1 Introduction
Since 2007, the urban population has exceeded the rural population for the first time in human history [1]. Urbanization is associated with various effects, which can directly affect biodiversity, e.g., habitat loss and fragmentation [2]. However, this phenomenon also induces alterations in human networks (social, economic, political) and thus in practices and land-use, generating indirect effects on biodiversity (e.g., resource use, waste generation, watercourse drainage, etc.) [3]. The impact of urban processes is not limited to cities or urban areas, but also affects rural land-uses and their functioning [4].
In Europe, land-use changes have accelerated in the last 50 years due to the agricultural recession of the 1950s that favoured urban sprawl over the rural hinterland while remnant agricultural areas were subjected to either intensification of agricultural practices or abandonment [5–7]. The spread of the urban footprint to the adjacent countryside also corresponds to an increase in the population's requirements in terms of the quality of life and has involved changes in rural living patterns during the last decades [8]. This urbanization process induces rapid changes of the organization of the rural landscape, with the setting up and spread of new anthropogenic ecosystems. These rapid alterations in land-use are considered as the main reason for the decline in both the biodiversity and surface area of species-rich ecosystems in Europe [7,9,10]. On the other hand, the expansion of private gardens and the associated development of horticultural flora have given rise to new habitats and resource types in the landscape [11,12] which can result in the potential enrichment of the local plant diversity [13]. The study of biodiversity in these urbanizing rural areas is of prime interest given the juxtaposition of spontaneous and domesticated nature they represent.
Butterflies, as phytophagous organisms, constitute one of the first integration levels in ecosystems of alterations in vegetation induced by land-use changes. Variations in the structure of butterfly assemblages (i.e., in abundance and diversity patterns) can be easily linked to human-generated disturbance [14,15], reflecting the quality of the environment and its changes [16,17]. The shortness of their generation time and their high habitat specificity result in high rapidity of response [18]. Moreover, their ecological niche is divided between several partial habitats (for mating, larval or adult feeding, etc.) [19] which makes butterflies a relevant indicator group for the study of the functioning of landscape mosaic [20].
This article attempts to characterize changes in the composition and dynamic of communities related to the evolution of available habitats in a mutating landscape. In this study, we characterize the butterfly community of four land-uses (forest, fallow land, private garden and vineyard) especially concerned by current changes. Our goal is to better understand the roles of these land-use types as biodiversity reservoirs. We also characterize the composition of the landscape surrounding each sampling location (further called “landscape context”) in order to evaluate the relative influence of land-use type and landscape context on the functional composition of butterfly communities. This multi-scalar approach constitutes an original and necessary way in order to predict the consequences for biodiversity of current and future changes in the composition and organization of the landscape mosaic. This is a point of crucial importance for the Luberon Regional Natural Park in which our study site is located. As a Man and Biosphere reserve [21], one of its focuses is to integrate human activities in the sustainable management of its natural heritage.
We aim to: (1) identify patterns in the structure of butterfly assemblages discriminating the different land-use types and/or landscape contexts, and (2) investigate whether assumed differences between land-use types and/or landscape contexts are expressed in the functional composition of butterfly communities.
2 Material and methods
2.1 Study site
Our study site is located at Lauris (44°44′N; 5°18′E), a village in southeastern France sited on a calcareous plateau on the banks of the Durance River. The region has a Mediterranean climate characterized by cool winters, irregular precipitation (between 700 and 1000 mm per year) and pronounced summer drought that limits the growing season to brief periods in spring and autumn [22].
In this study site, as in the Provencal rural hinterland, the abandonment of cultivated areas and pasture has been responsible since the 1950s for the development of fallow lands and forest fragments (23% more wooded area in France between 1965 and 1985 [23]). The evergreen brushwood of Quercus ilex (Linnaeus), formerly maintained by forest exploitation (pasture, coppicing, litter-collection, etc.), is now densifying. Forest fragments are also expanding, colonized by Pinus halepensis (Miller). The remnant cultivated areas consist in vineyards, olive and fruit trees and occasionally cereals. But vineyards, which contribute to the regional identity now that the “Côtes du Luberon” wines have been listed as an AOC (registered designation of origin), are in intensive competition with other crops. Since the 1970s, fallow lands (consisting here in dry calcareous grasslands) resulting from the abandonment of cultivated land, have been in decline in the face of pressure from construction. This urbanization process has induced major changes in the landscape organization patterns and the artificialization of the local flora, both associated with the development of private gardens in the landscape mosaic. Human pressures on this landscape mosaic have consequently evolved in both form and intensity during the last half century.
Today, the landscape mosaic of Lauris is composed of four main land-uses: 10% fallow lands, 12% built areas, 25% agricultural areas and 53% forest [11]. This landscape mosaic offers a variety of landscape contexts from the village centre to the forested hillsides surrounding the village.
Four land-uses have been investigated in this study: fallow land, private garden, vineyard and forest. These habitats, described above, also constitute different levels of anthropogenic pressure on habitats. Forests would appear to be the least disturbed habitat, whereas vineyards are a highly altered one. Like vineyards, gardens have resulted from high anthropogenic pressure. Finally, although they are agricultural areas that are left untilled, fallow lands integrate the effects of past disturbance and thus constitute an intermediate disturbance level.
