1 Introduction
Horticultural flora, characterised by ornamental plants and vegetables, contributes strongly to the diversity of urban environments. Several types of green space (parks, squares, green spaces of public buildings, car parks ...) are characterised by ornamental flowering plant species. In particular, private gardens in residential areas collectively represent between them the greatest extent of vegetated land in cities [1,2]. However, while horticultural species constitute the dominant flora of urban environments, ecological information, particularly interspecific relations and, to a lesser extent, biological traits, remains poorly documented [3]. Above all, horticultural species are appreciated for the benefits that they provide to man, including a means of contact with nature and improving physical and mental well-being [4,5]. The fact that horticultural flora reflects human influences may explain why it is rarely examined from an ecological perspective [3]. However, studies, dealing with biodiversity in city parks or gardens during the last few years, have recognized the potential value of horticultural flora to biological diversity [3,6,7]. It plays a key part in urban ecological dynamics and particularly on the pools of species that temporarily use, colonise, or persist in urban areas. Indeed, horticultural species constitute vital primary needs for urban wildlife in providing important habitats [8,9] and resources, and also in modifying the microclimate [10].
However, the effects of horticultural flora on wildlife are not all positive. Most horticultural species that have been introduced in green spaces are exotics, and some can escape cultivation, establish, and through naturalization become part of the natural vegetation [11,12]. They may have a negative impact on our semi-natural and man-made ecosystems by becoming invasive. Consequently, horticultural flora constitutes the main source of alien plant introduction, currently regarded as one of the greatest threats to biological diversity [13–15]. In the Mediterranean region, 90% of the most notorious invaders are ornamental plants (e.g., Cortaderia selloana (Schult.), Acacia dealbata (Link), Buddleja davidii (Franchet), Ailanthus altissima (Miller), Robinia pseudoacacia (L.)...), which cause damage in a multitude of ways: environmental, economic, social, and sometimes health problems [16,17]. In addition to the risk of invasion, these new escapees may also reproduce with spontaneous species, leading to a loss of genetic variability in native flora [18–20]. The common practice of creating hybrids in the horticulture and plant breeding industries poses additional risks to native plants, since these artificial hybrids may form a bridge for gene transfer between two formerly intersterile species [18]. It becomes problematic for the Mediterranean region, which is considered among the 34 hotspots of biodiversity in the world thanks to its exceptional number of endemic plants [21,22].
Moreover, it becomes more and more urgent to take into account the horticultural flora in an ecological perspective, given the constant rise of gardening as a hobby and the increasing urbanization. Urbanization is spreading rapidly in many areas of the world, especially in Europe, where urbanization has been growing particularly fast on the western coasts of Spain, France and Portugal [23–26]. Overpopulation of cities in coastal areas has led to an urban spread into the hinterland with isolated residential houses; housing estates are being established in the countryside surrounding these cities [27]. The development of gardens near to natural areas becomes a possible danger for natural vegetation, which is exposed to reservoirs of potentially invasive plants and genetically transformed species. Rural areas close to the large cities may also undergo the ecological consequences of urbanization in the near future.
Given the risks to Mediterranean biodiversity posed by horticultural flora, it is important to quantify the occurrence and abundance of plant taxa and acquire a basic knowledge of life-history traits for assessing the risks to habitats outside cultivation. This is all the more necessary, since there are only a small number of papers that document ornamental species composition as a whole. The majority partially analyse ornamental flora through studying only ligneous species [28–30].
It is also important to understand distribution of horticultural flora in various urban areas in order to improve our knowledge about changes in biodiversity induced by the various ways that landscape is structured by human activity. Studies dealing with spatial floral patterns have brought to the fore specific species associations between front or rear gardens, but rarely between gardens as a whole [28,31]. The few papers that examine gardens as a whole sometimes produce different results: Blanckaert et al. [32], in a study of 30 homegardens (an adjunct to the house where selected trees, shrubs and herbs were grown for edible product and cash income [33]) in Mexico conclude that there were no clear specific groupings of homegardens based on the presence/absence of data about floral composition. Conversely, Acar et al. [30] showed associations between species and residential types in Turkey, whilst Daniels and Kirkpatrick [34] identified distinct floral characteristics in different garden types. In addition, spatial floral patterns of garden flora have often been carried out in city gardens [29,30,35–38], but rarely in periurban and urbanizing rural areas. Nevertheless, the urbanization process, social history and management practices, which are the most important drivers of urban floral patterns [39], are very different between rural and urban areas. They should consequently influence garden flora in terms of composition (ornamental and edible species), richness (in relation to garden size) and structure (ornamental and fruit trees). The strongest juxtaposition between gardens and fallow land in urbanizing rural areas could also reinforce the impacts of horticultural flora on natural flora. It could increase the risks of colonisation by species introduced in a garden toward a neighbouring habitat [40]. Moreover, urban and urbanizing rural areas will lead to an increase of housing densities in years to come due to the decrease of available land. With the exception of Smith et al. [38], few studies have explored spatial patterns between gardens of different housing density types, even though housing densities could play a key part in the determination of floral patterns and more generally on new ecological dynamics in urban environments. It is therefore essential to learn more about the composition of horticultural flora through different housing density types in order to give additional information to decision-makers to preserve biological diversity in future building policies.
The present paper analyses the floral composition and distribution of the overall horticultural flora of 120 Mediterranean gardens in three housing density types in an urbanizing rural area of southern France. This study was designed to assess the pools of cultivated alien and native species, the degree and nature of the differences between them through different urbanization pressures. Our results will be valuable: firstly, for understanding the nature of the domestic garden resource in cultivated species and, secondly, by providing a baseline to analyse horticultural plants that have escaped outside gardens in a region where the conservation of biodiversity is a topic of utmost importance in the regional policy.
