1 Introduction
Silk is known to be produced by many Arthropod species in diverse phylogenetic groups from spiders or pseudoscorpions to insects including Diptera, Lepidoptera, Hymenoptera, and Trichoptera [1–3]. There are many kinds of silk with a large diversity of properties and chemical constituents [1]. Silk has many roles, from web building, protection, and thermal regulation, to communication between individuals [4,5]. Silk thread laid by an individual can also be used as a trail followed by other conspecifics as shown in social spiders and caterpillars [6–8]. These silk laying and following behaviours may trigger and amplify a recruitment process by creating a positive feedback mechanism. In social spiders, recruitment processes can be mediated by the use of silk trails [7,9]. For instance, during collective displacements, each individual of the social spider Anelosimus eximius contributes to the growth of a webbing structure through the addition of its silk thread. The opportunity for an individual to use a predecessor's dragline as a shortcut causes an amplification process. In this context, silk draglines laid by social spiders can be seen as trail marking and trail following usually associated with eusocial insects (e.g., ants and termites). Therefore, a network of silk can favour group formation and cohesion [9–12]. Furthermore, previous studies show in social arachnids, strong differences in the probability to follow a silk dragline between individuals coming from different populations of the same species [9,13]. They show that the probability to follow the silk secreted by individuals coming from another population is lower than the probability to follow the silk secreted by genetically close individuals. This means that the presence of individuals coming from different genetic pools reduces the cohesion of the group during the settlement process and thus increases the spatial distribution of spiders [9].
T. urticae is a phytophagous mite that produces silk from salivary glands and continuously excretes it at the pedipalp level while walking. All developmental stages are engaged in silk production [14]. Mites live in a common web that can cover entire plants and protects them against predators, [15,16] competitors [17,18], abiotic agents like humidity [19], wind [20], rain [21] and even acaricides [20,21]. As the quantities produced per individual are very low, building a common web requires a large number of conspecifics laying silk threads at the same place and time. This implies a kind of cooperative behaviour as seen in common web spinning [22]. In T. urticae, silk can also be used as a vector of locomotion and favours group formation and group cohesion [5,14]. Indeed, females are able to follow the silk trails (on which chemical cues are present) left by preceding females [5]. This behaviour induces an amplification process, resulting in aggregation at a new colony site. Furthermore, in T. urticae, the genetic background seems to play a great role in the organisation of a colony [23–25] and indicates that this mite can discriminate against individuals coming from another population.
In species dispersing partly or predominantly by walking, locomotor activity can be an indicator of their tendency to leave an unsuitable environment, and/or their ability to find new food resources and/or conspecific aggregates. Here, our aim was to observe and compare the locomotor behaviour of T. urticae, i.e. time in movement, in resting, and in exploration when placed on a site with or without silk. A previous paper [22] showed that, in this species, living in small groups increased silk production, fecundity, and survival. This implies that individuals can perceive the presence of their conspecifics through direct and/or indirect interactions. (In)direct perception through chemicals (pheromones) or tactile perception of conspecifics might constitute indicators of group size [26,27]. Indeed, this mite is a species in which tactile stimuli are very important [28]. Bostanian [29] described chemoreceptors and mechanosensory/olfactory receptors on the two first walking legs of T. urticae. Therefore, tactile contact with silk laid by other individuals could be a way to detect and identify them. In this study, we examine if T. urticae is able to discriminate the genetic origin of a silk producer, by testing the attracting and arresting properties of the silk of two genetically distant populations of T. urticae. Our assumption is that the presence of silk coming from its own population should decrease its locomotor activities while a place without silk or with silk from another population may trigger environmental exploration.
2 Materials and methods
2.1 Mites
Two populations of T. urticae were used for these experiments: a red form population (also called T. cinnabarinus) provided by Dr Kaouthar Lebdi Grissa (Institut National Agronomique, Tunis, Tunisia), and a green form population collected in the green-houses of our laboratory in Louvain-la-Neuve, Belgium. The most accepted taxonomical position does not recognize T. cinnabarinus as a valid species as in the World Catalogue of the Spider Mite Family [30], which cites it as a synonym of T. urticae. The two populations were reared on bean leaves (Phaseolus vulgaris L.) placed on damp cotton in Petri dishes. Breeding stocks were maintained in a climate room at 26 °C, 50–60% RH, and 16:8 (L: D). The two populations were reared in lab conditions for five years.
The genetic distance between the two forms used in this experiment was evaluated in a previous study using microsatellites. Both populations were considered inbred (FIS red form population confidence interval [CI]: 0.47 [0.32–0.58] and FIS green form population: 0.26 [0.15–0.30]) but were genetically distinct for all tested loci (FST = 0.47, Nei distance = 0.95) [31].