2.2 Butterfly surveys
Butterfly surveys were conducted at eight different sites, all located outside the village centre in patchy urban extensions (Appendix 1). These eight sites were chosen for gathering the four studied land-use types in a reduced area. In each of these eight sites, four sampling locations of approximately 500 m2 were sampled, corresponding to the four land-use types: fallow land, private garden, vineyard and forest fragment. These 32 sampling locations were visited (replicated) three times between May and July 2005, resulting in 96 sampling units. The size of the sampled locations (500 m2) has been chosen according to the size of the smallest studied private garden.
In order to standardise the climatic conditions, plots were sampled between 9 AM and 5 PM on days when the temperature was above 20 °C, cloud cover was less than 50% and wind speed was less than 20 km/h. During these surveys, the recorder covered the sampling location in 30 minutes catching every encountered individual with a butterfly net. Thirty minutes of recording seems to be a good compromise between realistic species richness assessment and reasonable cost in terms of time, since Steffan-Dewenter and Tscharntke [24] have shown that butterfly species accumulation curves increase rapidly to reach a plateau within the first 10 minutes, whatever the surface area of the site studied.
2.3 Data analysis
To characterize the landscape context around each of the eight sites, six landscape descriptors have been calculated in a 400 meters buffer [25] based on a land-use map (Appendix 1): percentage of ground occupied by dwellings, roads, forest, orchards, agricultural land and fallow lands. A Principal Component Analysis (PCA) was performed to assess differences in these landscape descriptors between the eight sites. This has allowed us to create two groups of landscape contexts. The discrimination of these two groups has been tested using a paired Hotelling's T2 test based on percentage of ground occupied by dwellings and forest.
Kruskall-Wallis tests were used to test for differences between land-use types in butterfly species richness, abundance, diversity (Shannon index) and evenness (Pielou index), based on the 96 sampling units. Where significant differences occurred, a Mann-Whitney post-hoc test was used to specify the nature of the differences.
In the same way, we used a Mann-Whitney test to test for differences in butterfly species richness, abundance, diversity and evenness between the two landscape contexts highlighted with PCA and Hotelling's T2 test.
In order to assess specific similarity between habitat types, the Jaccard index was calculated for each possible pair of samples based on the 32 sampling locations. In order to compare the importance of “landscape effect” and “land-use effect” on sample similarity, “within-land-use similarity” and “within-site similarity” have been compared. Mann-Whitney tests were used to assess whether there were significant differences between mean within-land-use similarity and mean within-site similarity for each land-use type.
To assess the influence of land-use type and landscape descriptors on functional composition of the community, a RLQ analysis has been performed (library ade4 in R, [26]). RLQ analysis is an extension of co-inertia analysis that performs a double inertia analysis of two arrays (R and Q) with a link expressed by a contingency table (L). Our analysis is based on the specific composition of the 32 sampling locations (Table L on which a correspondence analysis has been performed). Each sampling location has been characterized according to its land-use type and landscape descriptors (Table R on which a Hill-smith analysis has been performed). Each species has been described according to the following functional attributes (Table Q on which a multiple correspondence analysis has been performed).
Voltinism:
- • univoltine species (one generation per year);
- • bivoltine species (two generations per year) and;
- • multivoltine species (three or more generations per year).
Larval diet:
- • monophagous species (reported to feed on one host-plant species);
- • “strongly oligophagous species” (reported to feed on host-plants of one genus);
- • oligophagous species (species reported to feed on plant species belonging to one taxonomic family) and;
- • polyphagous species (species reported to feed on a variety of host-plants belonging to two or more taxonomic families) [17,24].
Over-wintering stage:
- • species that over-winter at egg or larval stage (wintering_st1) and;
- • species that over-winter at pupal or adult stage (wintering_st2).
Mediterranean affiliation has been also reported on the RLQ plot [27].
All statistical tests and analysis have been performed using R software [28] and PAST software [29].
3 Results
3.1 Landscape descriptors
On the basis of the PCA plot, landscape descriptors discriminate two landscapes contexts. These two groups of sites are well individualised on the first principal component which represents approximately 72% of inertia (Fig. 1). Sites E, F, G and H are regrouped on the left of the plot, associated with high forest density and high orchards density. Inversely, sites A, B, C and D are associated with high density of dwellings and fallow lands. Density of roads and agricultural lands do not really participate to discrimination. Values of environmental variables for each sampled site are shown in Appendix 2. The Hotelling's T2 test performed on percentage of forest and percentage of dwellings confirms the discrimination of the two groups of landscape context (T2 = 212; p = 0.014).
