1 Introduction
The white-clawed crayfish Austropotamobius pallipes (Lereboullet, 1858) is the biggest indigenous freshwater invertebrate living in Western and Central Europe [1–3]. Over the last few decades, European populations of native crayfish showed considerable fragmentation and decline on a widespread basis [4]. The decline is mainly due to the introduction of larger, quicker-growing and more aggressive non-indigenous species (such as Procambarus clarkii and Orconectes limosus) that carry the crayfish plague due to Aphanomyces astaci [5–9]. Moreover, the decrease in the populations of A. pallipes has been caused by such human activities as habitat fragmentation, deforestation and water deterioration, which represent more and newer threats for the survival of this species [10–12].
Like other native species, A. pallipes is considered a keystone species wherever it occurs [13]. Freshwater crayfish constitute an important component of many aquatic food webs in lake and stream ecosystems [14–17]. Crayfish are involved in the freshwater food chain both as prey for vertebrate predators [18] and as omnivorous feeders that have a significant impact on community structures [19–23]. Moreover, white-clawed crayfish play an important role in the wellbeing of freshwater systems [24] and contribute to the flow of energy and the cycling of matter [25]. A. pallipes have long been considered a valid bioindicator of water quality [6,26,27]. However, several recent studies have reported that the species can also be found in moderately polluted waters [28–35].
Hence A. pallipes is in need of special protective measures. The species is listed as “vulnerable” on the Red List of threatened animal species compiled by the International Union for the Conservation of Nature and Natural Resources [36]. It is also listed in annexes II and V of the Habitat Directive (Council of the European Communities, 1992, 1997). In Italy, A. pallipes is protected locally by regional laws, including Piedmont Region law L.R. no. 32 of 2 November 1982. This law prohibits the capture, transportation, commerce, detention, and selling of native crayfish. Nevertheless, the number of places where the species is found has decreased over the last 10–15 years as has the density of the populations in the places where it is found [37]. For these reasons, the Piedmont Region set up a plan of action to protect A. pallipes. The plan, in effect since 2005, calls for an accurate census of crayfish and an evaluation of the ecological factors that determine whether they are present or absent. The ecological factors that play key roles in this are the mineral component and the amount of organic matter in water [28,29,34,35]. In European streams, the distribution of white-clawed crayfish seems to depend mostly on calcium concentration because calcium is essential for the building of their exoskeletons [38]. Clean and well oxygenated mesotrophic waters with calcium levels not lower than 5 mg L-1 are required for the success of A. pallipes populations [39]. On the other hand, A. pallipes has a wide range of tolerance of pH levels, water conductivity, and all the main ion concentrations [13,30,32,34,35,39,40–43]. Besides, organic matter plays a very important role. Total Suspended Solids (TSS) tests, estimating the quantity of organic solids in water, have shown that there is a negative correlation between organic solids and white-clawed crayfish distribution in studies where Total Organic Carbon (TOC) tests were also used [34,35,41]. Another useful tool is the Biochemical Oxygen Demand (BOD5) index, one of the most common and reliable measures of pollutant organic material in water. The BOD5 test assesses the oxygen requirements of the bacteria present in a water sample in the oxidation of organic matter. This oxidation is the main process affecting the oxygen content of water. Although the BOD5 test is widely considered an important function in stream pollution-control activities, only Broquet et al. [11] have investigated the correspondence between the results of BOD5 tests and A. pallipes distribution. If we manage to understand the links between the distribution of endangered freshwater species and the ecological factors characterizing their habitats, we will be able to understand freshwater ecosystems better and thus use this important information in helping us set policy on management and conservation.
There is still little known about the mineral and organic components characterizing brooks inhabited by native crayfish in Northwestern Italy [37,44–46]. This study aims: (1) to investigate the chemical-physical demands of A. pallipes in Piedmont by means of multivariate statistical techniques; (2) to perform BOD5 tests; and (3) to evaluate the reliability of these tests.
