1 Introduction
Species belonging to the fungus-growing ants comprise the erstwhile tribe Attini, and were included recently in the clade Attini that corresponds to a wide group constituting a diverse selection of predominantly Neotropical taxa [1].
The leafcutter ants represented by the genera Atta and Acromyrmex are considered the dominant herbivores of the Neotropics [2,3]. These ants in particular are the most well-known and studied species of the genus Atta because they significantly impact human activity, because they cut plant material from cultivated plants in large quantities for the cultivation of the symbiotic fungus on which they feed [2,4]. However, in natural ecosystems, the leafcutter ants are especially important for ecosystem structure and dynamics [5].
Atta robusta Borgmeier, 1939, an endemic leafcutter ant of the “restinga” ecosystem (sandy open vegetation along the Brazilian coastline), occurs from northern regions of the state of Espírito Santo to the south of the state of Rio de Janeiro, Brazil [6]. It is even one of the few ants of this genus endemic to the restricted area [7]. The species was included in the list of threatened fauna, mainly because of the intense human occupation of the ecosystem in its region of occurrence, as well as because of the indiscriminate chemical use for control of the leafcutter ants [5]. Furthermore, it is possible that plant cover removal from the restinga directly influences the temperature and humidity control necessary for cultivation of the fungus on which they feed [5]. A. robusta plays a significant role in vegetation regeneration because it interacts with at least 36 plant species from the restinga of Guriri Island, state of Espírito Santo, throughout the year (reviewed in [5]). Because it does not exert any kind of economic impact and cannot be considered a pest, most of the studies conducted on this species are recent [5,6,8,9].
Of the various fields of genetics, cytogenetics is considered an important tool because chromosomal rearrangements may induce developmental changes in individuals without causing genomic alterations [10], thus directly participating in the evolutionary mechanisms generating variability and modifications in individual fitness [11,12]. Cytogenetic studies conducted on the family Formicidae highlight the importance of this field of genetics in evolutionary, phylogenetic and taxonomic studies of this diverse group [reviewed in 13]. Cytogenetic data on threatened species reveals the relevance of the referred tool for a better understanding of the structure and isolation of distinct populations, as well as in furnishing arguments for the species conservation programs [14,15]. For example, cytogenetic studies were performed on the threatened ant D. lucida at 15 sites of Bahia and Espírito Santo, revealing the occurrence of a great variation in the chromosome number: 2n = 106, 114, 116, 118 and 120 in the state of Bahia and 2n = 118 in Espírito Santo. In the southern Bahia forest, the fragments are smaller and much more isolated due to human occupation and deforestation, which increase the chance of the evolutionary divergence of karyotypes among the different populations. Cytogenetic results determined from a range of locations indicated the importance of this tool for conservation of this threatened species [15].
To date, chromosomal studies have been conducted in five taxa of leafcutter ants of the genus Atta. All presented 2n = 22 chromosomes, including the species collected in Brazil: A. bisphaerica, A. laevigata, A. sexdens rubropilosa [16,17], A. sexdens piriventris [18] and in Panama: A. colombica [19]. Data on chromosome banding are available for A. bisphaerica, A. laevigata, A. sexdens rubropilosa [17], and A. colombica [19]. Chromosome banding techniques are of fundamental importance for the localization of specific chromosomal regions, allowing for the performance of additional evolutionary inferences not permitted with conventional Giemsa staining alone. Cytogenetic data shows constant karyotypes for the different Atta spp. not only with respect to chromosome number, but also their morphology, pattern and heterochromatin composition [17].
The species of the genus Atta cytogenetically studied to date include ants presenting broad geographic distribution. No endemic ant species of this genus with restricted distribution has been studied at the cytogenetic level, which allows for comparative studies on the evolutionary dynamics of the group. Incorporating the cytogenetic results with those of other current studies on this ant (Teixeira MC, personal communication) will enable a better understanding of this group of leafcutter ants, and also assist in conservation strategies.
Knowledge on the degree of genetic variability within natural populations of threatened species can usually furnish arguments on structuring conservationist practices and politics, thus avoiding the disaster of species extinction. Habitat fragmentation and other anthropic interventions reduce the gene flow as well as genetic diversity [20], affecting the chances of survival of species, like A. robusta.
The present study sought to describe the karyotype of the threatened leafcutter ant A. robusta, endemic to restinga areas, in order to provide cytogenetic knowledge for its conservation and also to provide a better understanding of the evolution of the leafcutter ants.