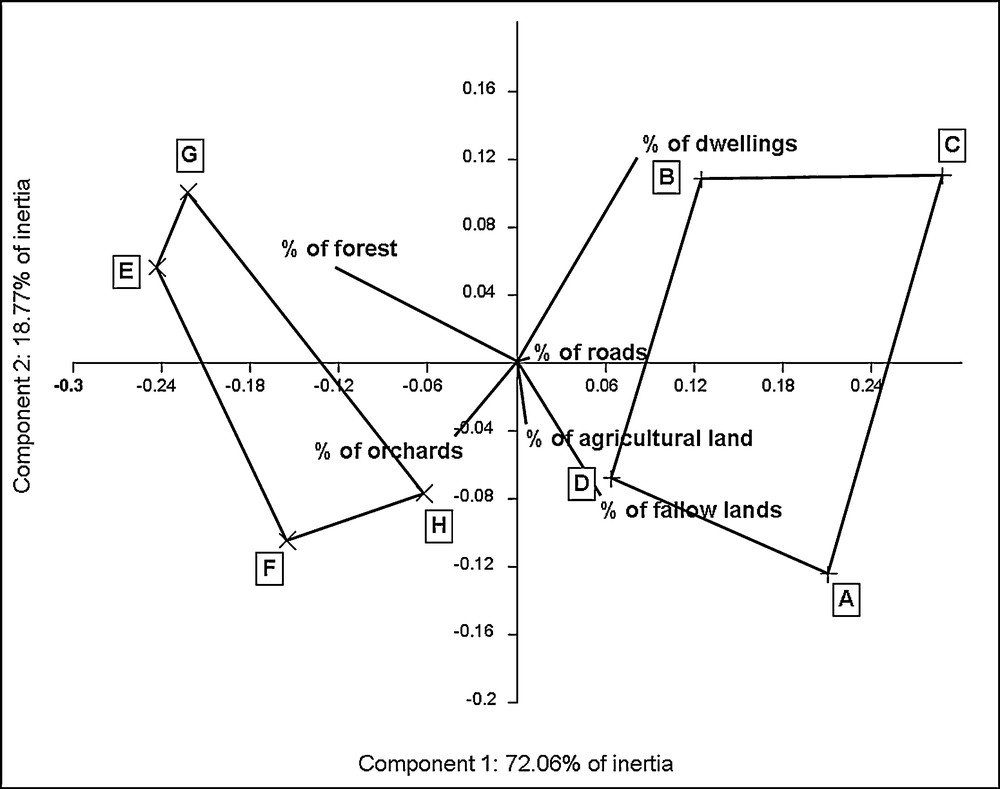
Graphical interpretation of the PCA computed on landscape descriptors.
3.2 Species richness and abundance of butterflies
One thousand three hundred and seventy eight individuals of 54 species were recorded over the three sampling periods (Appendix 3).
3.2.1 Landscape effect
Forty-two species were recorded in the most urbanized landscape context (685 individuals) and 45 species in the most forested landscape context (691 individuals).
No significant difference (in abundance, richness, diversity and evenness) appears between the two landscape contexts based on Mann-Whitney test.
3.2.2 Land-use effect
Fallow lands gather more individuals and species compared to other land-uses where vineyard is the poorest land-use relative to abundance and forest relative to species richness (Table 1).
Abundance, specific richness, diversity and evenness (± standard deviation) per sample and on overall samples for each habitat type.
| Fallow lands | Gardens | Vineyards | Forests | |
| Mean abundance/sample | 22.04 ± 8.26 a | 17.46 ± 7.70 a,b | 6.00 ± 3.76 c | 11.83 ± 4.31 b |
| Total abundance | 529 | 419 | 144 | 286 |
| Mean species richness/sample | 8.29 ± 3.00 a | 7.78 ± 2.84 a | 4.25 ± 2.01 b | 4.29 ± 1.55 b |
| Total species richness | 40 | 39 | 31 | 20 |
| Mean diversity/sample | 2.48 ± 0.62 a | 2.42 ± 0.70 a | 1.80 ± 0.74 b | 1.62 ± 0.62 b |
| Total diversity | 4.13 | 4.14 | 4.23 | 2.83 |
| Mean evenness/sample | 0.84 ± 0.009 a,b | 0.88 ± 0.08 a | 0.95 ± 0.05 c | 0.79 ± 0.16 b |
| Total evenneess | 0.75 | 0.78 | 0.85 | 0.65 |
The highest overall diversity occurs in vineyards. Shannon indices obtained for gardens and fallow lands are a little lower, while the lowest overall specific diversity occurs in forests (Table 1).
Abundance of butterflies, species richness, diversity and evenness per sample differ significantly between land-use types (respectively, Hobs = 47.40, p < 0.001; Hobs = 37.36, p < 0.001; Hobs = 26.70, p < 0.001; Hobs = 31.39, p < 0.001, in Kruskal-Wallis tests). Results of Mann-Whitney post-hoc tests are related in Table 1.
3.2.3 Specific similarity
Fallow lands and forests appear as the most homogeneous land-use types in terms of specific composition. They exhibit mean “within-land-use similarity” that is significantly higher than mean “within-site similarity” (p < 0.005) which shows a strong influence of land-use effect for these two land-use types (Table 2).
Mean between-land-use similarity (± standard deviation) between each pair of land-use types, mean within-land-use similarity and mean within-site similarity computed at site level using Jaccard distance.
| Fallow lands | Gardens | Vineyards | Forests | |
| Gardens | 0.42 ± 0.12 | |||
| Vineyards | 0.29 ± 0.06 | 0.31 ± 0.13 | ||
| Forests | 0.33 ± 0.08 | 0.26 ± 0.10 | 0.26 ± 0.11 | |
| Mean within-land-use similiarity | 0.43 ± 0.11 | 0.34 ± 0.10 | 0.26 ± 0.09 | 0.40 ± 0.10 |
| Mean within-site similiarity | 0.35 ± 0.10 | 0.33 ± 0.13 | 0.29 ± 0.10 | 0.28 ± 0.10 |
Although there is no statistically significant difference, gardens are more similar to fallow lands site by site than to other gardens (p = 0.087), and vineyards seem to be more similar to adjacent habitats site by site than to other vineyards (p = 0.29).