The multivariate statistical techniques have been widely used in various studies for the chemical/physical characteristics of water quality parameters [47–49] in comparison to uni-variant techniques that usually fail to give adequate information on multivariate dataset [50]. In the present study, multivariate techniques such as Principal Component Analysis (PCA), Discriminant Factor Analysis (DFA), and Logistic Regression (LR) were used for the water quality assessment and interpretation of the results.
Our ultimate goal is to contribute to the species management and conservation.
2 Methods
2.1 Study area and crayfish detection
First, we chose the sampling sites on the basis of both recent information and historical records about A. pallipes distribution. We did this through examining the literature and the museums as well as through contacting local town administrators, workers in natural-parks and wildlife reserves, foresters, WWF sections, fishermen and women, and local people. We chose 98 sites for our study system, which covered an area of 21.839 km2. These sites were located along brooks and small tributaries flowing into the Po River in Piedmont (Northwestern Italy). The sites are all characterized by running waters known as homes to native crayfish in the past and, supposedly, in the present too. They were investigated from spring to fall 2005–2008. The sites were distributed in such a way that they reflect the geological variety in Piedmont, where the substrata range from calcareous to siliceous. Thus there was no homogeneity in the physical and chemical characteristics of the river reaches among the sites. Species presence was assessed both during the day through manual surveys (2 people searching for 1 hour) and at night through the use of traps. The traps measured 50 × 25 × 25 cm with a 3 mm mesh size. They were baited with pig- or chicken-liver and left overnight. Each site was sampled three times before we considered it as not being inhabited by the crayfish.
2.2 Physical–chemical parameters
In each site pH, conductivity and dissolved oxygen (DO) were measured by means of a multi-parameter probe (mod. Hydrolab Quanta). Two 100 ml water samples were collected from the all sites at about a 15 cm depth in order to avoid floating material. The samples were stored in sterile polythene test-tubes and frozen until the time that chemical analyses were performed. We measured the concentrations of the most common inorganic ions used to assess water quality: Ammonium (NH4+), Nitrates (NO3-), Ortho-phosphate (PO43–), Chlorides (Cl–), Sulfates (SO42–), Calcium (Ca2+), and Magnesium (Mg2+) using a spectrophotometer DR LANGE Lasa 100 following IRSA [51]. We evaluated the BOD5 following Lenor et al. [52].
2.3 Statistical analyses
At first, for each parameter we adapted the Student's t-test with Bonferroni's correction to assess for differences between the “presence” and “absence” sites.
To improve the normal distribution fit, a log (x) transformation was performed on the absolute measurements. We wished to describe the variability among the water descriptors and to distinguish the sites inhabited by A. pallipes from those that were not. To do this, we conducted multivariate analyses using Principal Component Analysis (PCA), Discriminant Factor Analysis (DFA), and Logistic Regression (LR).
The PCA used all the chemical parameters that had been measured, so that the positive correlated variables were transformed into a smaller number of variables (principal components). Therefore, this is a coordinate transformation, which reduces the redundancy within the data by creating a new series of components. These Principal Components (PCs) are linear combinations of the original response vectors and are chosen to contain the maximum data variance and to be orthogonal.
We wished to verify whether it was possible to separate positive sites from negative ones. To do this, we conducted a DFA and a LR, only using PCs with eigenvalue > 1. The use of these last two techniques helped us to determine which PCs discriminate the positive and negative sites. Both the analyses were performed involving stepwise-forward-selection entry of independent variables. We used species presence or absence as the dependent variable and PCs as independent variables.
The performance either of DFA or LR were estimated from a leave-one-out jack-knifing cross-validated.
3 Results
3.1 Crayfish distribution
The streams that we examined have exhibited a wide variety of habitat structures. Some of the sampling areas were influenced little by human activities and others were influenced greatly. The areas of high anthropic impact were marked by discharges and by the modification of landscape features. The discharges came mainly from factories, sewers and farms. The landscape features were modified through plantations, canalization, dredging, reservoirs, and engineering works. Native crayfish were no longer observed in 57 previously inhabited watercourses. However, extant populations were still found in 41 of the 98 sampling sites. Fig. 1 shows the distribution of both the positive and the negative sampled sites.
