1 Introduction
Important features linked to the establishment and success of alien plants are often based on the propagule pressure, multiple introduction events and subsequent admixture, and residence time [1–5]. Another major threat arises from the frequent introductions of several congeneric species within the same region, which is the case for 61% of plant invaders in France (e.g., Senecio spp., Oxalis spp., Acacia spp. [6]). Hybridizations between non-native species, in addition to those between native and non-native species, may result in new alien-derived genotypes. This pattern of invasion-by-hybridization has been frequently recorded and discussed [7–15]. Such events may represent “evolution in action”, and constitute valuable case studies [16,17]. Inheritable trait evolution observed on contemporary timescales may play an important role in the success of certain alien plants and may explain their rapid spread [10,14,18,19]. Because new alien-derived genotypes or taxa are frequently competitive [9,20,21] or stress tolerant [22], they may differently affect the structure and function of native ecosystems. Comparisons between populations from native and introduced ranges are necessary to understand the role of changes and/or hybridization in the invasion process. Some studies have compared several life traits, and especially genetic structure and differentiation [10,14,22–25], but karyotype changes have never been considered.
Invasions by alien Carpobrotus spp. (Aizoaceae) have been recognized as one of the most severe threats to all the Mediterranean-climate coastal ecosystems [26–29]. Carpobrotus edulis (L.) N.E. Br. and C. acinaciformis (L.) L. Bol., which originated in South Africa, were introduced into Europe in the Botanical Gardens of Leyden (Holland) in 1680 [30], and Marseille (S-E France) in the early 1800s [31], then one century later elsewhere in Southern Europe [e.g. 32,33]. Everywhere, these long-lived, prostrate, trailing succulent plants were first planted as ornamentals, like 90% of alien chamaephytes in France [6], then also used to stabilize soils of coastal dunes and rocky slopes [28,29,34]. Their invasiveness in Provence (S-E France) is particularly due to the absence of natural enemies [35], their high competitive capacity [36], their dispersal via cuttings and seeds [27,37,38], and their high levels of genetic and clonal diversity [39]. In addition, the genus Carpobrotus shows various uncommon traits: (1) its worldwide invaders are endemic native species, like only 17% of invasive plants in France [6], (2) its native and invasive taxa are diploid, vs. 73% of polyploid invaders in France, and (3) it produces frequent diploid hybrids only in invasive ranges, vs. more than 80% of polyploids among invasive hybrids worldwide [6,8,15]. In South Africa, in situ hybridizations are quite rare because the seven endemic species are mainly allopatric and do not flower in the same period [40]. Interestingly, in invasive ranges, all recorded hybridizations occur between C. edulis and different species: native C. chilensis (Molina) N.E. Br. in California [41,42], native C. virescens (Haw.) Schwantes in Australia [43], and introduced C. acinaciformis in France [29].
In Provence, multilocus isozyme variations have shown that two distinct taxa occur: the species C. edulis, and “C. acinaciformis” that corresponds to a mixture of parental forms: hybrid (with C. edulis), backcross types and segregation products [29,39,44]. This large hybrid swarm has been referred to C. aff. acinaciformis by different authors [29,34,39]. In Provence, controlled pollination experiments showed that C. aff. acinaciformis is weakly self-fertile and maximizes seed production via hybridization, whereas C. edulis is completely self-fertile, with a flexible mating system, and always shows higher fruit, viable seed and seedling production [44,45].
Hitherto, no investigation on Carpobrotus invaders has taken into account the native species features. In the present study, we have undertaken the first comparison of morphological and karyological characters of C. acinaciformis, C. aff. acinaciformis and C. edulis between South African (native) and Provençal (invasive) populations in order to answer the following questions: (1) do morphological and/or karyological changes occur in the invasive range? (2) do all these changes result from hybridization? and (3) are these changes only a subset of parental characters or do they result in novelty in the invasive range?
2 Materials and methods
2.1 Taxa sampling
C. acinaciformis, like other South African taxa, displays vivid magenta flowers and inhabits coastal ecosystems, while C. edulis is the only species having yellow flowers and occurring in ruderal habitats [40]. For morphological and karyological studies, we randomly collected C. edulis and C. acinaciformis individuals throughout their native South African range from 7–13 and 11–15 populations, respectively (Fig. 1). This sampling was representative of the overall distribution of both species in South Africa sensu Wisura and Glen [40]. In spite of our thorough prospecting, no “hybrid-looking” individuals were found in situ. In the invasive Provençal range, C. edulis and C. aff. acinaciformis individuals were sampled from 7 and 5 populations, respectively, on the Port-Cros National Park islands and the adjacent mainland.