2 Materials and methods
Two colonies of A. robusta were collected in the city of Aracruz, located in the Brazilian state of Espírito Santo (19°51′S 40°04′W), during January 2012. Specimen collection was authorized by the Brazilian Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio), according to a special collecting permit (SISBio number 31861). This collecting permit was issued to Luísa Antônia Campos Barros. Ant vouchers (workers) were deposited in the reference collection of the Laboratório de Mirmecologia, Centro de Pesquisas do Cacau (CPDC/Brazil), under the record #5727.
Mitotic metaphases were obtained according to Imai et al. [21] by dissecting the cerebral ganglia of the larvae after the meconium elimination. A total of 50 individuals were analyzed. The metaphases were observed and photographed using a BX 60 microscope with a 100× objective, coupled with a Q-Color3 Olympus® image capture system. A total of 15 metaphases with similar degrees of condensation, without overlapping and evident centromeres, were organized by pairing the chromosomes in order of size, measuring and classifying them according to Levan et al. [22], based on the chromosome arm ratio (r) using the following features: long arm length (L), short arm length (S) and arm ratio between the long and short arms (r = L/S). The chromosomes were classified as: m = metacentric, sm = submetacentric, st = subtelocentric and a = acrocentric. Chromosomes were measured and organized using Image Pro Plus® and Corel Photopaint X3 software packages, respectively. The C-banding technique was performed according to Sumner [23]. Regions rich in GC and AT base pairs were detected using the fluorochromes Chromomycin A3 (CMA3) and 4′,6-diamidin-2-phenylindole (DAPI), respectively [24]. The Hsc-FA technique (secondary constriction heterochromatin-associated bands by fluorescence using Acridine Orange) was applied according to [25]. The NOR banding technique was performed according to Howell and Black [26]. Ribosomal gene clusters were detected by FISH according to Pinkel et al. [27], with the use of a 18S rDNA probe isolated from the bee Melipona quinquefasciata amplified by PCR using the primers 18SF1 (5′-GTCATATGCTTGTCTCAAAGA-3′) and 18SR1.1 (3′-TCTAATTTTTTCAAAGTAAACGC-5′) designed by Pereira [28]. The probes 18S rDNA were labeled by indirect method using digoxigenin-11-dUTP (Roche Applied Science) and the signal was detected with anti-digoxigenin-rhodamine (Roche Applied Science). The metaphases were analyzed with an epifluorescence microscope using WU (330–385 nm) and WG filters (510–550 nm), for DAPI and rhodamine, respectively.
3 Results
The chromosome number observed was 2n = 22 with the karyotypic formula: 18m + 2sm + 2st (Fig. 1a). A secondary constriction was observed on the fourth metacentric pair (Fig. 1a). The centromeric regions of the chromosomes indicated the presence of weak and little visible blocks of heterochromatin (Fig. 2). The long arm of the fourth pair of metacentric chromosomes showed a region rich in GC base pairs using CMA3 (Fig. 3a). Regions with differential staining using DAPI were absent, with a uniform pattern along the chromosomes (Fig. 3b). Markings resembling CMA3 pattern were also observed on the fourth pair of chromosomes when the Hsc-FA technique was applied using Acridine Orange (AO) (Fig. 3c), NOR banding technique (Fig. 3a – inbox) and FISH using 18S rDNA (Fig. 3d).
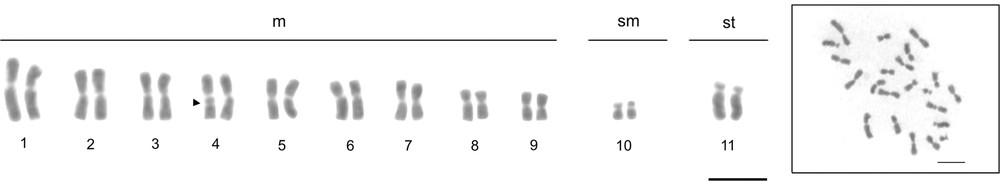
Conventional staining with Giemsa of Atta robusta. a: diploid karyotype 2n = 22; b: the respective metaphase. Arrowhead indicates the presence of a secondary constriction in the fourth metacentric pair. m = metacentric, sm = submetacentric, st = subtelocentric. Bar = 5 μm.