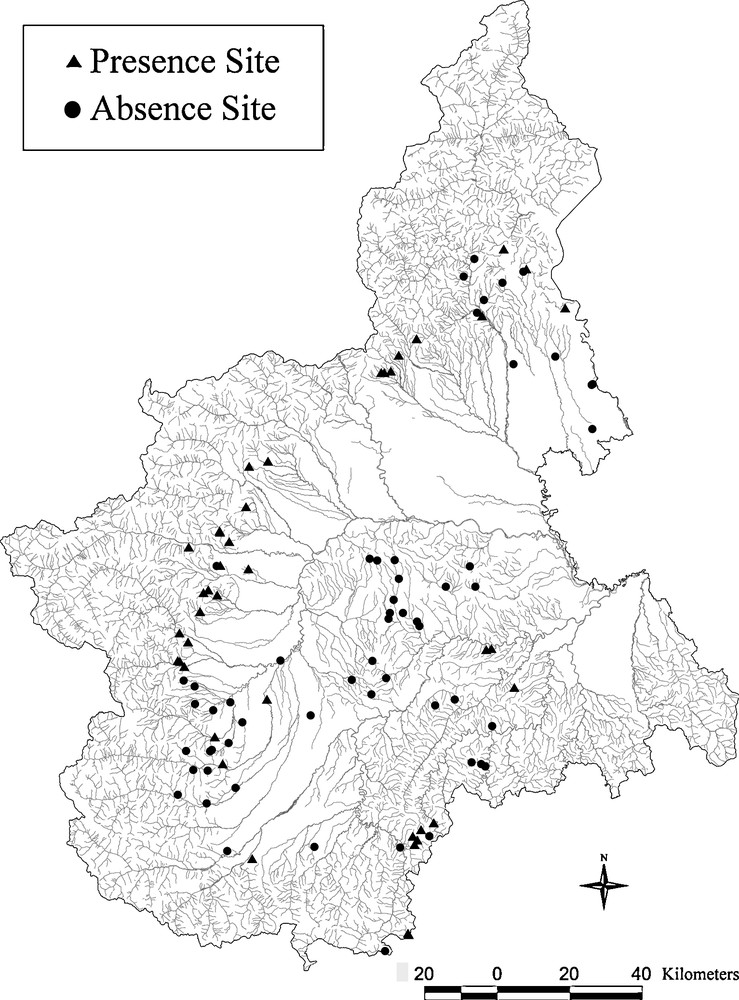
Map of the Piedmont region showing the distribution of the presence and absence sites of Austropotamobius pallipes.
3.2 Descriptive statistics
Descriptive statistics of physical–chemical water parameters, measured at both the positive and at the negative sites and obtained from the analyses were given in Table 1. The Student's t-test showed statistically significant differences in the BOD5 index, water hardness, Ca2+, and pH.
Descriptive statistics of water chemistry variables in “presence” and “absence” sites of Austropotamobius pallipes (mean + s.d. = mean + standard deviation).
| Parameter | Presence (n = 41) mean ± s.d. | Absence (n = 57) mean ± s.d. | Significance | |
| SO42- (mg/L) | 76.59 ± 136.76 | 100.323 ± 101.247 | t = 0.941 | p-value = n.s. |
| Cl- (mg/L) | 11.166 ± 12.469 | 15.787 ± 16.905 | t = 1.557 | p-value = n.s. |
| Mg2+ (mg/L) | 12.712 ± 11.717 | 16.556 ± 16.844 | t = 1.332 | p-value = n.s. |
| Ca2+ (mg/L) * | 28.378 ± 30.633 | 57.987 ± 46.027 | t = 3.821 | p-value < 0.05 |
| PO43- (mg/L) | 0.562 ± 0.970 | 0.680 ± 1.118 | t = 0.553 | p-value = n.s. |
| NH4+ (mg/L) | 0.427 ± 0.604 | 0.551 ± 0.777 | t = 0.885 | p-value = n.s. |
| NO3- (mg/L) | 7.725 ± 5.171 | 12.112 ± 12.211 | t = 2.426 | p-value = n.s. |
| DO (mg/L) | 8.335 ± 1.697 | 7.486 ± 2.362 | t = -2.072 | p-value = n.s. |
| BOD5 (mg/L) * | 5.228 ± 2.153 | 3.245 ± 1.995 | t = 4.637 | p-value < 0.001 |
| pH * | 7.887 ± 0.684 | 8.237 ± 0.565 | t = 2.677 | p-value < 0.05 |
| Cond. (μS/cm) | 224.463 ± 196.175 | 255.140 ± 289.268 | t = 0.625 | p-value = n.s. |
| Hardness (dH°) * | 7.777 ± 9.816 | 15.853 ± 16.481 | t = 3.027 | p-value < 0.05 |
* Parameters significantly (p < 0.05 after Bonferroni correction applied) differing the sites where crayfish are and are not present.