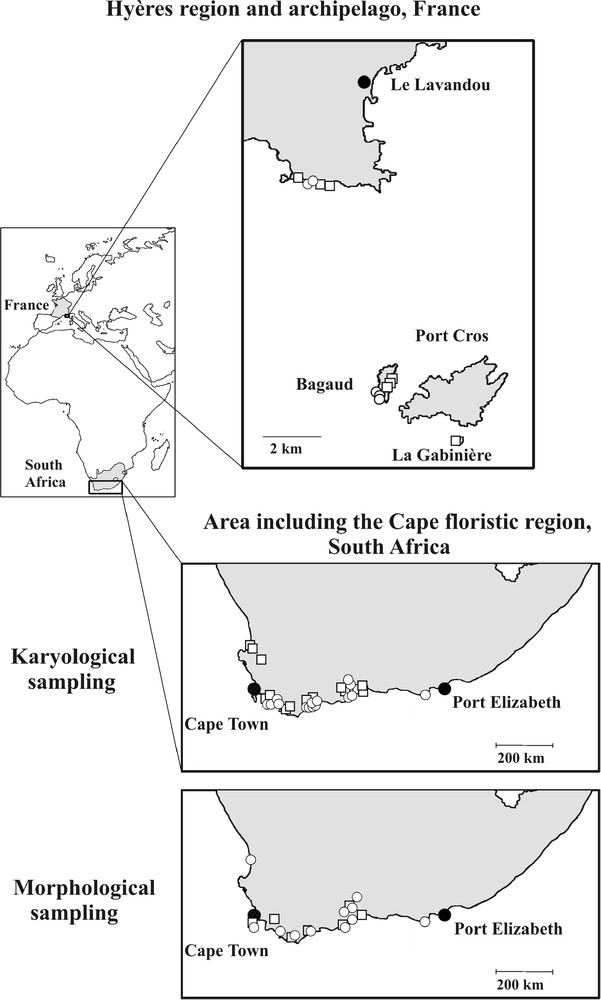
Location of karyological and morphological samplings for C. edulis (□), C. aff. acinaciformis and C. acinaciformis (○) in the Hyères region and archipelago (invasive range: Provence, S-E France) and the Cape Floristic Region (native range: South Africa).
2.2 Morphological and karyological parameters
First, morphological data were gathered for a total of 195 individuals, i.e., 109 in South Africa (46 for C. edulis and 63 for C. acinaciformis) and 86 in Provence (48 for C. edulis and 38 for C. aff. acinaciformis), with at least 5 individuals per population (Fig. 1). Only vegetative characters were measured because they are the best discriminant criteria [39,41] and are accessible at any time. Three replicates of seven characters were scored in laboratory, using callipers accurate to 0.1 mm and averaged for each plant: total leaf length (LL), leaf width (LW) and thickness (LT) of triangular leaf cross-sections 3 cm from the point of insertion, point width (PW) and point thickness (PT) of leaf cross-sections 1 cm from the leaf apex, and internode length (IL) and diameter (ID). Equilaterality indices for each cross-sectional triangle were also calculated: LE = LW/LT and PE = PW/PT.
Because plant size can vary according to habitats, we chose to perform statistical analyses on ratios in order to eliminate this effect as far as possible. Thus, all possible ratios were calculated from the above seven characters. Then, to reduce variable number and redundancy, ratios were removed in order to avoid significant correlations with a Spearman's r of > 0.8. Thirteen morphological ratios were thus retained: LL/(LL + LT), LL/(LL + ID), LL/(LL + LW), LL/(LL + PW), LL/(LL + IL), LT/(LT + LW), LT/(LT + PW), LT/(LT + PT), LT/(LT + IL), LW/(LW + ID), LW/(LW + PW), PT/(PT + PW), PT/(PT + ID).
Secondly, karyological analysis was carried out on a total of 54 individuals, i.e. 29 in South Africa (14 for C. edulis and 15 for C. acinaciformis, i.e. at least one individual per population) and 25 in Provence (12 for C. edulis and 13 for C. aff. acinaciformis). Stem nodes were rooted or seeds germinated in order to provide clean, young root material. All accessions were cultivated and collected under standard laboratory conditions. Root tips were fixed in a solution of absolute alcohol: glacial acetic acid (4:1, v/v), without pretreatment due to small chromosome lengths, and then stored at–18 °C. Root tips were stained in 45% aceto-carmine-ferriacetate, boiled for 3 minutes, and then squashed between slide and cover-slide. For each sample, ten mitotic metaphases were drawn (Wild M20, 15 × 100) with a camera lucida, which greatly magnifies the plates, with ca 0.15 μm error per chromosome (i.e. 1 mm on the paper). For parameter measurements, at least three high quality drawings were selected according to the following criteria: sharpness (centromeres and satellites in particular), chromosomes in the same plane and concentration phase, without overlaps or folds. Thus, for C. edulis and C. (aff.) acinaciformis, 45 and 48 metaphases were studied in South Africa, and 38 and 40 in Provence, respectively. Because chromosome sizes can vary according to squashes and phases, we have privileged indices and ratios.