2.2 Experimental set-up
Experiments were undertaken under the same environmental conditions as rearing. A microscope circular cover glass (ø = 15 mm) was divided into two equal areas by a piece of wet paper (1 mm in width). A virgin female (called a weaver female) aged less than 24 h from the red or the green form population was placed on a half part of the microscope cover glass. For 30 minutes, the female deposited silk trails (and possibly pheromones and faeces) by walking on this half part [13]; no eggs were laid during that period. Then, the wet paper and the weaver female were removed and the set-up was dried for 30 minutes at 26 °C.
We tested three treatments: (1) half glass with silk from the red form (RSP) and the other half free of silk (CP); (2) half glass with silk from the green form (GSP) and a half free of silk; and (3) half glass with silk from the red form (RSP) versus the other half with silk from the green form population (GSP).
For each treatment, a virgin female (called tested female) of less than 24 h from the red form or the green form population was placed at the centre of the cover glass after the set-up was dry. The mite behaviour was then video-recorded for 30 minutes. Twenty replications were made per treatment. The silk part was alternated on the right or on the left side of the set-up between two experiments. The same was done for the three treatments to avoid any environmental bias [5,32]. Four behavioural parameters were used to quantify the locomotor activity: (1) the total time spent on each part; (2) the time in movement; (3) the resting time; and (4) the time in static exploration (“statex”). Static exploration is a behaviour characterised by individuals staying at the same place but turning on themselves, probably to probe their environment [32]. These behavioural parameters were recorded in the tested females using jwatcher software (Copyright© 2000–2011 - Daniel T. Blumstein, Janice C. Daniel, and Christopher S.). Statistics on the durations of time in movement, time in resting and time in statex were done with their mean relative proportion on each part of the set-up. The relative proportion was calculated by dividing the time spent in movement, in resting, or in statex on one part of the set-up by the total time spent on that part (sum of the three times).
2.3 Statistical tests
To provide evidence for preference for the silk part or for the clean part for the treatments RSP vs. CP and GSP vs. CP, we subtracted for both populations the absolute value of the total time spent on the clean part to the absolute value of the total time spent on the silk part. In the third treatment (RSP vs. GSP), we subtracted the absolute value of the total time spent on the green silk part to the absolute value of the total time spent on the red silk part. If the mean result was significantly positive, mites spent significantly more time on the silk part, or on the red silk part in the case of the third treatment (RSP vs. GSP). On the contrary, if the mean result was significantly negative, mites spent significantly more time on the clean part or on the green silk part in the case of the third treatment (RSP vs. GSP). The third possibility was that the mean result was statistically similar to zero, in this case, it meant that the mites spent the same time on both parts of the set-up.
To test the influence of the genetic origin of the weaver female and of the tested female on the locomotor activity of T. urticae (time in statex, in resting, and in movement), we performed a two-way MANOVA to compare the relative proportion of two behavioural items (resting and movement) among modalities. Indeed, as the sum of the three behavioural items is always 100%, testing only two behavioural items will automatically include the result of the third behavioural item.
Tests and graphs were performed using GraphPad Prism version 5.01 for Windows (GraphPad Software, San Diego, CA, USA; www.graphpad.com) and R version 2.8.0 (©R Foundation for Statistical Computing, 2008). All tests were applied under two-tailed hypothesis and the significance level was set at 0.05.
3 Results
3.1 Red females
The silk woven by red females significantly influenced the total time spent by other red females on each part of the set-up. The total times spent on the red silk part minus the times spent on the clean silk part was significantly positive (RSP vs. CP: lower 95% CI of mean = 9.61, upper 95% CI of mean = 874.40). The total times spent on the red silk part minus the times spent on the green silk part was significantly positive (RSP vs. GSP: lower 95% CI of mean = 8.90, upper 95% CI of mean = 876.40). Red females were not influenced by the green silk (GSP vs. CP: lower 95% CI of mean = −463.70, upper 95% CI of mean = 473.30) (Fig. 1).