3.3 Principal Component Analysis
The PCA showed that the total variance of all four components with eigenvalue > 1 ranged up to 71.88%. A significant difference in the mean values of PC1 and PC3 between sites with and without A. pallipes was found (p < 0.01). Table 2 shows the weight of each variable in building the principal components. Fig. 2a illustrates the projection of the chemical and physical parameters on the principal plane. Fig. 2b shows the factor scores of the sites with and without native crayfish, plotted on the plane defined by PC1 and PC3.
Results of the preliminary Principal Component Analysis performed on the log transformed chemical variables. Eig, eigenvalues showing value > 1; Cl, component loadings; % var, percentage of the total variance explained for each of the Principal Components (PC1-PC4) found.
| PC1 | PC2 | PC3 | PC4 | ||
| Eig | 4.223 | 1.959 | 1.382 | 1.071 | |
| % var | 35.194 | 16.250 | 11.414 | 8.926 | |
| Cl | Mg2+ | 0.830 | –0.145 | 0.189 | –0.018 |
| BOD5 | –0.343 | 0.360 | 0.619 | 0.214 | |
| SO42- | 0.786 | –0.232 | 0.052 | –0.103 | |
| Cl- | 0.725 | –0.126 | 0.289 | 0.154 | |
| Ca2+ | 0.881 | 0.037 | –0.195 | –0.106 | |
| Hardness | 0.951 | –0.116 | –0.061 | –0.081 | |
| pH | 0.292 | 0.288 | –0.684 | 0.413 | |
| DO | –0.281 | –0.440 | –0.154 | 0.716 | |
| Cond. | 0.321 | 0.664 | –0.137 | 0.266 | |
| NO3- | 0.341 | –0.485 | 0.436 | 0.437 | |
| NH4+ | 0.174 | 0.707 | 0.362 | 0.102 | |
| PO43- | 0.422 | 0.512 | 0.057 | 0.136 |
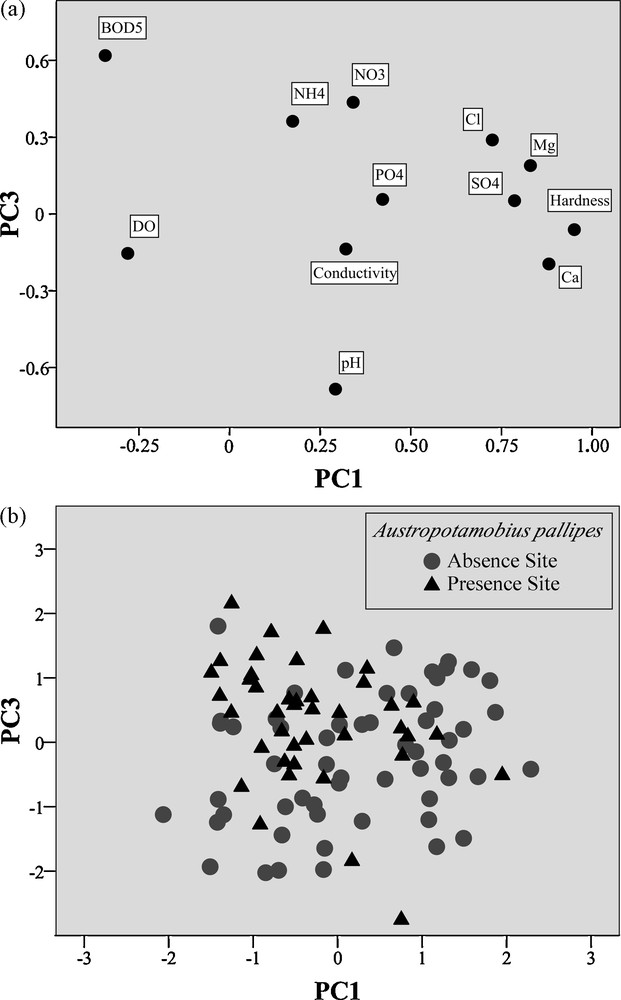
(a) Chemical-physical measured variables projected on the plane defined by PC1 and PC3; (b) scores of the 98 sampled sites projected on the plane defined by PC1 and PC3.