Karyotype formulas (KF) were established according to the ratio r = B/b for each homologous pair, where B and b are the lengths of the long and short chromosome arms, respectively. The nomenclature and abbreviations used for the description of the chromosome morphology are those proposed by Levan et al. [46]:
(1) 1 < r < 1.1: metacentric chromosome sensu stricto (M);
(2) 1.1 < r < 1.7: metacentric chromosome sensu lato (m);
(3) 1.7 < r < 3: submetacentric chromosome (sm);
(4) r > 3: subtelocentric chromosome (st).
The following parameters were also taken into consideration:
(5) mean chromosome length in μm (ML);
(6) centromeric index: , where n is the number of chromosome pairs;
(7) intrachromosomal asymmetry index:
A1 = 1–[∑(b/B)/n];
(8) interchromosomal asymmetry index: A2 = s/ML, where s is the standard deviation of ML [47];
(9) ratio of the shortest (S) to the longest (L) chromosome pair: S/L;
(10) 2n number of satellites (Sat) in the morphological sense (i.e. a pair of small spherical bodies attached at the chromosome by a slender thread).
The indices A1 and A2 quantify the karyotype asymmetry around the centromeres and the heterogeneity of chromosome lengths, respectively. Karyotypes were represented by idiograms for each native and invasive range taxon, with chromosome pairs arranged in order of decreasing size and aligned according to the centromere position.
2.3 Data analyses
Two principal component analyses (PCA) were performed, one using 13 morphological ratios and the other using 10 karyological characters, in order to discriminate native South African and invasive Provençal individuals in multivariate space (data were normalized before analysis). Discriminant analyses were then used to test taxon groupings for morphological and karyological data, respectively. Homoscedasticity was verified before analysis, and certain characters were transformed in order to minimize heteroscedasticity [48].
Due to invariance for certain characters or non-transformable heteroscedasticity [48], randomization tests were used to compare means and variance of the morphological and karyological characters between ranges of each taxon. Significance was determined as the proportion of 5000 randomly generated parameter differences as extreme or more extreme than the observed differences (one-tailed test, [49]). Probability values were adjusted using Bonferroni corrections for multiple comparisons [48]. Post-hoc Tukey tests were performed between native and invasive ranges within taxa. Statistical analyses were computed using the open source R software program [50].
3 Results
3.1 Morphological changes
In the absence of controlled cultures, morphological characters may be misleading, and ratios appear better suited to such comparisons. The PCA has been carried out using only the 13 morphological ratios. The first principal component (37.81%) separates C. acinaciformis from native range C. edulis individuals, according to LT/(LT + LW), PT/(PT + ID) and LL/(LL + LT), LL/(LL + PW), LL/(LL + IL), LT/(LT + PT), LW/(LW + PW), respectively (Fig. 2). Invasive range C. edulis individuals are well associated with native range C. edulis, whereas invasive range C. aff. acinaciformis occurs in an intermediate position relative to both native range species. Nevertheless, the discriminant analysis significantly differentiates two groups: C. edulis and C. acinaciformis (including C. aff. acinaciformis) (F = 113.82, df = 7, 187, P < 0.001), with five misclassified individuals in the invasive range. Invasive range individuals occupy less multivariate space than native ones, which suggests less morphological variation in the invasive range. Randomization results on 13 morphological ratios demonstrate that no significant range differences were found in native and invasive C. edulis, whereas they are significantly different between native range C. acinaciformis and invasive range C. aff. acinaciformis. In this latter taxon, five morphological ratios [LL/(LL + LT), LT/(LT + LW), LW/(LW + ID), PT/(PT + PW), PT/(PT + ID)] are clearly “intermediate” between the two species, the remaining eight are significantly lower and considered as “transgressive” (i.e., extreme/non-intermediate, sensu Schwarzbach et al. [51]).