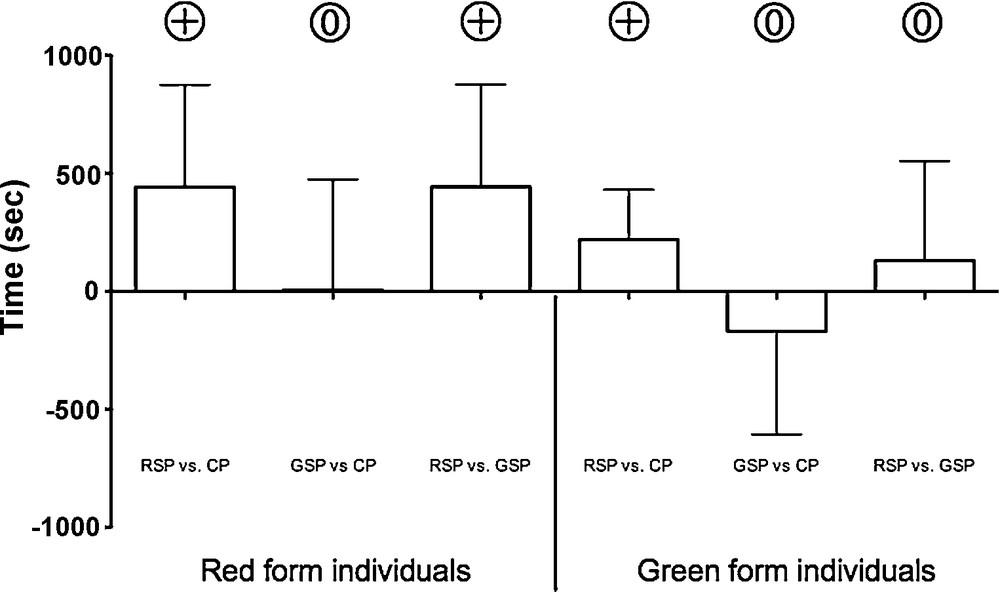
Mean result of the subtraction between the total time spent on the silk part and the total time spent on the clean part (RSP vs. CP and GSP vs. CP) and between the total time spent on the red silk part and the total time spent on the green silk part (RSP vs. GSP) for both red and green tested individuals. Error bars indicate the confidence interval (95%). indicates a result significantly positive and a result significantly similar to zero.
3.2 Green females
The total time spent on the green silk part minus the time spent on the clean/red silk part were statistically similar to zero (GSP vs. CP: lower 95% CI of mean = −606.50, upper 95% CI of mean = 268.30; RSP vs. GSP: lower 95% CI of mean = −291.30, upper 95% CI of mean = 551.10). Such as red females, green females seemed to be attracted by the red silk part as the difference of the time spent on the red silk part and the time spent on the clean part was significantly positive (RSP vs. CP: lower 95% CI of mean = 5.72, upper 95% CI of mean = 431.50) (Fig. 1).
3.3 Comparison
Two-way MANOVA showed that the relative proportion of each behavioural item either for the red or the green population was not influenced by the presence/absence of silk (F = 0.25, df = 1, P = 0.91). Furthermore, the same two-way MANOVA also showed a clear difference between the two tested populations (F = 11.12, df = 1, P < 0.001) concerning their locomotor activity. Indeed, for the three treatments the red form individuals spent less time in movement and more time resting than individuals of the green form, but this difference was statistically significant only for the first treatment (Table 1). Red tested females spent approximately 45% in movement, 40% in resting, and 15% in statex, whereas green tested females spent approximately 65% in movement, 20% in resting, and 15% in statex.
Mean relative proportion of time spent in statex, resting and in movement on the whole set-up for each modality (RSP vs. CP, RSP vs. GSP, BSP vs. CP) for both Tunisian red form and Belgian green form individuals.
| Statex (mean ± CI) | Resting (mean ± CI) | Movement (mean ± CI) | |
| Tunisian red form individuals | |||
| RSP vs CP | 13.35 ± 2.84 | 39.65 ± 10.03 | 47.01 ± 9.77 |
| RSP vs GSP | 13.67 ± 3.57 | 39.98 ± 8.96 | 46.36 ± 8.06 |
| GSP vs CP | 11.94 ± 4.10 | 40.03 ± 11.89 | 48.30 ± 10.29 |
| Belgian green form individuals | |||
| RSP vs CP | 16.51 ± 3.30 | 9.09 ± 3.18 | 74.41 ± 17.63 |
| RSP vs GSP | 13.22 ± 3.13 | 26.43 ± 14.60 | 60.36 ± 14.03 |
| GSP vs CP | 15.51 ± 3.93 | 25.73 ± 14.00 | 58.76 ± 13.09 |
4 Discussion
T. urticae is a silk producer and its web confers substantial advantages such as dispersal, colony establishment, mate finding, and protection against natural enemies and climatic conditions. Furthermore, T. urticae presents several aspects of an elementary social organisation such as kin discrimination capability [23,24,31]. Our study aimed to test whether T. urticae could recognise the silk produced from its own population.