3.4 Discriminant Function Analysis and Logistic Regression
The stepwise-forward DFA based on the aforementioned PCs revealed significant differences between positive sites and negative sites (Wilks’ λ = 0.851, F2-95 = 8.313, p < 0.001; PC1: F = 8.313, tolerance = 0.99; PC3: F = 8.11, tolerance = 0.99). Multivariate cross-validated discrimination classified 67.3% of the sampled sites correctly – i.e. 66.7% of the negative sites and 68.3% of the positive ones. Table 3 shows the eigenvalue and canonical discriminant functions standardized by within-variances of the DFA. Evidently, the factors obtained by the analysis are mostly due to PC1 (corresponding to water hardness, Ca2+, and Mg2+), and PC3 (pH, BOD5, and – to a lesser extent – NO3–).
Results of the Discriminant Factor Analysis performed on PC1-PC4 (Eig = eigenvalue; cc = canonical correlations; cdf = canonical discriminant functions standardized by within variances).
| cdf | |||
| Eig | cc | PC1 | PC3 |
| 0.175 | 0.386 | -0.722 | 0.753 |
Although only four of the physical–chemical water parameters showed significant differences between the presence and absence locations, the Logistic Regression Analysis showed the best classification results with two PCs (PC1 and PC3), retained in the equation at the step 2 (Table 4). These last, as mentioned above for the DFA results, correspond to six different physical–chemical water parameters. Overall, cross-validated regression classified 74.43% of investigated locations correctly (Table 5). The most important PC was PC3 (Table 4), corresponding basically to pH and BOD5.
Results of the Logistic Regression Analysis for water chemistry variables (performed on PCA factors); variables in the equation. Variable(s) entered on step 1: PC3. Variable(s) entered on step 2: PC1.
| B | S.E. | Wald | df | p | Exp(B) | ||
| Step 1 (a) | PC3 | 0.624 | 0.234 | 7.193 | 1 | < 0.01 | 1.866 |
| Constant | –0.365 | 0.215 | 2.879 | 1 | > 0.05 | 0.694 | |
| Step 2 (b) | PC1 | –0.628 | 0.237 | 7.021 | 1 | < 0.01 | 0.534 |
| PC3 | 0.671 | 0.246 | 7.467 | 1 | < 0.01 | 1.957 | |
| Constant | –0.387 | 0.224 | 2.989 | 1 | > 0.05 | 0.679 |
Classification table of Logistic Regression Analysis for water chemistry variables. The cut-off value is 0.500.