2 Methods
2.1 Study site
The study was carried out in a rural area of southeastern France, in the Mediterranean Basin region (43°44′N, 5°18′E) (Fig. 1). The site of Lauris is located in the Natural Regional Park of Luberon, bordered to the north by the watershed of the Petit Luberon and to the south by the Durance River. The entire study area was 2181 ha in size and essentially made up of 53% of woodland, 25% of agricultural areas, 10% of fallow lands and 12% of urban areas; it is under the influence of the meso-Mediterranean bioclimate. It is in the zone of influence of two big cities, Aix-en-Provence and, to a lesser extent, Marseille, where urbanization has been spreading to the surrounding agricultural and natural countryside since 1975. In 30 years, the population of Lauris has doubled, rising from 1620 inhabitants in 1975 to 3143 inhabitants in 2005.

The study area showing the village of Lauris located in the Natural Regional Park of the Luberon in the French Mediterranean area.
Three different housing densities were defined by locally estimating the built-up site density (proportion of built surface per unit area) with Arc View GIS (r. 3.2 software) (Fig. 2). The three types corresponded to the different phases of the village urbanization and were related to housing and garden types [38]:

Map of the distribution of the three housing density types and the location of the 120 study gardens in Lauris (France). High housing density type (built-up area >20%) corresponds to the center of Lauris; Medium housing density type (built-up area 10–20%) corresponds to two residential areas close to the center; Low housing density type (built-up area <10%) corresponds to villas scattered in pine forest or encroaching on fallow land.
- – type 1 corresponds to a high housing density (built-up area >20%) and particularly to the old centre of Lauris. It is composed of small houses (two or more adjoining dwellings) built during the 12th and 13th centuries. They have front gardens of 20 m2 on average;
- – type 2 corresponds to a medium housing density (built-up area between 10 and 20%). It concerns two residential areas close to the village centre, built between 1965 and 1975. Each area is composed of detached or semi-detached estates, incorporating private gardens of 600 m2 on average;
- – type 3a corresponds to a low housing density, characterised by villas scattered in pine forest (built-up area <10%). These were built from 1975 to 1995 when, in an attempt to preserve agricultural activities, natural and semi-natural forests composed of Pinus halepensis (Mill.) were targeted by urbanization [41]. The garden size of these dwellings ranged from 2000 to 10,000 m2;
- – type 3b corresponds to a low housing density (built-up area <10%), characterised by modern villas encroaching on fallow land following the cessation of agricultural activity. The garden size of these dwellings ranged from 2000 to 10,000 m2.
The size of each housing density area is 9 ha for type 1, 34 ha for type 2 and 216 ha for type 3.
2.2 Garden selection
In order to provide a homogeneous distribution of gardens, 30 houses from five main streets within each housing density type were chosen for survey. Each street was then exhaustively visited so that the entire length and both sides of each street were examined and each house visited. After requesting permission to undertake the survey on the resident's property, we sampled immediately the garden. In order to reduce refusal or absence of homeowners, a publicity campaign was carried out using local papers and the local council, prior to visits. Given that socio-economics status is an important driver of floral patterns in urban areas [39,42], each area was systematically visited between April and July 2005 during the week and the weekend, in order to sample the gardens of people working and of people who have a second home. Moreover, less than 10% of homeowners refused the floral sampling of their garden, so that the study did not concern an only social class. The combined area of all the sampled gardens was 215,000 m2.
2.3 Vegetation sampling
In each garden, native and alien cultivated plants (excluding the constituent species – grasses – in lawns) were recorded during an exhaustive survey of the garden. The inventory was drawn up distinguishing 13 landcovers of the garden: lawn, gravelled path, flower bed, pot and tub, hedge, wall, borders of swimming pool, playing field, pine forest, oak grove, orchard, vegetable garden, and olive grove. Taxonomic identification was carried out on the basis of the Universal Encyclopaedia of 15,000 garden plants and flowers [43]. Plants that could not be identified were labelled as ‘unknown’ or ‘Genus sp.’ when the genus was identifiable or ‘Genus group’ when the group of cultivar was identifiable. The intergeneric hybrids were recorded as ‘x Genus species’, interspecific hybrids ‘Genus x species’. Some horticultural plants involve species that are difficult to distinguish from varieties, such as taxa of Arum, Aubrieta, Chrysanthemum, Dahlia, Dianthus, Iris, Gazania, Gladiolus, Heuchera, Lilium, Mandevilla, Narcissus, Ostheospermum, Paeonia, Petunia, Pyracantha, Tulipa, Rhododendron and Rosa; these were identified only to the genus level.
2.4 Data analysis
We specified the most frequently recorded families, genus and species by giving frequency values ( frequency). The abundance of each species was also given, except those of Iris sp. and Hypericum calcynum, which have high rates of vegetative reproduction. A frequency distribution of species abundance was presented. The proportion of growth forms (annual, biennial, perennial) and Raunkiaer's form was given [44]. Each species was also assigned to one of three status defined by Aboucaya (1998) [45]: “known to be invasive”, “potential invasive”, “on waiting list” in France, in order to assess the number of invasive species that are introduced to gardens. To detect any significant difference in the proportion of invasive species among housing density types, χ-square tests were performed.