3.3 RLQ analysis
The first two axes of RLQ account for 94% of the total inertia (78 and 16%, respectively). The Monte-Carlo test indicates that the co-structure between R and Q is significant (p < 0.0001, based on 999 permutations). The first two axes of RLQ analysis accounted for most of the variance of the corresponding axes in the separate analyses of environmental descriptors (91% for Hill-Smith analysis of Table R) and species traits (85% for multiple correspondence analysis [MCA] of Table Q), which testifies to the strength of the link between Tables R and Q (Fig. 2).
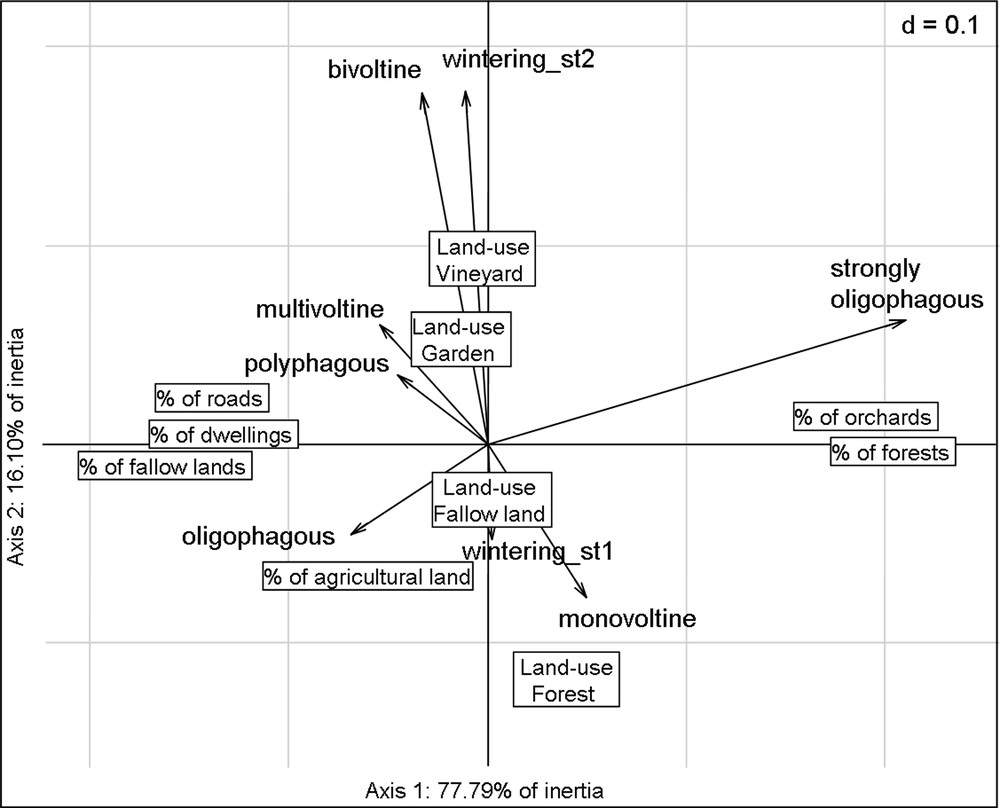
The results of RLQ analysis indicating associations along the two first axes between environmental descriptors (boxes) and species traits (arrows).
Correlations between environmental descriptors and the axes of RLQ are related in Table 3. The first axis of RLQ corresponds to an urbanization gradient from the most forested sites (right of the plot) to the most urbanized sites (left of the plot), while the second axis discriminates sampling location according to land-use type (Table 3 and Fig. 2). The high percentage of inertia related to the first axis of the RLQ analysis underlines that landscape context is more discriminating than land-use type.
Correlations between environmental descriptors and the two first axes of the RLQ analysis.
| Axis 1 | Axis 2 | |
| Land-use_Fallow land | −0.055 | −0.384 |
| Land-use_Garden | −0.078 | 0.583 |
| Land-use_Vineyard | 0.000 | 0.890 |
| Land-use_Forest | 0.102 | −0.812 |
| % of fallow lands | −0.959 | −0.001 |
| % of forest | 0.996 | 0.001 |
| % of agricultural land | −0.503 | −0.339 |
| % of orchards | 0.981 | 0.007 |
| % of roads | −0.931 | 0.022 |
| % od dwellings | −0.973 | 0.004 |
Correlations between species traits and the axes of RLQ are related in Table 4. The species traits that correlate best with the first axis are voltinism and larval diet. On the second axis, the best correlated traits are over-wintering stage and voltinism. These functional attributes discriminate generalist species (polyphagous, multivoltine species that over-winter at adult or pupal stage) found in the left top of the plot from more specialist species (oligophagous, monovoltine species that over-winter as egg or larvae) found in the right bottom of the plot (Figs. 2 and 3). Monophagous species have been pooled with strongly oligophagous species because of their weak number of individuals.