| Predicted | ||||
| Absence | Presence | % correct | ||
| Step 1 | Absence | 45 | 12 | 78.9 |
| Presence | 24 | 17 | 41.5 | |
| Overall % | 63.3 | |||
| Step 2 | Absence | 47 | 10 | 82.5 |
| Presence | 19 | 22 | 53.7 | |
| Overall % | 70.4 |
4 Discussion
Our findings underline that the white-clawed crayfish are distributed in a heterogeneous and fragmentary way over the area that we studied. Over the last few decades, Piedmont populations of native crayfish declined considerably [37], as did all those in Europe [3–5,8,9,42]. In the present study, in particular, native crayfish were no longer observed in 57 previously inhabited watercourses. This phenomenon may be explained as the result of deforestation, habitat fragmentation, engineering works, stream canalization and increased water pollution. Although A. pallipes can be found in high quality waters [e.g. 26,53,54], the physical–chemical analyses carried out in the 98 sites (Table 1) and the multivariate statistics (Tables 2–5) show that A. pallipes is a valuable bioindicator of the presence/absence of dissolved organic matter, fine particles and - to a lesser extent – NO3–.
Moreover, the correct classifications we obtained are similar to those by several authors [29,43,55], but they are higher than the ones reported by Trouilhé et al. [35], who reported the first three PCs explained 55% of the total variance. It is to be underlined also that the only authors using PCs to run a DFA, as we did, are Rallo and Garcìa-Arberas [29], who obtained 73% of correct classification using four PCs.
Moreover, our DFA and LR models showed similar percentages of overall classification (67.3 and 70.4, respectively) but different sensitivity and specificity. In fact, while DFA reached similar percentage of presences and absences correctly predicted (68.3 and 66.7, respectively), LR showed much lower sensitivity (53.3%) than specificity (82.5%), in spite of the occurrence of A. pallipes at 40.18% of the sampled sites. The same trend is reported by Manel et al. [56]. In general, predictions are more accurate when there are more occurrences of the species under examination [56–59], especially when the number of presences and absences is just about the same [59]. This is obviously a problem because in ecology, especially for rare species, absences are, of course, more frequent than presences. High levels of correctly predicted presences are particularly important in instances where the presence of scarce species is predicted for conservation purposes – for example, in identifying areas for protection or management of rare species.
4.1 Organic matter
The organic matter dissolved in the water is a key factor in explaining white-clawed crayfish distribution, together with pH. This is something that is made evident by our research project as well as by the earlier research [34,35,41]. In fact, both the DFA and LR show that the BOD5 is the most important parameter, one that allows us to separate positive from negative sites. Broquet et al. [11] and Trouilhé et al. [35] had already underlined that organic matter is one of the most important factors characterizing brooks with native crayfish. In this research project, we measured – through BOD5 – the organic matter that bacteria can attach to biologically. (This mainly consists in dissolved organic matter and fine particles.) We confirmed that BOD5 is an index that is reliable to use for characterizing sites where A. pallipes lives. This hypothesis has been confirmed by only one other research previously [11], where the BOD5 index was used to obtain values only slightly lower than the ones we measured.
4.2 The mineral component of the underling geology
4.2.1 Ca2+ concentration
There is another factor, we concluded, that is very important for A. pallipes presence: the mineral component of the underlying geology. The Ca2+ concentration in all our sampled sites was > 2.56 mg L–1. This concentration corresponds to the lowest values of Ca2+ reported in the literature to be necessary to build the exoscheleton [29,35,60]. Ca2+ is very important for A. pallipes life because it plays a key role in building the exoskeleton [38,61]. In fact, the Student's t-test showed that the concentrations of Ca2+ in positive and negative sites were significantly different statistically. These findings conflict with those of Renai et al. [43]. Hard water, as is known, has a high mineral content, primarily consisting of Ca2+ and Mg2+ concentrations. Hence water hardness is proportional to Ca2+ concentration. In fact, we found that the water in the streams inhabited by A. pallipes is softer in a statistically significant way than the water in streams where the crayfish are absent (see Table 1). The Ca2+ level is higher in limestone areas than in granite or siliceous areas. The mean Ca2+ concentration in sites without A. pallipes is almost double that of sites with A. pallipes. Therefore, not limestone but granite or siliceous substrates are essential for the presence of white-clawed crayfish, as we asset. Other research projects also underline the importance of the underlying the geological substrates for the presence of crayfish both in rivers and lakes [29,62,63]. Our study is the first to report that A. pallipes presence is so closely related to granite or siliceous substrates.