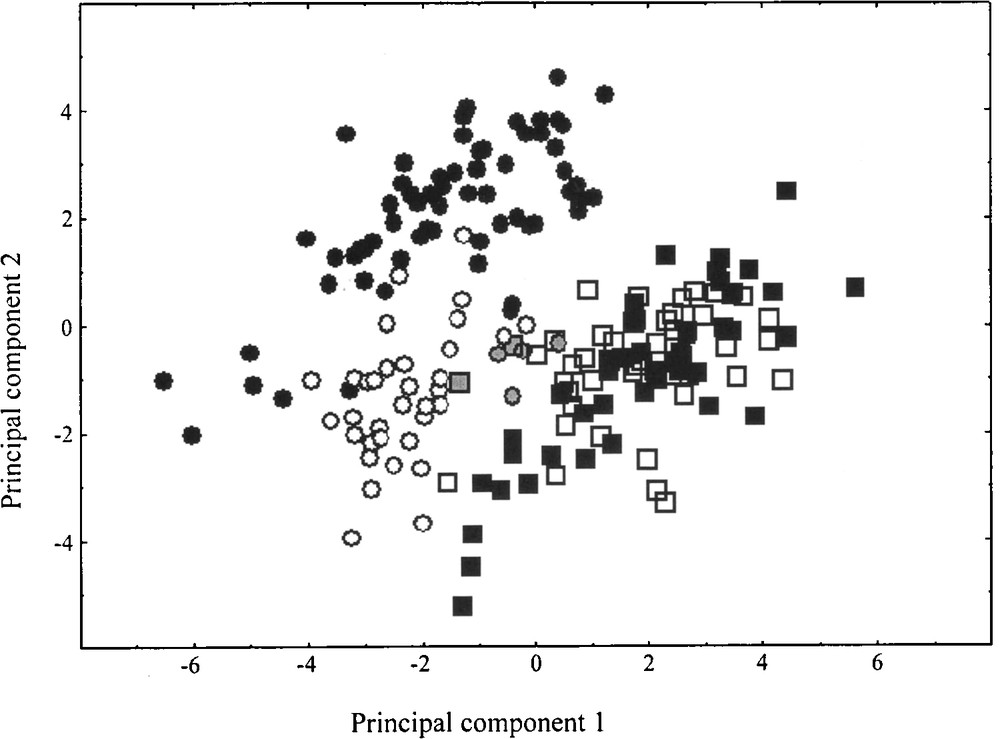
Principal component scores using morphological ratios for Carpobrotus taxa in native ranges, C. edulis (■) and C. acinaciformis (●), and invasive ones, C. edulis (□) and C. aff. acinaciformis (○). Principal components 1 and 2 represent 37.81 and 25.24% of the variation, respectively. Five invasive individuals (grey symbols) are misclassified following the discriminant analysis results.
On the other hand, randomization tests demonstrate that, between native and invasive ranges, 5/9 morphological characters are significantly different in C. edulis, and 8/9 in C. acinaciformis (Fig. 3). All five differences are size increases in invasive C. edulis, while C. aff. acinaciformis shows increasing (5/8) and decreasing (3/8) sizes. Of these differences, two in invasive C. edulis (LT, PT) and five in C. aff. acinaciformis (LT, PT, ID, LE, PE) are clearly intermediate characters, while three in C. edulis (LL, LW, PW) and C. aff. acinaciformis (LL, PW, IL) may be considered as “transgressive” characters.
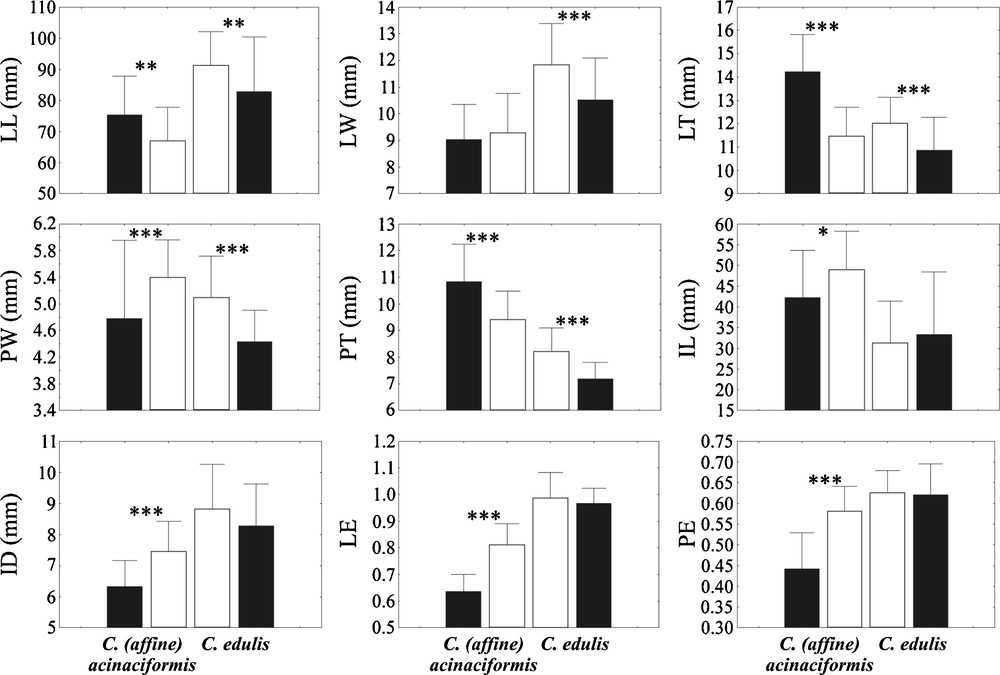
Means and standard deviations for the morphological characters measured in native (black) and invasive (white) ranges for C. edulis, and native (black) and invasive (white) ranges for C. (aff.) acinaciformis. LL: leaf length; LW: leaf width; LT: leaf thickness; PW: point width; PT: point thickness; IL: internode length; ID: internode diameter; LE: leaf equilaterality; PE: point equilaterality. Asterisks indicate significant post-hoc Tukey tests between native and invasive ranges within each group at the P < 0.05 (*), P < 0.01 (**), and P < 0.001 (***) levels.