A surprising result of this article is that the ability of the silk to attract individuals from their own population has been observed in only one of the two tested populations. Individuals from the green form population were not attracted by their own silk, while contrarily, individuals of the red form population spent more time on the silk laid by conspecifics. Indeed, it is known that T. urticae use the silk as an indicator of the presence of conspecifics [5,32]. This situation might be explained by two non-exclusive hypothesis: differences in the quantity and/or quality of silk or differences in other products (other than the silk) laid by the two populations could cause different behavioural responses in tested mites. Indeed, in T. urticae the silk is used as protection against external aggressions (wind, rain, predators, pesticides) [15–21]. We can therefore imagine that in safe and controlled conditions with no exposure to rain or predators, such as our lab conditions, populations may lose or decrease qualitatively and/or quantitatively their silk production [33].
In 2008, Yano showed that the attraction of T. urticae for silk increased with the quantity of silk laid [5]. Here, our data on tested females show that the green and the red form populations present different locomotor activity patterns on similar set-ups and potentially different patterns of silk production. Indeed, we show that individuals of the green form population move longer and rest less frequently than individuals of the red form population. If the secretion of the silk was continuous during walking [14], the green form population should produce more silk than the red form population as, on average, they spent more time walking. However, it is the silk from the red form population that presents the higher attractiveness. In this case, the difference in attractiveness observed during our experiments should be linked to other properties of the silk (qualitative and/or quantitative). Indeed, for a similar period of time in movement, an individual walking faster should produce more silk than another individual. Time in movement is not the only indicator of locomotor activity that can inform us on the silk production of T. urticae. Testing an individual's locomotor activity in each population and more particularly the speed on set-ups without silk could allow us to quantify the covered distance and therefore to estimate the link between silk production and displacement.
Another hypothesis is that the silk laid by the green form differs qualitatively from the silk laid by the red form and presents physical and/or chemical properties disfavouring displacements. Indeed, we can imagine that, as the secretion of silk is a continuous process [14], a more sedentary mite (red form) might produce thicker silk that is a substrate favouring displacements while a more mobile mite (green form) might produce thinner and stretched silk that complicates displacements. Chemical compounds laid on the silk may also explain why mites prefer to spend more time on the silk laid by the red form. Indeed, the red form population seems to present a higher level of sociality with a higher propensity to live in groups (higher silk production, higher survival rate), than the green form population, which is penalised by group living (lower number of eggs) [32]. One can guess that a more effective chemical communication has been selected in the red form population with a higher quantity or a higher persistence of pheromones on the silk than in the green form population. Isolating these chemical compounds and testing their attraction on several populations could also be an interesting next step for this study.
A second surprising result is that one population of mites is able to transform its environment in such a way that both forms spend more time on its silk. This surprising result, if it is confirmed by other studies, might improve the probability of colony survival. As related and unrelated mites spend longer periods of time on the silk woven by one population, they can reinforce the nest and lay eggs. This might be a strategy to rapidly increase the population of the colony and to rapidly build a protective web. Such a phenomenon has already been observed in social spiders, where the absence of antagonistic behaviour towards non-kin can increase the growth rate of the colony and can increase its probability of survival [22,34]. In spider mites, this ability of the silk to attract individuals from another population has been observed in only one of the two tested populations. Therefore, this strategy cannot be extrapolated to the whole T. urticae species. However, it would be interesting to see if this strategy is common among T. urticae by testing other populations with the same set-up.
Currently, we do not know which properties of the silk (qualitative and/or quantitative) differ as a function of the weaver population. For example, testing an individual's locomotor activity from each population on set-ups without silk should allow us to test whether mite behaviour is modified by their own silk production.
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.
Acknowledgements
We are very grateful to Pr Kaouthar Lebdi who supplied the red form of Tetranychus urticae, which was used in our experiments and to George van Impe for the useful discussions about T. urticae. We would like to thank all the team of the “Laboratoire d’écologie et biogéographie” for their help in data analysis. G.J. Le Goff is supported by a Grant from the FRIA (Fonds pour la formation de la recherche dans industrie et l’agriculture). The authors are also indebted to the National Fund for Scientific Research (FNRS, Belgium) for funding through the Fund for Fundamental and Collective Research (FRFC, convention 2.4622.06). A.C. Mailleux is financially supported by Institut d’encouragement de la recherche scientifique et de l’innovation de Bruxelles (IRSIB). This article is a publication BRC242 of the Biodiversity Research Center (université catholique de Louvain).