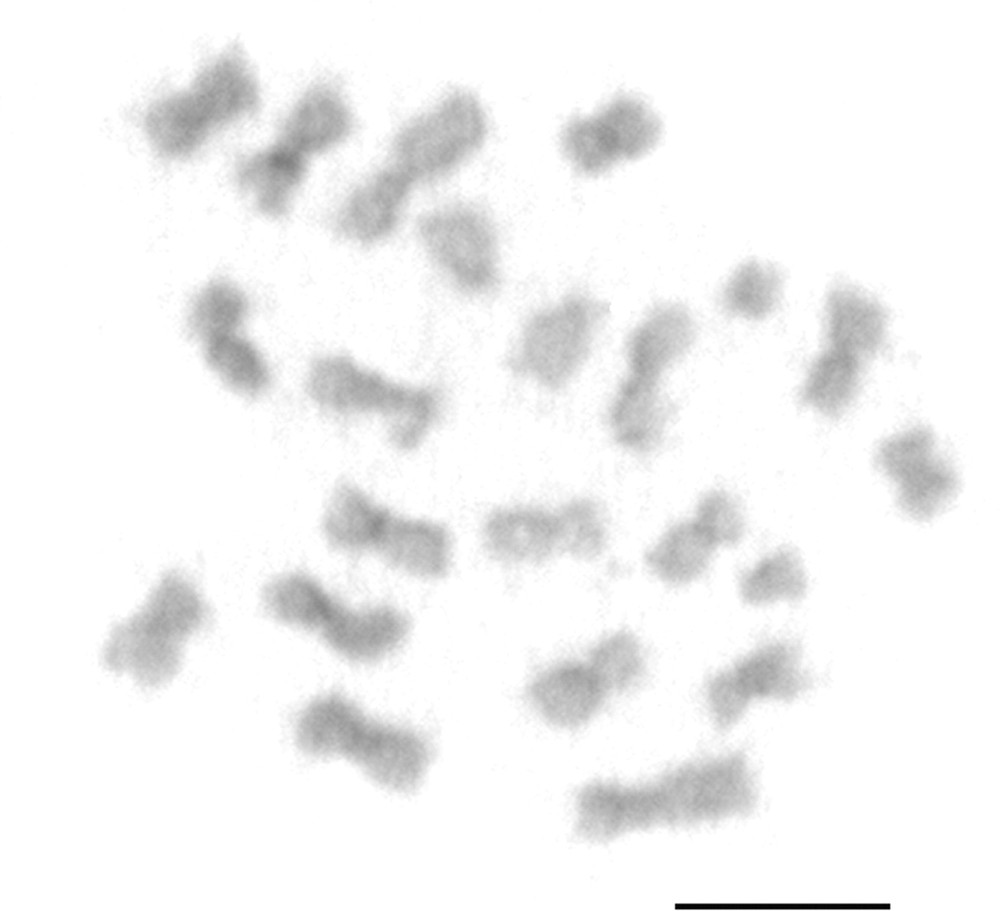
Metaphase of Atta robusta submitted to the C-banding protocol. Centromeric markings denote weak heterochromatin. Bar = 5 μm.
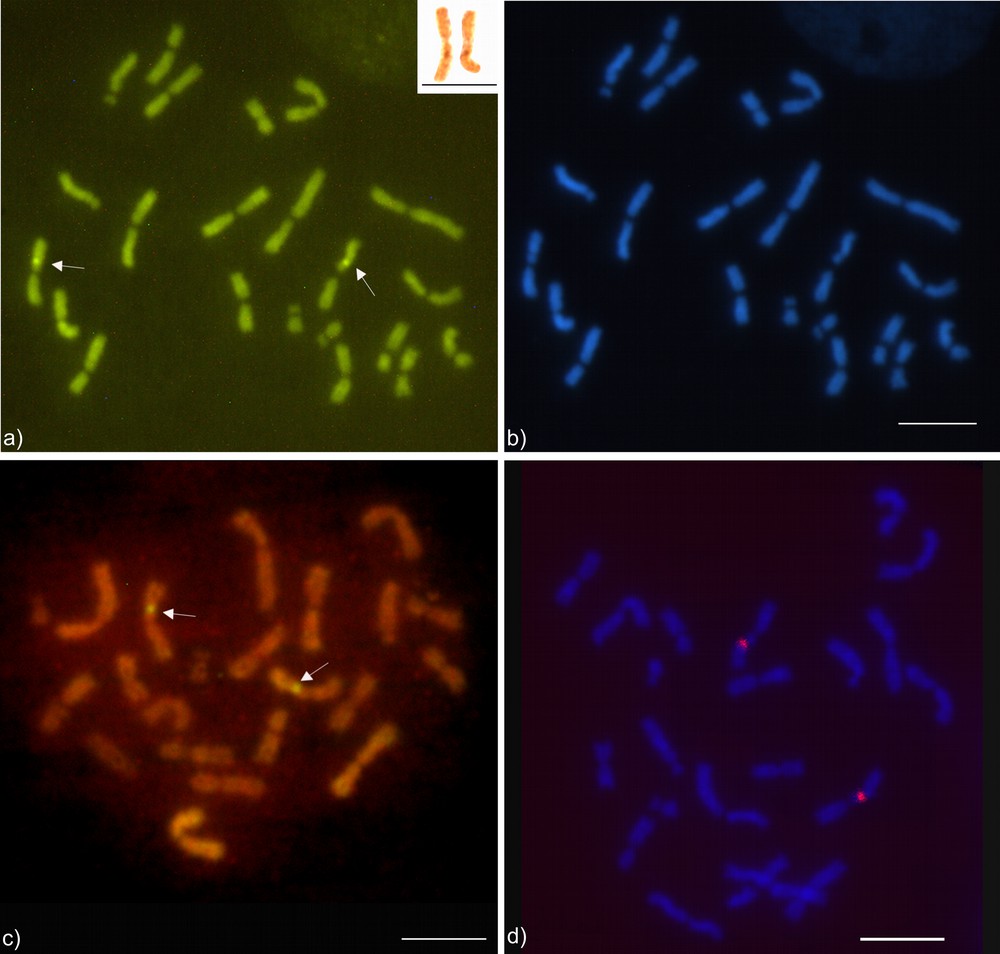
(Color online.) Cytogenetic markings in the fourth chromosome pair of Atta robusta. Metaphase stained with fluorochromes (a) CMA3 that match GC-rich regions indicated by the arrows and (b) DAPI with uniform pattern of AT base pairs along the chromosomes. NOR banding (b, inbox), indicates the nucleolus organizer regions bearer pair. Hsc-FA using Acridine Orange marks the NOR-associated heterochromatin indicated by the arrows (c) and the FISH technique to detect ribosomal genes 18S (d). Bar = 5 μm.
4 Discussion
The chromosome number of A. robusta showed the same general pattern described for other Atta spp.: 2n = 22, with a predominance of metacentric chromosomes. The referred species also presented a conserved morphology of chromosomes when compared with the other species of the genus [17], which was based on the chromosome arm ratio according to Levan et al. [22]. Cytogenetic results of this study confirm the information available for Atta and allow for confirming the conserved chromosome number, morphology and banding pattern within the genus for taxa studied to date, which included species from three of the four groups of Atta indicated by molecular data [29]. It included species in three of the four groups of the genus Atta pertaining to A. laevigata and A. bisphaerica in the Epiatta group; A. colombica in the Atta sensu stricto group; and A. sexdens rubropilosa, A. sexdens piriventris, and A. robusta in the Neoatta group.