A species-accumulation curve was plotted to assess completeness of sampling effort at the combined area and at each housing density type. The cumulative number of species is plotted against some measure of the effort expended to find them. Species accumulation curves based on ‘proxy’ units such as area of quadrats, trap-hours or hours of observation represent a uniform process; this is the reason why the sampling effort must be standardized [46]. The order in which samples are added to the species accumulation curve affects the shape of the curve produced; the use of randomization procedures is useful to overcome this problem [47]. Contrary to Thompson et al. [36] and Loram et al. [48], who used a standardized measure of sampling effort in each garden (quadrats), we used in our study a non-uniform sampling process. As we wanted to sample exhaustively native and alien cultivated species of all garden landcovers on the total area of each garden, our sampling unit was the total garden area, which ranged from 2 to 11,000 m2. The use of a randomization procedure in this case leads to an overestimation of species richness due to the heterogeneity of sampling units. Therefore, we could not use the traditional method of plotting a species accumulation curve. In order to plot a species accumulation curve that corresponds to a plot of the cumulative number of species discovered, within a defined area, as a function of some measure of the increasing effort expended to find them, we had to classify in our case the studied gardens in increasing order of size, interpreting an increase in sampling effort, and for each cumulative garden area, we added the number of new species. The 30 gardens of each housing density area were used to make up each cumulative area. This methodology, used to plot the species accumulation curve, does not permit to determine the minimum sampling effort required for estimating the species richness in urban areas, but it is useful to assess the completeness of the sampling effort in our particular case.
A principal correspondence analysis (PCA) was also performed in order to identify the most widely represented species in gardens of the different housing density types. This analysis was conducted with a species frequency matrix (62 rows corresponding to taxa and four columns corresponding to urban areas) and only with taxa whose frequency was greater than 20% (see Appendix A). We clarified then in which landcovers the most frequent species were present. A simple correspondence analysis (CA) was also performed with the same species frequency matrix in order to identify whether or not specific groups fit with housing density types. A Monte Carlo permutation test based on 1000 random permutations was used to test the null hypothesis, i.e. species were unrelated to the housing density type.
In order to assess the ratio of regional (Mediterranean Basin) and exotic species, information on the origin of the species was taken from Brickell and Mioulane (2004) and classified according to 12 origins [43]: Europe, America, Asia, Africa, Oceania, Mediterranean Basin, Tropical, Eurasia, Eurafrica, Northern Hemisphere, mixed (species coming from more than three different continents) and horticultural (species resulting from artificial hybrid selection). To detect any significant differences in the proportion of species origins among housing density, analysis of variance (ANOVA) tests were performed.
All statistical analyses were run with Minitab (r.14 software).
3 Results
3.1 Diversity of horticultural flora
Nine hundred and seventy-three horticultural plants were collected in the combined area of all gardens (215,000 m2). Out of the 114 plant families recorded, those with the highest number of taxa were Asteraceae (7.2%), Rosaceae (6.6%), Liliaceae (4.8%), Crassulaceae (4.6%), Lamiaceae (4.2%) and Caprifoliaceae (3.4%). All other plant families had a frequency of less than 3.4%. Taxa were also divided into 376 genera, 80% of which were represented by only one species. Ninety-one percent (519 species) were much less frequent than 20% (Appendix A); 82% of species were recorded fewer than 50 times (Fig. 3). The most abundant species were x Cupressocyparis leylandii (1548), Cupressus arizonica (1243), Pyracantha sp. (1104), Prunus laurocerasus (1011), which were all planted in hedges, and Rosa sp. (1034).
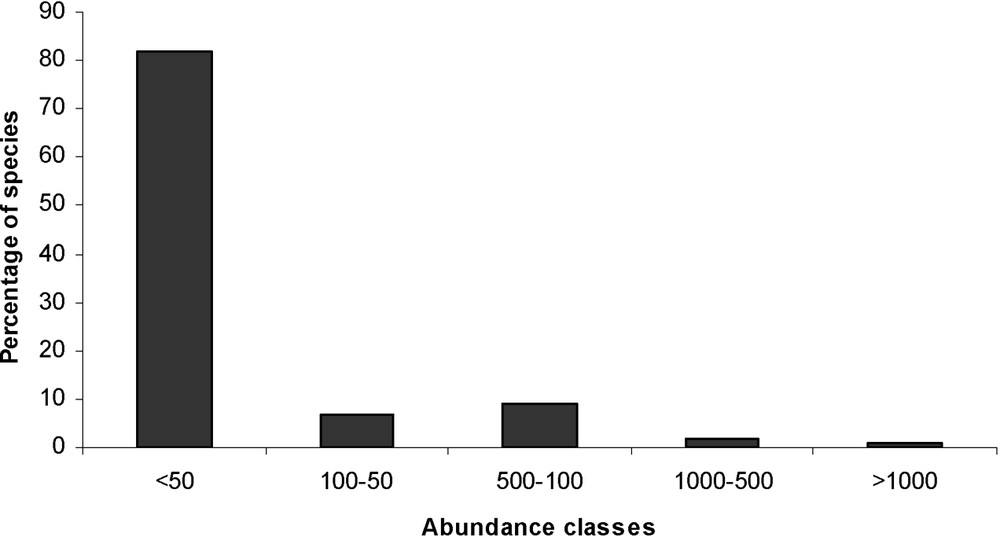
Frequency distribution of abundance classes of horticultural species in 120 gardens, sampled 2005 in the urbanizing rural area of Lauris, southern France.
With regards to the growth pattern of species, 92% were perennials, 7% annuals and 1% biannuals. An overwhelming number of taxa were trees and shrubs, divided as follows: phanerophytes 55.1%, chamaephytes 21.4%, geophytes 12.4%, hemicryptophytes 6.3% and therophytes 4.8%.