Finally, native C. acinaciformis significantly differs from native C. edulis by five characters: the highest LT and PT, and the smallest ID, LE and PE, whereas four characters separate C. aff. acinaciformis from invasive C. edulis: the highest IL, and the smallest LL, LW and LE. Consequently, the leaf equilaterality index (LE) remains the only single character in common between the two groups allowing their identification in both native and invasive ranges. This major character corresponds to the cross-section leaf form: isosceles (C. acinaciformis) or equilateral (C. edulis) triangular.
3.2 Karyological changes
Mean karyological characters and idiograms are given for each taxon in Table 1 and Fig. 4. Our counts agree with previous diploid reports for Carpobrotus spp.: 2n = 2x = 18 [52], the chromosomes are rather small ranging in size between 1.8 and 3.2 μm. Both native South African species show high stability, since a single karyotype formula characterizes each species, clearly separating C. acinaciformis from C. edulis. In contrast, both invasive taxa show wide karyological variability, with three different karyotype formulas for each taxon, and sometimes two chromosomes difficult to pair (only in few samples of C. aff. acinaciformis from Bagaud Island). One metacentric pair (M: no. 9) characterizes both C. acinaciformis and C. aff. acinaciformis, but the subtelocentric pair (st: no. 5) is found only in the native range. These two types of chromosome are absent in C. edulis, both in native and invasive ranges. Another evident character is that C. acinaciformis and C. aff. acinaciformis have significantly fewer satellites compared to C. edulis, both in native and invasive ranges. Nevertheless, invasive C. aff. acinaciformis has significantly more satellites than its native counterpart, and inversely for the invasive C. edulis.
Mean of karyological characters for native and invasive Carpobrotus taxa [mean (± SD)]: karyotype formulas (KF), mean chromosome length in μm (ML), centromeric (CI), intrachromosomal (A1) and interchromosomal (A2) asymmetry indices, ratio of shortest/longest pair (S/L) and 2n satellite numbers (Sat).