Correlations between species traits and the two first axes of the RLQ analysis.
| Axis 1 | Axis 2 | |
| Monovoltine | 0.630 | −0.315 |
| Bivoltine | −0.104 | 0.614 |
| Multivoltine | −0.739 | 0.187 |
| Wintering_st1 | 0.015 | −0.789 |
| Wintering_st2 | −0.015 | 0.789 |
| Strongly oligophagous | 0.979 | 0.018 |
| Oligophagous | −0.892 | −0.080 |
| Polyphagous | −0.644 | 0.078 |
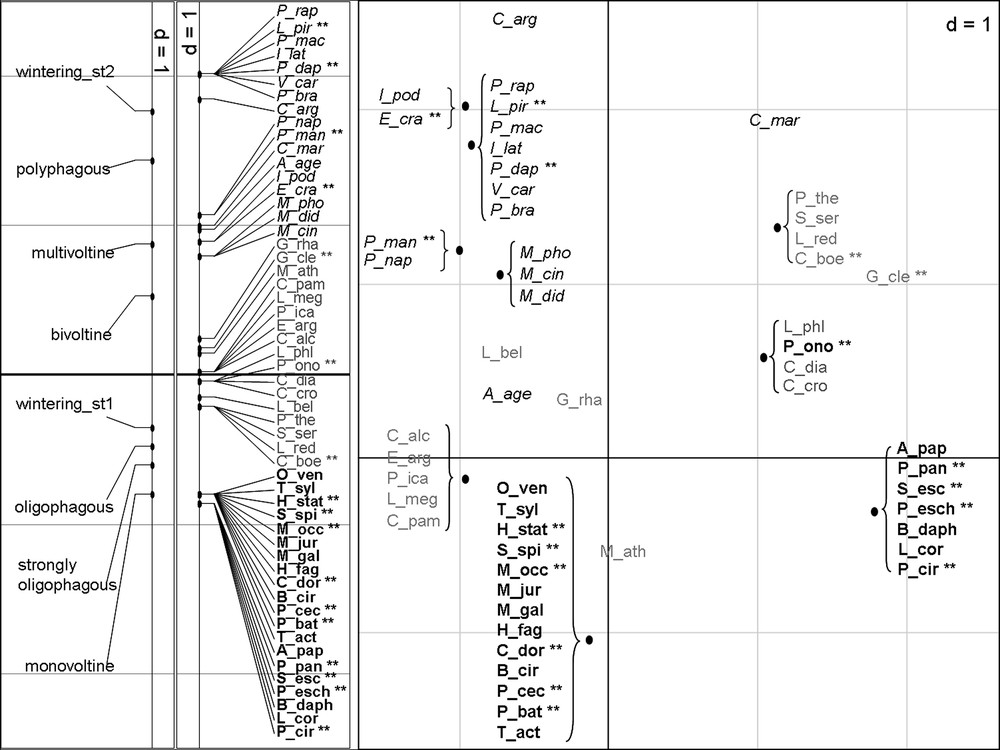
Ordination of species on the first axis of the separate MCA of species traits (plot on the left) and on the two first axes of the RLQ analysis (plot on the right).
Species with generalist traits are in italic black, species with specialist traits are in bold black and species with intermediate traits are in grey. Mediterranean species are marked with double asterisk.
The separate MCA of the Table Q highlights three groups of species according to their trait assemblage (Fig. 3, plot on the left): specialist species (in black and bold), generalist species (in black and italic) and intermediate species (in grey). This discrimination has been reported on the RLQ plot and is consistent with the correlations between species traits and the two first axes of the RLQ analysis (Table 4). All the species classified as specialist in the MCA and 13 of the 17 Mediterranean species appear in the right bottom of the RLQ plot, and all the species classified as generalist species appear in the left top of the plot.
4 Discussion
4.1 Variations in community structure
Our results highlight that abundance of butterflies, species richness, diversity and evenness differ significantly between the four land-use types, even if they do not allow us to highlight significant differences due to landscape context.
Fallow lands constitute the richest habitat type in both species and individuals, directly followed by gardens. Vineyards and forests host poorer communities, which differ from one another in individual abundance (the number of recorded individuals in forests is twice as high as in vineyards).
These differences could be linked to assumed levels of anthropogenic pressure on habitats. Actually, Connell suggested the strong diversity of intermediately disturbed habitats is due to intermediate frequency of disturbance that reduces the pressure of dominant species on others and allows less competitive species to establish themselves [30]. In a same way, Niell et al. observed peaks of richness of butterfly species at intermediate levels of residential development in an area historically dominated by oak woodland. Blair and Launer also demonstrate that this idea of maximal diversity at intermediate levels of disturbance can be extended to include anthropogenic pressure on habitats [15]. Intermediate levels of human development could constitute less constrained habitats that can harbour richer communities than natural areas (strong biotic limitations) or highly human development impacted areas (strong physical limitations) [31]. Thus, the high species richness of fallow lands, compared to forests and vineyards, could be explained by the fact that fallow lands represent an intermediate habitat between natural and highly impacted habitats. Fallow lands could thus attract individuals from these two communities (natural and highly developed areas) that would find favourable conditions to achieve their development. This idea supports the central position of fallow lands on the RLQ plot (Fig. 2). They appear as an important diversity reservoir in the landscape mosaic.
Vineyards appear as the most diverse habitat, although the abundance of butterflies is the lowest. They constitute a heterogeneous habitat gathering an important number of butterfly species despite a low abundance of individuals. These results lead us to conclude that vineyards are likely to play the role of crossing areas, this assumption being confirmed by the high evenness showing an absence of dominating species in vineyard samples.
Forests appear as the least diverse habitat with only 20 species. Although they accumulate fewer butterflies than fallow lands and gardens, the number of individuals is twice as high as in vineyards. Moreover, some species such as Satyrium esculi (Hübner) dominate the community, indicating a more specialized community than in other habitats. This is supported by the high discrimination of forests in RLQ analysis, and by the homogeneity of forest samples which shows a certain stability of this habitat compared to other land-uses.
