architectural structure as observed in previous species. Van der Burgh and de Vogel [11] indicate a higher root number per module than for the species previously discussed. Additional information on habitat may allow us to better understand the possible causes.
Our studies therefore suggest that the architectural pattern described for C. ornatum is similar in all others species of this genus, with only small variations of size and morphology between species.
4.5 Comparison with the study of Gongora quinquenervis Ruiz and Pav
Our description of the architectural development on species of the genus Chrysoglossum confirmed that it is similar to that observed by Barthélémy [9] in the species Gongora quinquenervis Ruiz and Pav. (Fig. 4A). Nevertheless, several differences exist between these two growth patterns. The number of internodes per vegetative module is different, with 4 in C. ornatum and up to 10 in G. quinquenervis. Roots originate from the nodes in Gongora, while they arise from the internodes in Chrysoglossum. The pseudobulb in G. quinquenervis carries two apical, sessile, articulated leaves, while the pseudobulb of Chrysoglossum carries a single, petiolate, not articulate leaf. The presence of a petiole may be related to the competition pressure from surrounding vegetation. The generative module arises in these two species from the distal portion of vegetative modules; in Gongora it is inserted on the rhizome, while in Chrysoglossum it is at the base of the pseudobulb. The inflorescences are pendulous in the epiphytic G. quinquenervis, while they are erect in the terrestrial C. ornatum. The plagiotropic part of the generative module carries roots in Chrysoglossum, while this does not seem to be the case in Gongora.
4.6 Occurrence and systematics
The genus Gongora, which belongs to the subtribe Stanhopeinae, has not yet been sufficiently studied to determine if the growth pattern initially described by Barthélémy [9] occurs in other species of this genus. However, additional observations made by this author suggest that this type of architecture is also present in species of Peristeria, in the subtribe Coeliopsidinae. Recent phylogenetic analyses within Maxillarieae have shown that these 2 subtribes are phylogenetically very close [27]. This brings us to reflect on the relevance of vegetative and architectural characteristics for a better understanding of the phylogeny among this family in some tribes, such as Collabieae and Maxillarieae. Freudenstein and Rasmussen [28] stressed the importance of vegetative characters in their cladistic analysis at the level of the entire orchid family. These characters have also already been identified as better phylogenetic indicators than floral characters among Malaxideae by Cameron [29]. That is why research is currently underway on the Collabieae tribe (on the genera Collabium and Tainia). This work could enhance results achieved by Johnson [30] on Annonaceae, introducing architectural characters as reliable phylogenetic indicators.
Acknowledgements
We thank Dr. Phasy, Dean of the faculty of Science at the National University of Laos for access to the living plant collection, and his assistance to the preparation of the field trips; Mr V. Lamxay and Mr S. Lanorsavanh, from the Department of Biology of the National University of Laos and all local authorities for their assistance during field trips; Dr. E.F. Smets for access to the collection of the National herbarium of the Netherlands; Dr. P. Kessler for access to the orchid collection of the Hortus botanicus of Leiden; Dr. E.F. de Vogel for his suggestions and constructive criticism, and Dr. J.J. Vermeulen for his cordial welcome in his laboratory. We also thank an anonymous reviewer for some very constructive suggestions and insights. Our fieldwork, as part of the ORCHIS project (http://www.orchisasia.org/), was financed by grant LA/Asia Invest II/03 (114285) under the AsiaInvest programme of the European Union. AMAP (Botany and Computational Plant Architecture) is a joint research unit associates CIRAD (UMR51), CNRS (UMR5120), INRA (UMR931), IDR (R123), and Montpellier 2 University (UM27); http://amap.cirad.fr/.