| Taxon (Region) | KF | ML (± SD), μm | CI (± SD) | A1 (± SD) | A2 (± SD) | S/L (± SD) | Sat (± SD) |
| C. affine acinaciformis (Provence) | 1M + 5m + 3sm 1M + 6m + 2sm 1M + 7m + 1sm |
2.23 (0.27) | 12.31 (0.52) | 0.24 (0.02) | 0.16 (0.03) | 0.64 (0.05) | 6.15 (0.37) |
| C. acinaciformis (South Africa) | 1M + 4m + 3sm + 1st | 2.22 (0.11) | 14.82 (0.87) | 0.34 (0.02) | 0.13 (0.02) | 0.67 (0.05) | 2,87 (0.99) |
| Range comparison | ns; **; *; ** | ns | ** | *** | * | * | ** |
| C. edulis (Provence) | 5m + 4sm 6m + 3sm 7m + 2sm |
2.23 (0.22) | 13.02 (0.46) | 0.28 (0.02) | 0.15 (0.04) | 0.62 (0.07) | 12.33 (0.88) |
| C. edulis (South Africa) | 4m + 5sm | 2.22 (0.20) | 14.37 (0.45) | 0.35 (0.02) | 0.16 (0.02) | 0.64 (0.04) | 14.21 (1.05) |
| Range comparison | ***; *** | ns | ** | ** | ns | ns | * |
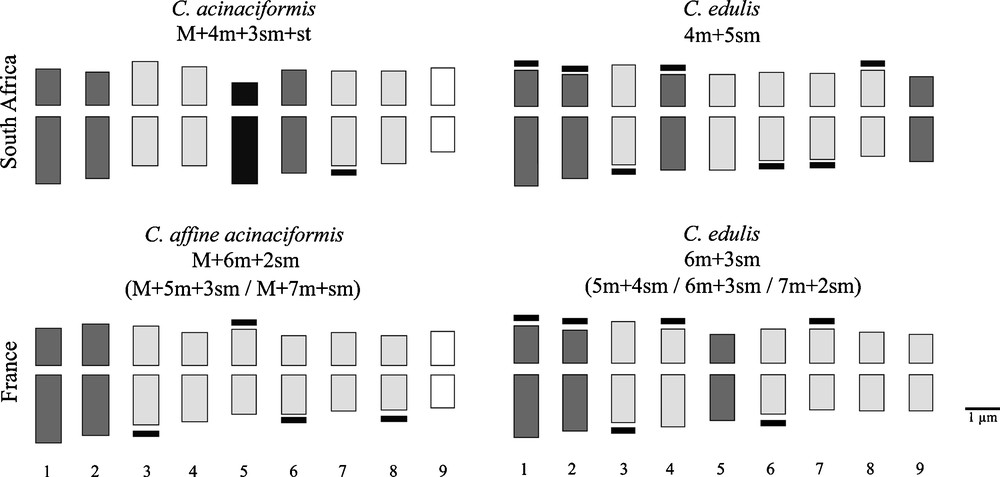
Comparison of mean karyotype formulas and idiograms of Carpobrotus taxa in native (South Africa) and invasive (Provence, S-E France) ranges. Satellites are indicated (in black) at the end of some chromosomes. Colours indicate the different types of chromosome pairs according to the centromere position: Metacentric sensu stricto M (white), metacentric sensu lato m (light grey), submetacentric sm (dark grey) and subtelocentric st (black).
The karyological PCA well separates: (1) the two native species C. acinaciformis and C. edulis through the second axis, and (2): both these natives from invasive taxa through the first axis (Fig. 5). The first principal component (37.14%) represents gradients primarily of m, sm, CI and A1, and the second one (31.33%) of M, st, S/L, and Sat (i.e. variable contributions to principal components > |0.5|). In contrast to morphological PCA, invasive range individuals occupy more multivariate space than native ones, which indicates more karyological variations in the invasive range.
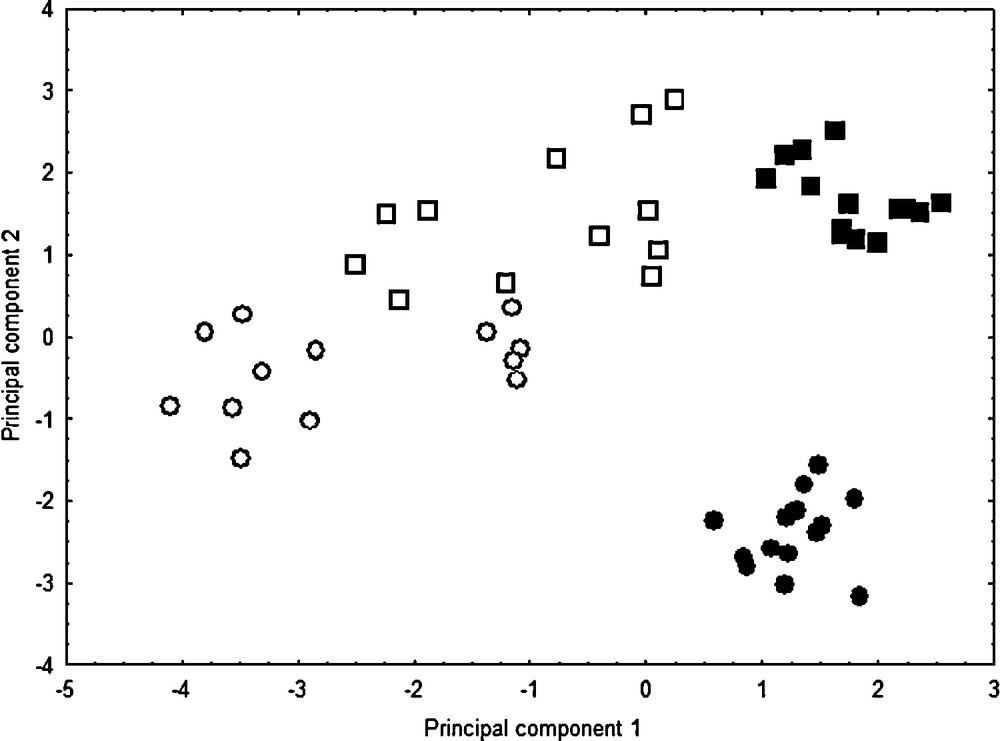
Karyological principal component scores for Carpobrotus taxa in native ranges, C. edulis (■) and C. acinaciformis (●), and invasive ones, C. edulis (□) and C. aff. acinaciformis (○). Principal components 1 and 2 represent 37.14% and 31.33% of the variation, respectively.