The morphological constancy of the chromosomes revealed for Atta spp. differs from that observed for species of the other leafcutter ant genus Acromyrmex, which presented 2n = 38 chromosomes [Barros et al. in prep.]. Morphological differences in the chromosomes may be a consequence of the greater chromosomal number of Acromyrmex spp., which, according to the most robust chromosomal evolution theories of Formicidae proposed by Imai et al. [21], would result from centric fissions that allow for variations in the heterochromatin blocks between different species. The karyotype of A. striatus, however, bears closer similarity to Atta spp. (2n = 22) studied earlier and, associated with the molecular data, it provided new insights into the phylogenetic position of this species among leafcutter ants [30]. According to the authors, it is a sister group of the remainder leafcutter ants that split before the divergence between Acromyrmex.
The heterochromatin distribution pattern of A. robusta is similar to those of other Atta spp., weak centromeric markings, differing from the pattern observed for A. striatus (2n = 22), which is the only Acromyrmex having the same chromosome number as Atta spp. In this ant, cytogenetic studies not only presented weak centromeric markings, but also more evident additional pericentromeric, interstitial, and telomeric heterochromatic GC-rich blocks [27]. Markings observed using the Hsc-FA technique was similar to those observed using the fluorochrome CMA3 in the present study. Hsc-FA using (AO) marks the NOR-associated heterochromatin (for details, see [31]). The same pattern was also observed for the ants Dinoponera lucida [31] and Wasmannia auropunctata [32]. The only pair of A. robusta chromosomes that showed heterochromatic blocks rich in GC base pairs using the fluorochromes CMA3 and AO has a pattern similar to that observed in other Atta spp. The negative interstitial region of the fourth chromosome pair was observed with DAPI, indicating complementarity with CMA3. The same non-specific pattern observed with DAPI along the chromosomes was observed for other fungus-growing ants, e.g., Mycocepurus goeldii [33], A. striatus [30], Trachymyrmex fuscus [34] and Atta spp. [17].