3.2 Species richness in different housing densities
The species accumulation curve for all the studied gardens (120 gardens) had a logistical shape that tends toward an asymptote (Fig. 4a) and suggests that our sampling effort provided a good representation of horticultural flora. Curves vary with housing density types with that for species of high housing density, rapidly reaching an asymptote after 600 m2 (Fig. 4b), with 217 plants collected. This accumulation curve was also steeper near the origin, reflecting the high local richness of cultivated species communities. A larger sample area would thus provide very little additional information. Rather, greater numbers of plants were recorded in gardens of medium- and low-density housing types because of the larger cumulative garden areas. A total of 272 horticultural plants were collected on 19,000 m2 in the gardens of medium housing density (Fig. 4c), while in gardens of low housing density, 412 and 310 species were respectively collected on 79,000 m2 in an agricultural environment and 116,000 m2 in a forest environment (Fig. 4d).
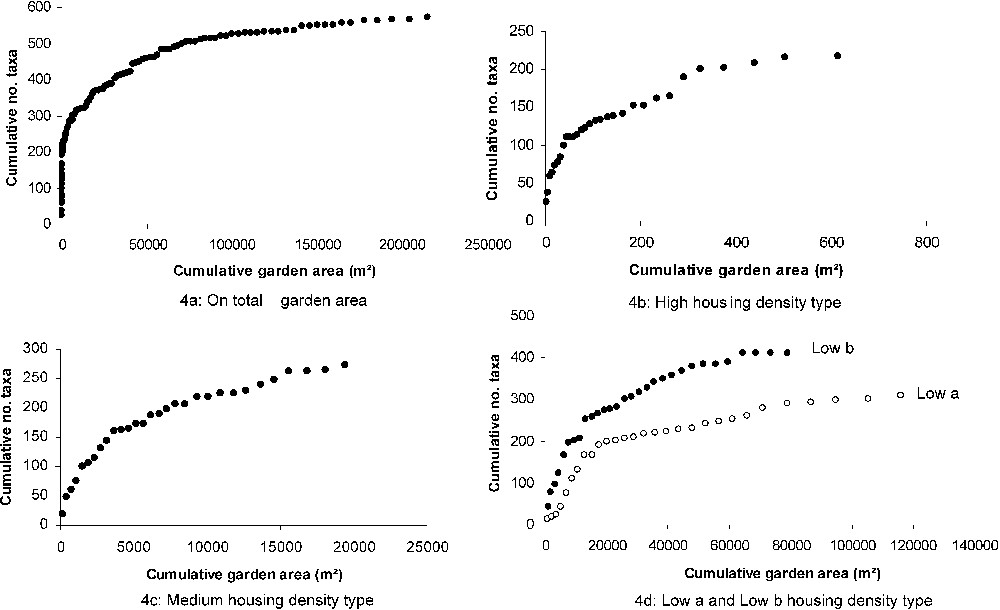
Horticultural plant taxa accumulation curve on cumulative garden area in different housing density types, sampled in 2005 in the urbanizing rural area of Lauris, southern France.
3.3 Common species in different housing densities
The PCA performed on all species gave no relevant result, because in total area, there were numerous species with a frequency lower than 20%. Fig. 5 shows results of PCA for types of housing densities with taxa whose frequency was greater than 20%. The results were presented only for the first two axes, which represent 86.1% of the variance (Fig. 5a and b). The first axis of PCA accounted for 63.10% of the variance, while the second axis accounted for an additional 23%. The first axis clearly sorted taxa by frequency, whilst the second separated gardens by housing density type. The most frequent species in gardens of type 1 were Rosa sp., Pelargonium ‘zonal group’, Petunia sp. The gardens of types 2 and 3a showed similarities in the most frequent species and were characterised by Syringa vulgaris, Rosmarinus officinalis, Olea europea, Cupressus sempervirens, Iris sp. and Forsythia x intermedia. In the gardens of type 3b, Pyracantha sp., Viburnum tinus, x Cupressocyparis leylandii, Althaea sp., Prunus cerastifera, Laurus nobilis, and Ficus carica were the most frequent species. Two species, Nerium oleander and Lavandula angustifolia, were common in all gardens, and consequently did not belong to a specific housing density type.
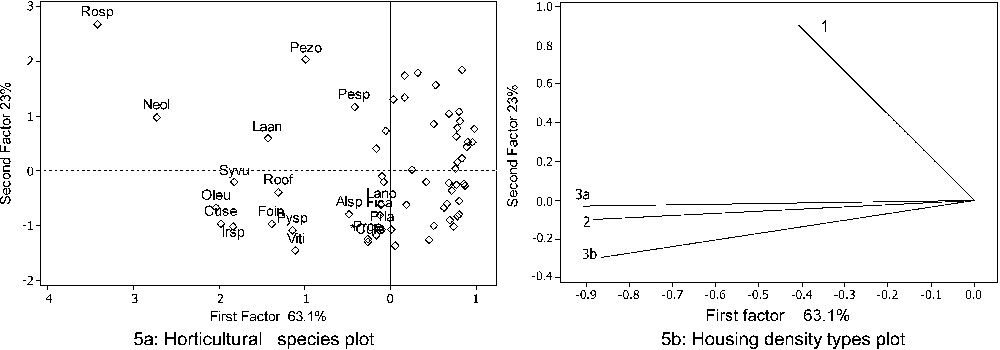
PCA ordination of the species on the different housing density types: 1: high housing density type; 2: medium housing density type; 3a: low a housing density type; 3b: low b housing density type. Alsp: Althaea sp.; Cuse: Cupresssus sempervirens; x Cule: x Cupressocyparis leylandii; Fica: Ficus carica; Foin: Forsythia x intermedia; Laan: Lavandula angustifolia; Lano: Laurus nobilis; Neol: Nerium oleander; Oleu: Olea europea; Pesp: Petunia sp.; Pezo: Pelargonium ‘zonale’; Prce: Prunus cerasifera; Prla: Prunus laurocerasus; Pysp: Pyracnatha sp.; Roof: Rosmarinus officinalis; Rosp: Rosa sp.; Syvu: Syringa vulgaris; Irsp: Iris sp.; Viti: Viburnum tinus.