Thus, the pattern of increased richness in fallow lands compared to vineyards and forests supports the idea of increased species richness at intermediate levels of anthropogenic pressure. Recent studies on butterfly communities also show this pattern of increased species richness at intermediate levels of an anthropogenic disturbance gradient [17,32]. But the high species richness of gardens is confusing: this man-made habitat supports a species richness equivalent to that of fallow lands. Actually, gardens gather as many species as fallow lands with 21% fewer individuals. Their samples also appear as more heterogeneous than those of fallow land which contributes to linking gardens to vineyards in terms of functioning. Gardens could thus constitute an intermediate habitat between fallow lands (“diversity reservoir”) and vineyards (“crossing area”) which is congruent with their position on the RLQ plot (Fig. 2). This idea of crossing area is consistent with Young's observations in a residential garden where the duration of butterfly visits was characteristically short, with a mean visit time of nine seconds [33]. Finally, it is worth noticing that exchanges between gardens and fallow lands seem to be particularly important highlighted by the high specific similarity between these two land-uses. Actually for Vickery, gardens of rural and suburban areas almost always have nearby habitats where butterflies can spend a large part of their life cycle only visiting gardens for nectar plants they contain [34].
4.2 Variations in functional composition
Butterfly assemblages of our study site show a consistent pattern in the distribution of functional attributes of species on the main gradients of landscape descriptors. Composition of the landscape is directly related to species traits that discriminate generalist species (polyphagous, multivoltine species that over-winter at adult or pupal stage) associated with urbanized sites from more specialist species (oligophagous, monovoltine species that over-winter as egg or larvae) associated with forested sites. Moreover, this landscape context interacts with land-use types to influence functional composition of the community. Based on these species traits, two communities can be discriminated.
In vineyards and gardens of the most urbanized sites, polyphagous, multivoltine and bivoltine species are over-represented. This particular trait assemblage corresponds to generalist characteristics attributed to disturbed habitats in many studies [13,35–37]: good dispersing abilities and weak host-plant specialization. This community also exhibits an over-representation of species over-wintering as adult or pupa. This strategy can represent an advantageous foraging behaviour at the favourable season. The latter species are able to move to find resources, whereas species over-wintering as egg or larvae depend on the presence of their host-plants where they over-winter. Moreover, gardens bring into the landscape new exploitable resources for butterflies, in particular ornamental and/or exotic plants, water sources which extend nectaring plant availability, particular configurations of vegetation such as hedgerows or flower beds [13,34]. These management practices, and especially watering practices, may explain multivoltine species’ over-representation in gardens in our study.
Inversely, in fallow lands and especially forests of the most forested sites, Mediterranean species and species with specialist traits, such as Pyronia cecilia (Vallantin) or Maniola jurtina (Linné), are over-represented.
This variation of species traits according to land-use types and landscape contexts is also congruent with Connell's reasoning who stated that too infrequent disturbance regime would favour good competitors, resulting in the dominance of few specialist species in the community, whereas too frequent or too severe disturbance would favour good dispersing and/or quickly maturing species [30].
The representation of the generalist characteristics increases with landscape context from forested sites to urbanized sites, and with land-use type from forests to gardens and vineyards with fallow lands in intermediate position. So the number of generalist butterflies in our results would appear to reflect the level of disturbance at both landscape scale and habitat scale [24,38–40]. This result is consistent with observations by Niell et al. along an anthropogenic disturbance gradient consisting in forest fragment decline with rural residential development [17]. They also suggest that an increase in the number of generalist butterflies is a more sensitive indicator of changes implied by anthropogenic disturbance than a decline in the number of specialists. Vulnerability to landscape degradation (fragmentation, disturbance and habitat loss) is known to be greater for specialist species than for generalist species [38–40] which may benefit from reduced competition with specialists [41]. That's why the very weak representation of the specialist species in our urbanized sites underlines a risk of functional homogenization associated with urbanization processes [40,42]. In a same way, Kuussaari et al. show that increasing species in Finland are more mobile, use a wider range of host-plants and live in more eutrophic habitats than declining species [39] which can be worrying as this overall trend in butterfly communities seems to reflect a more general phenomenon in Europe.
5 Conclusion
We succeeded in discriminating the four land-use types according to community structure (abundance, species richness, diversity and evenness). Our results also show that land-use type and landscape context interact to influence the functional composition of the community.
From the conservation point of view, our study reveals that quality of the environment for butterflies is strongly conditioned by landscape context. However, we also show that land-use type, i.e. habitat management, can partly balance this influence.
The situation of potential functional homogenization, associated with urbanization, is all the more worrying as fallow lands, which constitute the best diversity reservoir in the landscape mosaic, are declining. This habitat is exposed to a double threat in relation with current land-use changes [6]. It is threatened by house construction, but also by agricultural decline. If fallow lands are not maintained anymore by traditional agricultural or pastoral activities, this change may result in decreased biodiversity [7,43,44], whereas this type of habitat is recognized as one of the most species-rich habitats in Europe [9,6].
An increase in vineyards will also have a negative effect on butterfly diversity and will be likely to favour generalist species. As shown in our results, crops are known to be habitats of low biodiversity [45]. But the role of vineyards as crossing areas may be of considerable importance in terms of ecosystem functioning. For Shreeve, conditions which minimise resting and basking but facilitate flight increase the probability of individuals locating new habitats (independently of factors that determine whether they leave an area) [46]. From this point of view, vineyards could efficiently contribute to the interconnection of habitat patches or the colonization of new habitats.