4.2.2 SO42- and Mg2+ concentration
There were other factors that we considered – the SO42– and Mg2+ ions. In this case, we found no statistical differences in the SO42– and Mg2+ measurements in positive sites and in negative sites (see Table 1). However, previous research projects offer us conflicting results. On the one hand, Rallo and García-Arberas [29] noted that SO42– and Mg2+ are the factors that play the most important roles in separating positive from negative sites. They found that SO42– concentrations followed the levels of concentrations of Mg2+ and that the concentrations of SO42– and Mg2+ were higher (in a statistically significant way) in the sites inhabited by the crayfish than in those not inhabited by them. On the other hand, Trouilhé et al. [35], did not find any statistical differences in the concentrations of SO42– and Mg2+: there were no differences that related to the presence or absence of crayfish. However, the mean values they found in the sites they analyzed were much lower than the mean values we found. Moreover, the present research underlines the importance of the Mg2+ in building PC1, which is – together with PC3-retained in both DFA and LR models at step 2.
4.3 Water conductivity
There is another factor that we considered, the level of conductivity. This level is not a factor that limits A. pallipes presence, according to our research. Our findings are reinforced by the fact that conductivity has no weight in building the PCs used to run the DFA and LR. All the values we recorded fit in with those recorded in the literature [29,35,43,60].
4.4 pH values
Another important fact that we examined is the pH. There were statistical differences in the mean pH values in positive and negative sites, according to our research (Table 1). DFA and LR confirmed the importance of the pH as well as of the nitrates. In fact, the pH is the most important variable in building PC3, used to distinguish positive from negative sites for the crayfish. The inhabited sites are those that have a lower pH (= 7.89) than the negative ones. This is mainly due to two reasons: the pH is correlated with the Ca2+ concentrations positively and the water coming from areas with granite or siliceous rocks is less basic than water coming from areas with limestone. The pH values of the positive sites are within the ranges reported in the literature [35].
4.5 Pollution
A. pallipes distribution is not affected by pollution due to ammonia and ortho-phosphate. In fact, we observed streams inhabited by A. pallipes that were polluted by these ions, which flowed into them. Our findings concur with earlier research projects [11,28–30,34,35]. Hence, we agree with Füreder and Reynolds [33] and Trouilhè et al. [35] that we should no longer consider A. pallipes as a valid bioindicator of water quality as a whole. We make one qualification, though: A. pallipes is still a valuable bioindicator of the presence/absence of dissolved organic fine particles and NO3-.
4.6 Other environmental features
There are other environmental features that can explain the distribution of A. pallipes, at least partially. These are the features that help A. pallipes avoids potential predators and find food – e.g. bank-side shrubs, tree roots, shade, shelters, and riverbeds suitable for burrowing activities. Previous research projects take these features into account [13,41,64].
4.7 Conclusion
The data reported here can be applied to white-clawed crayfish management and conservation. We realize that natural soil conditions cannot be modified. However, we can modify human activities. We can strive to reduce those human activities that increase the organic matter in water. This is one simple way to preserve extant crayfish populations. Our data lead us to conclude in this way and so does the data on the marked reduction of native crayfish in Piedmont [37]. We therefore strongly urge that conservation measures should be taken for the populations present today and for their habitats. On the level of scientific research, we urge researchers to apply decision trees and Artificial Neural Networks to A. pallipes modelling, techniques already applied widely in freshwater fish management [65,66], which were not applicable in the present research because of the too few number of cases. Only with the exploitation of these and other more reliable and more modern techniques can we fully understand the habitat requirements of A. pallipes and help in managing this species.
Acknowledgements
This work was funded by the Piedmont Regional Government through the project “Action plan for the crayfish Austropotamobius pallipes complex (Crustacea Decapoda Astacidae) in Piedmont” and by CRT (Cassa di Risparmio di Torino) foundation through the Alfieri project.
Special thanks are due to Giulia Bemporad for her assiduous participation in samplings.