Considering all gardens, Rosa sp. was the most frequent species in ‘flower bed’ and ‘wall’ landcovers, with respectively 66% and 38% frequency, while the Pelargonium ‘zonal group’ was the most frequent species in ‘pot and tub’ landcover (31%). The most commonly and frequently found in ‘flower bed’ landcover were Iris sp. (53%), Lavandula angustifolia (49%) and Nerium oleander (43%), in ‘lawn’, Olea europea (64%), Syringa vulgaris (45%) and Prunus cerasifera (41%), and in ‘hedge’ Pyracantha sp. (48%) and x Cupressocyparis leylandii (41%).
3.4 Specific species in different housing densities
Species were related to the housing density type (Monte Carlo test, , , ). Results of CA on housing density types are presented only for the first two axes, which represent 86.75% of the total inertia, respectively 61.20% and 25.55% (Fig. 6). Begonia ‘semperflorens group’, Dianthus sp., Lonicera japonica, Delosperma cooperi, and Pelargonium ‘zonal group’, which contributed to the first axis, were related to gardens of type 1. Prunus dulcis, Vitis sp. and Prunus armeniaca were the main contributors to the second axis and characterised gardens of type 3, while Morus kagayamae, which also contributed to the second axis, was the typical garden taxa of type 2.

CA ordination of the species on different housing density types. 1: High housing density type; 2: Medium housing density type; 3a: Low a housing density type; 3b: Low b housing density type. Bese: Begonia ‘semperflorens’; Disp: Dianthus sp.; Loja: Lonicera japonica; Deco: Delosperma cooperi; Pezo: Pelargonium ‘zonale’; Prdu: Prunus dulcis; Prar: Prunus armeniaca; Moka: Morus kagayamae; Visp: Vitis sp.
3.5 Native and alien cultivated species
Twelve percent of the horticultural taxa originated from the Mediterranean Basin. The alien cultivated taxa (88%) came mainly from Asia (23%), America (19%), Europe (11%), and Africa (7%). Among American and African taxa, 12% originated from the southwest of North America and South Africa.
Fig. 7 shows the spectrum of origin of the species that occur in gardens in each housing density type. Gardens of type 1 showed a significantly different proportion (ANOVA, ; ) of African taxa such as Pelargonium ‘zonal group’, Pelargonium ‘ivy group’ and Delosperma cooperi as mixed taxa such as Dianthus sp. and Hydrangea macrophylla. The highest percentage of taxa from the Mediterranean Basin were recorded for gardens of types 2 and 3a, with respectively on average 25% and 21% (ANOVA, , ). The most strongly represented Mediterranean taxa were Cupressus sempervirens, Lavandula angustifolia, Nerium oleander, Olea europea, Rosmarinus officinalis, Viburnum tinus.

Origins of horticultural taxa in gardens on different housing density types (with standard error). Med.Bas: Mediterranean Basin, Am.: America, North. Hemisph: Northern Hemisphere, Afr.: Africa, Unkn.: Unknown. * significative differences (ANOVA test, p<0.05).
Twenty-one out of the 573 taxa were registered on the three lists of invasive species established by Aboucaya [45] (Table 1), seven being notoriously invasive in the Mediterranean area. Their frequencies in gardens were highly variable, ranging from 1 to 48% of 120 gardens. The proportion of invasive taxa were not significantly different between housing density types ( test, , , ). The abundance of notorious invaders in gardens was low. The most abundant species were hedges species such as Pyracantha sp., Pittosporum tobira, and Elaeagnus x ebbingei.
Lists of “called” invasive (owing three status defined by Aboucaya (1998) [45]) horticultural taxa with their frequency values (F) and abundancy (A) in 120 gardens of Lauris
| List 1: Known invasive species | F | A |
| Pittosporum tobira | 23% | 134 |
| Buddleja davidii ⁎ | 12% | 22 |
| Robinia pseudo-acacia ⁎ | 12% | 32 |
| Cortaderia selloana ⁎ | 10% | 17 |
| Acer negundo | 7% | 10 |
| Acacia dealbata ⁎ | 3% | 4 |
| Ailanthus altissima ⁎ | 3% | 4 |
| Carpobrotus acinaciformis ⁎ | 2% | 2 |
| List 2: Potential invasive species | F | A |
| Pyracantha sp.1 | 48% | 1104 |
| Lonicera japonica | 28% | 60 |
| Cedrus atlantica | 21% | 98 |
| Opuntia sp.⁎ | 8% | 13 |
| Aptenia cordifolia | 5% | 20 |
| Yucca filamentosa | 1% | 2 |
| List 3: Species on waiting list | F | A |
| Elaeagnus x ebbengei | 22% | 468 |
| Agave americana | 13% | 42 |
| Mirabilis jalapa | 9% | 51 |
| Broussonetia papyrifera | 8% | 15 |
| Pinus nigra | 8% | 23 |
| Polygala myrtifolia | 3% | 8 |
| Elaeagnus angustifolia | 2% | 11 |
1 Pyracantha sp. groups together with P. coccinea (potential invasive species) and P. rogersiana and several hybrids.
⁎ Notorious species invaders in the Mediterranean region [16].