The presence of forest fragments in the landscape seems to be a guarantee for the presence of Mediterranean and specialist butterfly species. Forests appear as a specialized habitat, although their community shares many species with other habitats, and especially with fallow lands. In fact, some species such as S. esculi can exploit both forests and other habitats. These species can disperse into other habitats because of their local proximity, but probably could not persist in a large area without forest fragments [47].
Finally, the role of gardens in this landscape mosaic has still to be elucidated. Considering their current expansion due to urbanization processes, understanding the role of gardens in the functioning of the landscape mosaic is of prime importance. In our study, gardens constitute, as fallow lands, a potential reservoir of diversity, whereas they are composed of only 12% native plant species [11]. Actually, gardens bring in landscape new exploitable resources for butterflies [15,34]. As a matter of fact, the strong diversity of garden community in our results could be linked to the internal heterogeneity of this habitat [11] and the network of small patches all maintained at different successional stages it constitutes. However, many authors doubt that they might constitute breeding habitats [34,48] and some studies show that gardens are likely to act as population sinks or selection filter [15,36,48,49], only favouring species able to exploit these new resources (e.g., exotic plants). As butterfly species richness of fallow lands can be linked to the spontaneous vegetation dynamic of this habitat and the nectaring and larval host-plant richness it induces [9], the question of the influence of management practices in gardens would be of prime importance. Could gardens constitute substitution reservoirs for species facing the regression of semi-natural habitats as fallow lands?
Conflict of interest statement
There is no conflict of interest.
Acknowledgements
We would like to thank the municipality of Lauris and the Luberon Regional Natural Park for their contribution to this work. We also acknowledge the help of Bernard Brun in reading the paper, André Chauliac for species identification and Audrey Marco for the land-use assessment. We are grateful to Mickael Paul for improving the English language and Nicolas Pech for improving the statistical background.
Appendix 1
Map of Lauris with land uses (A. Marco, pers. comm.) and the eight studied sites.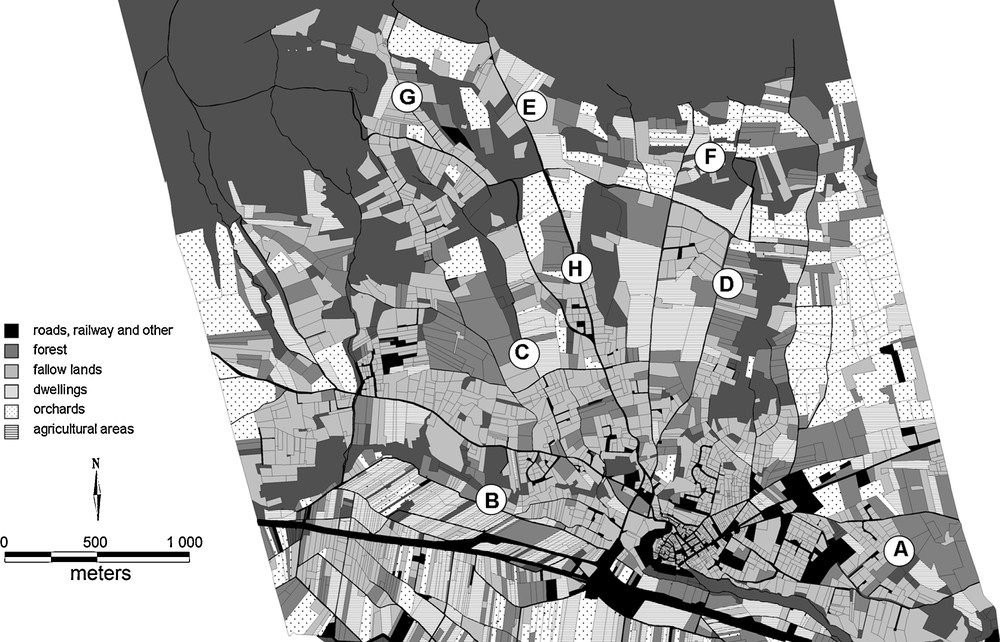
Appendix 2
Landscape descriptors for each sampled site.
| Percentage of ground occupied by: | ||||||
| Site | Dwellings | Forest | Orchards | Agricultural land | Fallow lands | Roads |
| A | 0.3324 | 0.0486 | 0.0738 | 0.1094 | 0.3277 | 0.0383 |
| B | 0.4551 | 0.1735 | 0.0083 | 0.1915 | 0.1178 | 0.0465 |
| C | 0.5568 | 0.0478 | 0.0352 | 0.1117 | 0.2012 | 0.0423 |
| D | 0.3072 | 0.1669 | 0.0832 | 0.2212 | 0.1977 | 0.0209 |
| E | 0.2417 | 0.4637 | 0.1070 | 0.1378 | 0.0766 | 0.0153 |
| F | 0.1807 | 0.2886 | 0.2202 | 0.1694 | 0.1161 | 0.0243 |
| G | 0.2910 | 0.4286 | 0.1674 | 0.0471 | 0.0437 | 0.0196 |
| H | 0.2334 | 0.2569 | 0.1194 | 0.1992 | 0.1596 | 0.0341 |
Appendix 3
Butterfly species recorded in the four land-use types.