For each range comparison, randomization tests (Table 1) indicate that invasive range individuals differ from native ones, by having significantly more metacentric (m) pairs, and consequently fewer submeta- and subtelocentric (sm, st) chromosome pairs. Compared to native species, the range differences of invasive taxa for subtelocentric pair (st) and satellite (Sat) numbers may be considered as intermediate characters (on axis 2 of the PCA; Fig. 5). On the other hand, the range differences for meta- and submetacentric (m, sm) pair numbers as well as centromeric index (CI) and intrachromosomal asymmetry (A1) may be considered as “transgressive” (on axis 1 of the PCA). In fact, the significant decrease of these two indices relative to the centromere position and the increase of metacentric pairs (m) show that both invasive taxa have much more symmetrical karyotypes than the two native ones. Nevertheless, the discriminant analysis supports the presence of two distinct groups: C. acinaciformis (including C. aff. acinaciformis) and C. edulis (F = 86.37, df = 6.47, P < 0.001), with all individuals correctly classified. Discriminating characters include A2 (P < 0.01), S/L (P < 0.05) and Sat (P < 0.001). Thus, the C. acinaciformis group has significantly more homogeneous chromosome lengths (i.e., mean lower A2 and higher S/L scores) compared to the C. edulis group.
4 Discussion
In the present study, morphological and karyological discriminant analyses significantly differentiated two groups: C. edulis and C. acinaciformis (including C. aff. acinaciformis). Each group conserves some specific characteristics, particularly the satellite number (C. edulis group > 11, C. acinaciformis group < 7) and the leaf equilaterality index. Nevertheless, if the two native South African species exhibit very distinct characteristics, evident changes have occurred in Provence. Intermediate characters are considered as the direct result of hybridization between genetically divergent lineages, while transgressive characters (i.e. extreme/non-intermediate) are unexpected consequences, which can contribute to hybrid vigour and heterosis [51].
4.1 Morphological changes
With five intermediate ratios and characters, C. aff. acinaciformis has undergone the most extensive morphological changes in Provence. So we can confirm that this taxon results from interspecific hybridizations between C. acinaciformis and C. edulis, followed by introgressions [39,44,45]. In California, isozymes and cpDNA data have also demonstrated a unidirectional hybridization/introgression with C. edulis as pollinator and C. chilensis as maternal contributor [42,53]. In addition, with eight transgressive ratios and three transgressive characters, morphological variations of C. aff. acinaciformis are not a subset of parental traits, but correspond to a new phenotypic variant. On the other hand, in spite of some significant changes in morphological characters, invasive C. edulis is still close to its native counterpart, because all its ratios and equilaterality indices do not significantly differ from native individuals. In contrast, invasive Californian Carpobrotus hybrids only showed intermediate characters [41].
In fact, morphological changes have to be linked to the history of Carpobrotus spp. introductions in Europe. To acclimatize and improve these weakly frost-tolerant taxa, human interventions have been necessary, as documented for many exotic plants [8,10,54]. Such old and efficient practices mainly involve inter- and intraspecific crosses to obtain hybrid vigour, but also cutting, grafting and seed treatments, in order to select required characters [55]. For Carpobrotus spp., the best illustration is the flower diameter: 8–12 cm in invasive Provençal taxa [32,56] vs.7–10 cm in both native South African species [40].
4.2 Karyotype changes
Compared to the morphological data, the high karyotype variability of both invasive taxa is the clearest and most unexpected change, involving many transgressive characters linked to the centromere position (i.e. decreased intrachromosomal asymmetry). Furthermore, both invasive taxa have undergone restructuration to the extent that their karyotypes now resemble each other more than they resemble native species. The rarity of heterozygous karyotypes in our invasive populations proves: (1) the oldness of hybridization processes between karyologically distinct progenitors (disappeared a long time ago in Provence), (2) ongoing active introgressions between various hybrids, and (3) the existence of several successive generations.