4 Discussion
4.1 Diversity and floristic patterns of horticultural species
Our study confirms that private gardens contain a large cultivated biodiversity, strongly dominated by perennial and woody alien species. With 573 taxa recorded from a combined area of 215,000 m2, private gardens present a high level of horticultural floral richness in urbanizing rural areas. In comparison, the level of introduced plant richness in gardens of rural areas is nevertheless lower than in city gardens. A study of 400 domestic gardens in Mexico City revealed that 525 out of 750 species recorded were aliens in a total garden area of 55,000 m2 [49]. Similarly, Smith et al. [3] inventoried 798 alien species on 12,700 m2 of gardens in Sheffield. This difference of level of introduced plant richness between rural and urban areas can be explained by the differences in garden size, rural gardens being much bigger than those of cities and, therefore, the species density of cultivated plants lower. Moreover, the concentration of houses in cities also induces a stronger need to be in contact with nature, unlike in rural environment close to natural areas. In the artificial environment of urban areas, introductions of horticultural plants are more important for the physical and mental well-being of the human population that lives in urban areas [4,5]. If we consider the rapid development in housing density in the years to come, especially from low to medium housing density types, the flora of rural areas will undergo the same evolution as that seen in cities, particularly the increase in alien species.
Our study also shows that the accumulation of new cultivated species is variable under different urbanization pressures, especially in high housing density type, where it is greater at the local scale. These differences may be related to the garden's structure in each housing density types [36], and more particularly to the diversity of garden landcovers.
Although the horticultural flora of rural gardens is rich, it is also very heterogeneous, like in urban gardens. In the 120 gardens of Lauris, most of the taxa have low frequency values, apart from a few very popular species. The study of the species abundance showed also that the number of introduced individuals was low, except for species commonly planted in hedges. The majority of the species occurred as solitary specimens; therefore, the opportunities for the large majority of horticultural plants to colonise outside gardens may also be relatively few. These differences in frequency values have already been evaluated by studies dealing with ornamental woody species in urban gardens. Acar et al. [29] mentioned that in different residential gardens of Trabzon city in Turkey, out of 274 woody species, 231 were much less frequent than 15%. Similarly, in studying patterns of trees in a residential neighbourhood of a low-density housing estate in Hong Kong, Jim [28] highlights two groups of tree species, one composed of a few common species and a second of many uncommon species. In a recent study of Sheffield garden flora, Smith et al. [3] show that a large proportion of alien species were recorded only once, as well. The similarities between the spatial floral patterns of ornamental trees in Turkey and Hong Kong, of the Sheffield garden flora and of the ornamental flora in Lauris may reflect the operation of two opposite social attitudes, namely conformity versus individualism [28]. On the one hand, conformity, generally defined as the tendency to act or think like other members of a group, could be expressed by spatial similarities through the most popular species. On the other hand, an individualism phenomenon could be expressed by the heterogeneity of horticultural flora.
Our study shows that similarities in species composition can be observed between gardens of a same housing density type. Zmyslony and Gagnon [31] found the same result with the floral composition of front yards in a residential street of Hochelaga–Maisonneuve District, Montreal. They suggested that it was related to a conformity phenomenon through mimicry activities at a local scale. Residents in the street section are influenced by the shape, colour and location of the vegetation they see in gardens of their nearest neighbours. Our study also shows that the composition of popular species observed within a housing density area was not the same as the species composition of another housing density area. This result could suggest that the conformity phenomenon is not expressed in the same way through different housing density types.
4.2 Influence of regional factors
The results concerning the floral composition of Mediterranean private gardens show us that the influence of regional factors on plant introductions must be taken into account. Firstly, taxa such as Prunus cerasifera, Prunus armeniaca and Vitis sp. are remainders in the gardens of past agricultural activities (cherry orchards, olive groves, vineyards). The history of land use appears, consequently, determining the distribution and composition of horticultural flora, as in the Mediterranean flora [50]. Secondly, private gardens were characterised by a strong proportion of species specifically adapted to the Mediterranean climate. A quarter of the horticultural taxa cultivated in Mediterranean gardens (24%) were either from the Mediterranean Basin or from climatic regions under Mediterranean influence. This trend has also been observed in other Mediterranean gardens, like in Trabzon city in Turkey, where 25% of taxa originated from Turkey and Mediterranean regions. In northern temperate gardens throughout the world, Mediterranean taxa are sometimes present, but by no means common. These results show that the choice of homeowners could be influenced by regional climatic conditions, but also by the regional market availability of Mediterranean taxa. In order to assess the relative importance of social and economic factors on gardening practices, a socio-economic study should be conducted.
4.3 Risks for natural flora
Rural gardens also constitute sources of many notorious invasive species. More than half of the notorious invasive species in the Mediterranean region are cultivated in rural gardens. Also, the probability of colonisation from a garden to a neighbouring habitat might be stronger in urbanizing rural areas than in cities if we take the highest proportion of interfaces between gardens and uncultivated habitats in urbanizing rural areas into account. These houses create more urban/wildland interfaces. The French Mediterranean countryside could be threatened by biological invasions. It would be interesting to monitor areas adjoining gardens in order to identify which species had escaped from gardens and in which part of the landscape mosaic they grow. Field observations suggest that recent fallow, very frequent in rural areas, may be favourable sites for invasive species to seep into the landscape.