| Species | Fallow lands | Gardens | Vineyards | Forests | Total |
| Maniola jurtina (Linnaeus, 1758) | 54 | 46 | 12 | 130 | 242 |
| Satyrium esculi (Hubner, 1804) | 65 | 63 | 16 | 33 | 177 |
| Polyommatus icarus (Rottemburg, 1775) | 47 | 59 | 4 | 6 | 116 |
| Pieris rapae (Linnaeus, 1758) | 33 | 40 | 28 | 10 | 111 |
| Aricia agestis (Denis & Schiffermuller, 1775) | 55 | 14 | 4 | 35 | 108 |
| Brintesia circe (Fabricius, 1775) | 38 | 22 | 6 | 12 | 78 |
| Pyronia cecilia (Vallantin, 1894) | 54 | 10 | 2 | 66 | |
| Melanargia galathea (Linnaeus, 1758) | 37 | 12 | 10 | 3 | 62 |
| Melitaea didyma (Esper, 1779) | 28 | 25 | 4 | 57 | |
| Coenonympha pamphilus (Linnaeus, 1758) | 18 | 36 | 2 | 56 | |
| Lycaena phlaeas (Linnaeus, 1761) | 19 | 9 | 3 | 13 | 44 |
| Pieris brassicae (Linnaeus, 1758) | 9 | 8 | 2 | 19 | 38 |
| Pontia daplidice (Linnaeus, 1758) | 13 | 5 | 5 | 23 | |
| Lasiommata megera (Linnaeus, 1767) | 3 | 8 | 9 | 1 | 21 |
| Polyommatus escheri (Hubner, 1823) | 10 | 2 | 3 | 6 | 21 |
| Limenitis reducta (Staudinger, 1901) | 4 | 6 | 8 | 1 | 19 |
| Carcharodus alceae (Esper, 1780) | 2 | 7 | 3 | 12 | |
| Pieris napi (Linnaeus, 1758) | 4 | 6 | 1 | 1 | 12 |
| Argynnis paphia (Linnaeus, 1758) | 2 | 1 | 6 | 9 | |
| Pieris mannii (Linnaeus, 1758) | 2 | 6 | 1 | 9 | |
| Iphiclides podalirius (Linnaeus, 1758) | 2 | 6 | 8 | ||
| Cacyreus marshalli (Butler, 1898) | 2 | 4 | 1 | 7 | |
| Gonepteryx cleopatra (Linnaeus, 1767) | 2 | 5 | 7 | ||
| Melitaea cinxia (Linnaeus, 1758) | 2 | 4 | 1 | 7 | |
| Polyommatus thersites (Hubner, 1834) | 2 | 1 | 4 | 7 | |
| Mellicta athalia (Rottemburg, 1775) | 4 | 1 | 1 | 6 | |
| Melitaea phoebe (Denis & Schiffermuller, 1775) | 2 | 1 | 2 | 5 | |
| Brenthis daphne (Denis & Schiffermuller, 1775) | 1 | 3 | 4 | ||
| Gonepteryx rhamni (Linnaeus, 1758) | 2 | 1 | 1 | 4 | |
| Thymelicus sylvestris (Poda, 1839) | 2 | 2 | 4 | ||
| Cacharodus boeticus (Rambur, 1839) | 2 | 1 | 3 | ||
| Hipparchia statilinus (Hufnagel, 1766) | 1 | 2 | 3 | ||
| Pyrgus cirsii (Rambur, 1839) | 3 | 3 | |||
| Satyrium spini (Denis & Schiffermuller, 1775) | 2 | 1 | 3 | ||
| Colias crocea (Geoffroy, 1785) | 1 | 1 | 2 | ||
| Hipparchia fagi (Scopoli, 1763) | 2 | 2 | |||
| Issoria lathonia (Linnaeus, 1758) | 1 | 1 | 2 | ||
| Argynnis pandora (Denis & Schiffermuller, 1775) | 1 | 1 | 2 | ||
| Papilio machaon (Linnaeus, 1758) | 1 | 1 | 2 | ||
| Spialia sertorius (Hoffmannsegg, 1804) | 2 | 2 | |||
| Celastrina argiolus (Linnaeus, 1758) | 1 | 1 | |||
| Clossiana dia (Linnaeus, 1767) | 1 | 1 | |||
| Coenonympha dorus (Esper, 1782) | 1 | 1 | |||
| Euchloe crameri (Butler, 1869) | 1 | 1 | |||
| Everes argiades (Pallas, 1771) | 1 | 1 | |||
| Leptotes pirithous (Linnaeus, 1767) | 1 | 1 | |||
| Lysandra bellargus (Rottemburg, 1775) | 1 | 1 | |||
| Lysandra corindon (Poda, 1761) | 1 | 1 | |||
| Melanargia occitanica (Esper, 1793) | 1 | 1 | |||
| Ochlodes venatus (Bremer & Grey, 1853) | 1 | 1 | |||
| Pyrgus onopordi (Rambur, 1839) | 1 | 1 | |||
| Pyronia bathseba (Fabricius, 1793) | 1 | 1 | |||
| Thymelicus acteon (Rottenburg, 1775) | 1 | 1 | |||
| Vanessa cardui (Linnaeus, 1758) | 1 | 1 | |||
| Abundance | 529 | 419 | 144 | 286 | 1378 |
| Species richness | 40 | 39 | 31 | 20 | 54 |
| Diversity: Shannon index | 4.13 | 4.14 | 4.23 | 2.83 | 4.21 |
| Evenness: Pielou index | 0.78 | 0.78 | 0.85 | 0.65 | 0.73 |