Nevertheless, the increased karyotype asymmetry is usually considered as a derived character within a given lineage, because it strengthens specific isolation barriers [57], as stated by Levin [58]: “the fertility of hybrids declines as the number of chromosomal differences between species increases”. Thus, the decreased asymmetry found in invasive Carpobrotus karyotypes is in the opposite direction to the general evolutionary trend. In the Western Mediterranean Basin, this case has been rarely reported, and only in a few endemic species, such as Lomelosia cretica (L.) Greuter et Burdet. Isolated northern populations (Minorcan cliffs, Balearic Islands) of this species exhibit decreased karyotype asymmetry, satellite loss, morphological changes, and severe meiotic and pollen abnormalities [59, as Scabiosa cretica L.], like invasive Provençal Carpobrotus [60]. It is worth noting that specimens of Carpobrotus spp. first introduced at Marseille in the early 1800s (MARS Herbarium) have normal pollen grains [60].
The karyological PCA clearly supports the notion that karyotype changes and different crosses had taken place in the introduced range. Compared to the invasive C. edulis, C. aff. acinaciformis karyotypes differ more widely from those of its native counterpart, and have undergone extensive changes as a result of its hybridization/introgression with invasive C. edulis. This is confirmed by: (1) the karyological PCA showing a clear convergence of C. aff. acinaciformis towards invasive C. edulis forming a hybrid swarm; (2) the increase in the number of satellites (2–4 to 6–7); (3) the loss or restructuration of the subtelocentric pair; (4) the excess of heterozygotes and the higher genetic diversity of C. aff. acinaciformis [39]. In contrast, invasive C. edulis exhibits a “simple drift”, without obvious signs of interspecific hybridization. The only intermediate character (satellite number decrease: 12–16 to 11–14) rather seems to be linked to intraspecific chromosomal rearrangements. In European botanical gardens, crosses between distinct and distant populations of C. edulis probably occurred, facilitated by the extensive range of this native ruderal species in South Africa, compared to other coastal endemic and more restricted congeners.
Diverse mechanisms such as intra- (C. edulis) and interspecific (C. aff. acinaciformis) crosses and introgressions, man-made selection, founder effects, but also thermal shocks (e.g. impact of drastic low temperatures on meiosis) [61,62], may explain these unexpected karyotype restructurations, and their opposite direction to general evolutionary trends. If the origin of the asymmetry decrease remains unknown, this change has facilitated various crosses, and has even been amplified through interspecific hybridizations. Furthermore, it is worth noting that these changes have occurred within a short time lag corresponding to the naturalization of Carpobrotus spp., i.e. two centuries on southern English coasts [30] and less than one century in Provence [32,63]. Comparisons between Provençal populations and oldest or youngest introductions from England and Spain [33,34], respectively, are necessary in order to evaluate the extent of this rare phenomenon.
4.3 Evolutionary potential
Chromosomal changes in invasive Carpobrotus have led to an increase in intraspecific variability, and an interspecific convergence of karyotypes that might facilitate further hybridization, probably coupled with fertility selection. This may also explain the lack of tetraploid individuals, whereas this is usually the rule in hybrid lineages from distinct karyotype diploid species [57,58,64]. Furthermore, given the different mating systems of the two invasive taxa [44,45], their co-occurrence in coastal ecosystems, and their simultaneous flowering in Provence, hybridization and introgression events are likely to continue in situ, resulting in a snowball effect. Thus, there is a potential for C. aff. acinaciformis hybrid swarm to be assimilated into C. edulis to form a “coalescent complex” [15,65–67], especially as the native parents are absent. The case of Carpobrotus in Provence can be compared to that of the invasive Senecio squalidus L. (2n = 20) in Britain: “a unique example of recent ecogeographic homoploid hybrid speciation facilitated by spatial isolation following human-mediated introduction” [68,69]. Furthermore, karyotype restructurations, particularly efficient in diploid taxa, can provide a source of genetic variability on which selection can act during the colonization of new habitats [67]. Such restructurations should be added to the list of “rapid evolutionary changes” [19] used to predict invasive capacities of introduced species. Further investigations of molecular polymorphism of native and invasive Carpobrotus spp. are now needed to be set against our karyological and morphological insights.
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.
Acknowledgements
We are very grateful to the reviewers for their valuable comments. This study was supported by EPIDEMIE (www.ceh.ac.uk/epidemie), INVABIO (Ministère de l’Écologie et du Développement Durable, subvention no. 01113), the National Park of Port-Cros (contract no. 97.029.83400), and is based upon work supported under a National Science Foundation Graduate Research Fellowship to C.M.S. The sampling in South Africa was supported by a grant from the Centre National de la Recherche Scientifique (CNRS) within the framework of the cooperation project CNRS-NRF no. 13276, in 2003. The authors thank S. Charpentier, F. Bretagnolle and P. Roche for their help in the field, and M. Verlaque for figures and comments. Thanks also to our South African colleague, R.M. Cowling (Univ. Port Elizabeth) for his kind help during our fieldwork in South Africa.