The seasonally xeric nature of the Mediterranean climate is often a barrier to the establishment of introduced species. Our study showed that 12% of horticultural taxa cultivated in Mediterranean gardens come from climatic regions under the Mediterranean influence like the southwest of North America and of South Africa. The proportion of water-stress-tolerant species (South-African taxa, often planted in pots and tubs) is higher in gardens of high housing density, which are exposed to a more xeric environment than the surrounding area [51]. Thus garden owners, planting species adapted to the Mediterranean climate, reinforce the risk of alien species invading the Mediterranean countryside [52].
Moreover, 12% of cultivated taxa are native. More than half of them are among the most common horticultural taxa: Cupressus sempervirens, Ficus carica, Lavandula angustifolia, Laurus nobilis, Nerium oleander, Olea europea, Rosmarinus officinalis and Viburnum tinus. They can potentially contribute to overall genetic diversity and maintain gene flow between remnant populations, thus buffering small and otherwise isolated populations from extinction. However they can also threaten the genetic integrity of natural populations: gene flow from such plantings can limit local adaptation and lead to population decline and local extinction if foreign genes disrupt locally adapted gene complexes [20].
5 Conclusion
This study shows that rural gardens exhibit wide horticultural diversity, firstly characterised by a high level of richness and a strong heterogeneity, probably linked to the individualism of western societies. However, common floral groups have been found in gardens of a same housing density type, a small number of popular species being found in all gardens types. This could be linked to a conformity phenomenon that seems to be different for varying housing density types. The study of cultivated species accumulation rate makes us conclude that the sampling effort must be variable for different housing density types and can be related to the diversity of garden structures. A detailed analysis of the relationship between garden attributes and floral patterns willprovide information on the importance of this factor in the diversity of garden flora. The study of species composition suggests an influence of regional factors on plant choice (history of land use, climate ...). Gardening practices should be investigated by a social inquiry in order to understand the factors that influence plant introductions. The species composition shows also a strong proportion of well-adapted species and the presence of many notorious Mediterranean invasive species in Mediterranean rural gardens. This increases the risk of ornamental species establishing in surrounding fallow, very frequent in rural areas due to agricultural decline. It is now necessary to learn more about the interspecific relations among horticultural flora in order to understand how gardens interact with the wider environment and, consequently, play a role in the ecological dynamics of the rural landscape mosaic.
The results presented here show that if the urbanization of rural areas continues at the rate of the last few years, floristic diversity in Mediterranean rural areas will undergo the same evolution as that seen in cities (i.e. an increase in the proportions of alien and invasive species). Our study shows that the floral composition, richness and distribution of introduced species are variable under different urbanization pressures. Consequently, these results should be taken into account in urban policies in order to control the pool of introduced species and consequently preserve biological diversity in urban areas.
Acknowledgements
We are grateful to the municipality of Lauris for their contribution to this work and to the garden owners for access to their estate, to Mr. P. Le Fauconnier, from the urban department of the Natural Regional Park of Luberon, for providing urban data, to N. Pech for statistical analysis, to Mr. J.-C. Mathieu from Jean Rey's tree nursery for species identification, and to Miss Matthey and Mr. Paul for improving the English language. This work was supported by the Association for the Development of Teaching and Research in the Provence–Alpes–Côte d'Azur region.
Appendix A Frequency values of the species recorded in different housing density types of Lauris village (only those above 20% were given)
| Plant species | Family | Relative frequency value in each housing density type | ||||
| High | Medium | Low a | Low b | Total frequency | ||