The markings obtained with the FISH technique confirmed the results obtained when employing the fluorochromes CMA3 and AO, as well as the NOR banding, complementing the data and allowing us to conclude that there is a single NOR for A. robusta. Studies conducted with ants that presented markings in one pair of chromosomes coincided with single NOR e.g., D. lucida [15,31]. It is possible that a single NOR will be found in the genus Atta. Only M. goeldii among fungus-growing ants had its 18S rDNA sites mapped [35], and this study presents the first data for leafcutter ants.
Multiple markings rich in GC base pairs were described in A. striatus [30]. However, they were absent in A. bisphaerica, A. laevigata and A. sexdens rubropilosa [17] and also in the chromosomes of A. robusta stained with CMA3. A single NOR was confirmed for A. robusta; however, the characteristics of the heterochromatin blocks rich in GC base pairs remain unknown for A. striatus and only further molecular cytogenetic techniques (FISH) could confirm the presence or absence of rDNA in the chromosomes. The fungus-growing ant M. goeldii showed single NOR [35], although it presented multiple GC-rich regions [33].
At the long arm of the fourth chromosome pair markings were observed in its interstitial region using the NOR banding technique, indicating the presence of active ribosomal genes in the last interphase in this pair. The repeatability obtained in the metaphases subjected to this technique is low. However, the clear secondary constriction associated with the presence of heterochromatic blocks rich in GC base pairs, using the CMA3 and AO, in addition to 18S rDNA-FISH, confirm that this chromosome pair is the NOR bearer. Imai et al. [36] raised the possibility that, somehow, the chromatin of the ribosomal genes was little accessible, which hinders the argentophilic proteins from associating with the chromatin and that silver nitrate later binds to them.
The endemic status of A. robusta suggests peculiarities in its biology if compared to other leafcutter ants. Moreover, strong anthropic pressure on the biome of this species may greatly reduce the variability of its populations by subjecting them to a bottleneck effect capable of reducing its frequency of occurrence. This effect could be expected in function of its cytogenetic configuration; however, the results of this study, which represent the first cytogenetic information on an endemic species of Atta with a restricted distribution, showed no differences in the karyotype compared to other Atta spp. Although samples were not collected in other populations of A. robusta, the constancy of the chromosomal characters found in Atta suggests that variations in the karyotype of A. robusta are unlikely.
Populations of A. robusta have been management target for conservation purposes since 2008 due to the intense process of plant cover reduction in restinga areas. The use of cytogenetic data in animal relocation and breeding programs is of great significance to prevent the reduction of fitness or infertility, because chromosomally divergent groups need to be avoided [14,37]. Therefore, it is important to ensure, especially when dealing with threatened species, that the animals used as founders do not carry chromosomal rearrangements [28]. Considering the constant karyotype (number, morphology and banding patterns) of the threatened A. robusta and other Atta spp., cytogenetic data do not indicate restrictions in relocation or reintroduction in areas where populations were extinct due to the conserved karyotype. However, other data need to be considered, including molecular biology that indicates haplogroups for the species [13,17]. It differs from the giant ant Dinoponera lucida that presents high chromosome number variation in some populations of the state of Bahia (Brazil). In this case, cytogenetic data need to be considered for relocation or reintroduction in areas where the populations were extinct in association with other fields of knowledge. Considering the status of the threatened A. robusta, all information is important for conservation and chromosome data is not a restriction. Furthermore, efforts for preserving restinga environments are needed to preserve A. robusta populations along with many other organisms.
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.
Acknowledgments
We are grateful to Filipe Pola Vargas and José Teixeira for field support at Aracruz, Riudo de Paiva Ferreira for technical support. Access to the biological material was allowed by the Brazilian Instituto Chico Mendes de Conservação da Biodiversidade (permit SISBio number 31861). LACB and JHCD acknowledge the grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). This research was supported by Fundação de Amparo à Pesquisa de Minas Gerais (FAPEMIG) process: APQ-00141-2012 and Fundação de Amparo à Pesquisa da Bahia (FAPESB).