| Rosa sp. | Rosaceae | 70.00 | 100.00 | 83.33 | 93.33 | 86.67 |
| Nerium oleander | Apocynaceae | 43.33 | 83.33 | 83.33 | 86.67 | 74.17 |
| Olea europea | Oleaceae | 20.00 | 66.67 | 70.00 | 93.33 | 62.50 |
| Cupressus sempervirens | Cupressaceae | 13.33 | 70.00 | 80.00 | 80.00 | 60.83 |
| Syringa vulgaris | Oleaceae | 23.33 | 76.67 | 63.33 | 76.67 | 60.00 |
| Iris sp. | Iridaceae | 10.00 | 73.33 | 83.33 | 66.67 | 58.33 |
| Lavandula angustifolia | Lamiaceae | 30.00 | 50.00 | 73.33 | 63.33 | 54.17 |
| Forsythia x intermedia | Oleaceae | 10.00 | 70.00 | 63.33 | 66.67 | 52.50 |
| Rosmarinus officinalis | Lamiaceae | 16.67 | 60.00 | 66.67 | 63.33 | 51.67 |
| Pelargonium ‘zonale’ | Geraniaceae | 46.67 | 33.33 | 63.33 | 53.33 | 49.17 |
| Pyracantha sp. | Rosaceae | 6.67 | 56.67 | 66.67 | 63.33 | 48.33 |
| Viburnum tinus | Caprifoliaceae | 3.33 | 70.00 | 50.00 | 70.00 | 48.33 |
| Althaea sp. | Malvaceae | 10.00 | 46.67 | 36.67 | 66.67 | 40.00 |
| Petunia sp. | Solanaceae | 33.33 | 20.00 | 53.33 | 53.33 | 40.00 |
| x Cupressocyparis leylandii | Cupressaceae | 3.33 | 46.67 | 26.67 | 70.00 | 36.67 |
| Hedera helix | Araliaceae | 20.00 | 43.33 | 43.33 | 36.67 | 35.83 |
| Morus kagayamae | Moraceae | 3.33 | 66.67 | 16.67 | 56.67 | 35.83 |
| Prunus cerasifera | Rosaceae | 3.33 | 30.00 | 40.00 | 70.00 | 35.83 |
| Mentha viridis | Lamiaceae | 26.67 | 30.00 | 33.33 | 50.00 | 35.00 |
| Buxus sempervirens | Buxaceae | 13.33 | 40.00 | 36.67 | 46.67 | 34.17 |
| Euonymus fortunei | Celastraceae | 13.33 | 36.67 | 46.67 | 40.00 | 34.17 |
| Ficus carica | Moraceae | 6.67 | 33.33 | 40.00 | 56.67 | 34.17 |
| Laurus nobilis | Lauraceae | 6.67 | 50.00 | 40.00 | 40.00 | 34.17 |
| Campsis grandiflora | Bignoniaceae | 30.00 | 23.33 | 50.00 | 30.00 | 33.33 |
| Prunus laurocerasus | Rosaceae | 0.00 | 53.33 | 43.33 | 36.67 | 33.33 |
| Delosperma cooperi | Aizoaceae | 36.67 | 26.67 | 33.33 | 33.33 | 32.50 |
| Hydrangea macrophylla | Hydrangeaceae | 33.33 | 26.67 | 26.67 | 43.33 | 32.50 |
| Cupressus arizonica | Cupressaceae | 0.00 | 43.33 | 43.33 | 40.00 | 31.67 |
| Prunus armeniaca | Rosaceae | 0.00 | 16.67 | 40.00 | 66.67 | 30.83 |
| Dianthus sp. | Caryophyllaceae | 36.67 | 13.33 | 36.67 | 33.33 | 30.00 |
| Thymus vulgaris | Lamiaceae | 6.67 | 40.00 | 30.00 | 43.33 | 30.00 |
| Sempervirum tectorum | Crassulaceae | 13.33 | 26.67 | 40.00 | 36.67 | 29.17 |
| Lonicera japonica | Caprifoliaceae | 33.33 | 26.67 | 20.00 | 30.00 | 27.50 |
| Salvia officinalis | Lamiaceae | 10.00 | 26.67 | 33.33 | 36.67 | 26.67 |
| Verbena x hybrida | Verbenaceae | 23.33 | 26.67 | 26.67 | 30.00 | 26.67 |
| Prunus dulcis | Rosaceae | 0.00 | 0.00 | 33.33 | 66.67 | 25.00 |
| Gazania sp. | Asteraceae | 23.33 | 30.00 | 26.67 | 16.67 | 24.17 |
| Punica granatum | Punicaceae | 0.00 | 10.00 | 40.00 | 46.67 | 24.17 |
| Convallaria majalis | Liliaceae | 10.00 | 23.33 | 20.00 | 40.00 | 23.33 |
| Juniperus x media | Cupressaceae | 3.33 | 26.67 | 26.67 | 36.67 | 23.33 |
| Antirrhinum majus | Scrophulariaceae | 23.33 | 20.00 | 16.67 | 30.00 | 22.50 |
| Begonia semperflorens | Begoniaceae | 33.33 | 13.33 | 26.67 | 16.67 | 22.50 |
| Erysimum cheiri | Brassicaceae | 23.33 | 26.67 | 23.33 | 16.67 | 22.50 |
| Oxalis articulata | Oxalidaceae | 20.00 | 26.67 | 23.33 | 20.00 | 22.50 |
| Pelargonium ‘lierre’ | Geraniaceae | 20.00 | 13.33 | 23.33 | 33.33 | 22.50 |
| Pittosporum tobira | Pittosporaceae | 3.33 | 20.00 | 33.33 | 33.33 | 22.50 |
| Abelia x grandiflora | Caprifoliaceae | 10.00 | 20.00 | 33.33 | 23.33 | 21.67 |
| Elaeagnus x ebbingei | Elaeagnaceae | 0.00 | 13.33 | 33.33 | 40.00 | 21.67 |
| Lagerstroemia indica | Lythraceae | 0.00 | 20.00 | 20.00 | 46.67 | 21.67 |
| Philadelphus coronarius | Hydrangeaceae | 6.67 | 6.67 | 36.67 | 36.67 | 21.67 |
| Rhododendron sp. | Ericaceae | 13.33 | 13.33 | 26.67 | 33.33 | 21.67 |
| Viola x wittrockiana | Violaceae | 13.33 | 36.67 | 13.33 | 23.33 | 21.67 |
| Vitis sp. | Vitaceae | 10.00 | 6.67 | 23.33 | 46.67 | 21.67 |
| Cedrus atlantica | Pinaceae | 0.00 | 23.33 | 26.67 | 33.33 | 20.83 |
| Chrysanthemum sp. | Asteraceae | 13.33 | 20.00 | 33.33 | 13.33 | 20.00 |
| Cistus albidus | Cistaceae | 6.67 | 16.67 | 26.67 | 30.00 | 20.00 |
| Coronilla emerus | Papilionaceae | 6.67 | 16.67 | 30.00 | 26.67 | 20.00 |
| Hypericum calycinum | Clusiaceae | 0.00 | 13.33 | 33.33 | 33.33 | 20.00 |
| Impatiens walleriana | Balsaminaceae | 16.67 | 6.67 | 30.00 | 26.67 | 20.00 |
| Parthenocissus quinquefolia | Vitaceae | 20.00 | 23.33 | 13.33 | 23.33 | 20.00 |
| Primula sp. | Primulaceae | 16.67 | 23.33 | 16.67 | 23.33 | 20.00 |
| Santolina chamaecyparissus | Asteraceae | 0.00 | 26.67 | 40.00 | 13.33 | 20.00 |


