1 Introduction
Reproductive strategies are supposed to be shaped by evolution to increase the fitness of reproducing individuals. It has been suggested that reproductive strategies are constrained by the spatial distribution of resources such as food, mates, appropriate habitat to build a nest, or location of nests in which to lay parasitic eggs. Variation in both brood parasitism and extrapair fertilizations could result from conspecific space use through relatively simple processes [1]. Territorial individuals are more likely to interact with the individuals established on neighboring territories and population density could increase the likelihood to encounter an extrapair mate, or locate a host's nest. Extrapair offspring could be sired by adjacent male neighbors [1–8], and brood parasites could preferentially select immediate neighbors as hosts [9,10]. However, individuals seeking extrapair copulations and nests to parasitize should choose the best option in terms of fitness benefits. Therefore, these individuals should be willing to assume substantial costs under some circumstances, such as traveling long distance or invest time to find extrapair mates or host nests of particularly good quality [11]. A long-term study in the common golden eye also showed that females visit nests to collect information on breeding success and subsequently parasitize the nests with lower predation probabilities [12,13]. In the Blue tit (Parus caeruleus), Kempenaers et al. [3] found that extrapair males were also usually close neighbors, as found in the Hooded warbler (Wilsonia citrina) [2]. On the contrary, female superb fairy-wrens (Malurus cyaneus) and tree swallows (Tachycineta bicolor) have been shown to engage in costly long distance movements to encounter extrapair mates [11,14]. However, experimentally increasing the costs of flight in females changed the spatial distribution (but not the level) of extrapair fertilizations [11]. All these studies demonstrate that the spatial distribution of territories can shape, at least partially, the reproductive decisions.
Reproductive decisions, such as engaging in extrapair copulations and/or brood parasitism, can strongly change the composition of nests. Such a change of nest composition has two major consequences:
- • it can change the strength of the relatedness between parents and offspring, which can change the relationship between the offspring and the social parents;
- • it modifies the relatedness between offspring sharing a nest, which can modify cooperation between offspring (e.g. helping behaviours).
Here we analysed reproductive strategies and genetic mating systems of the Common moorhen Gallinula chloropus, a sexually monomorphic water bird belonging to the rail family. Interestingly, although monogamy is the main social mating system adopted by moorhens within a population, alternative strategies have been observed in high breeding densities, such as intraspecific brood parasitism, polyandrous trios (a female paired with two males; 11% [15]; 9–14% [16]), and communal nesting [9,16]. The latter involves a mother and her daughters (who mated with their fathers) building a communal nest and rearing a single brood altogether [9,16]. In this species, intraspecific brood parasitism was reported in two studies with 4–9% of the offspring being parasitic and 27% of nesting females laying one or more eggs in a neighbor's nest [17,18]. It has been suggested that moorhen brood parasites almost exclusively select immediate neighbors as hosts [9,10]. When investigating brood parasitism with molecular tools (fingerprinting), McRae and Burke [17] found no cases of extrapair paternity. This result is surprising given the apparent flexibility in the moorhen's mating system. Our investigation of a small population of Common moorhens on a microspatial scale aimed to reveal new insights in reproductive strategies by examining the genetic relationship of nest mates and to investigate whether the nest composition is influenced by the microspatial structure, as given/defined by nest proximity and nest position within the population.
2 Material and methods
2.1 Subjects
The Common moorhen is a sexually monomorphic water bird belonging to the rail family. Moorhens are predominantly socially monogamous, with pairs changing from year to year. During winter, they live in large flocks where pair formation occurs before they leave the flock in the spring to establish territories [15]. Females initiate courtship more frequently than males and compete with each other to pair with small males with large fat reserves. Heavy, more competitive females are thus paired with preferred males [19]. Males compete for territories and ensure territory defence [20]. They select nest sites, build the nest, vigorously defend it against intruders and perform most of the incubation. After raising a successful brood, pairs usually raise a second one, partly with help from juveniles of the first brood. The chicks are precocial; they are able to leave the nest on the first day of hatching and begin to feed by themselves soon after hatching. However, during 6 weeks, they remain dependent on their parents for food and often stay another three weeks on their natal territory [9].
2.2 Sampling
Our aim was to perform exhaustive sampling of moorhen chicks on a microspatial scale. During the summer 2005, we collected blood samples from 59 chicks from 15 nests along 5780 m of the Loir river, which had a width between 40 and 50 m in summer time (Fig. 1). Nests in the geographic center were on average 541 m apart, while the mean distance among two nests was 1921 m across all nests. The sampled population is a low-density population situated in the northern region of Angers called the “Basses Vallées Angevines” (47°27′N, 00°32′W, Département de Maine et Loire, France, Fig. 1). The habitat consists of a vast floodplain wetland crossed by the Loir river and many channels connecting residual ponds to the river. This wetland is flooded during winter and changes into pasturelands during summer. This means that nests 3 and 12, despite being located in ponds/small lakes, are well connected to the Loir river. We extensively searched and mapped all nests before hatching and are confident that our sample is exhaustive for the Loir population. In our population, we observed 4 ± 1 egg per nest. Nests were visited every 2 days until the first egg hatched, and were then re-visited daily to sample all chicks. Sampled chicks were marked with a small dot on the beak using a waterproof pen to avoid resampling at later visits of the same nest. Further, we counted how many adults were observed in the vicinity of the nest and never found more than two adults. However, moorhen adults are impossible to distinguish by observations and sexual dimorphism is weakly expressed. We therefore cannot be sure that the two adults we saw in the vicinity of a nest were always the same and of the same sex. Our observations may suggest that social pairs were socially monogamous.
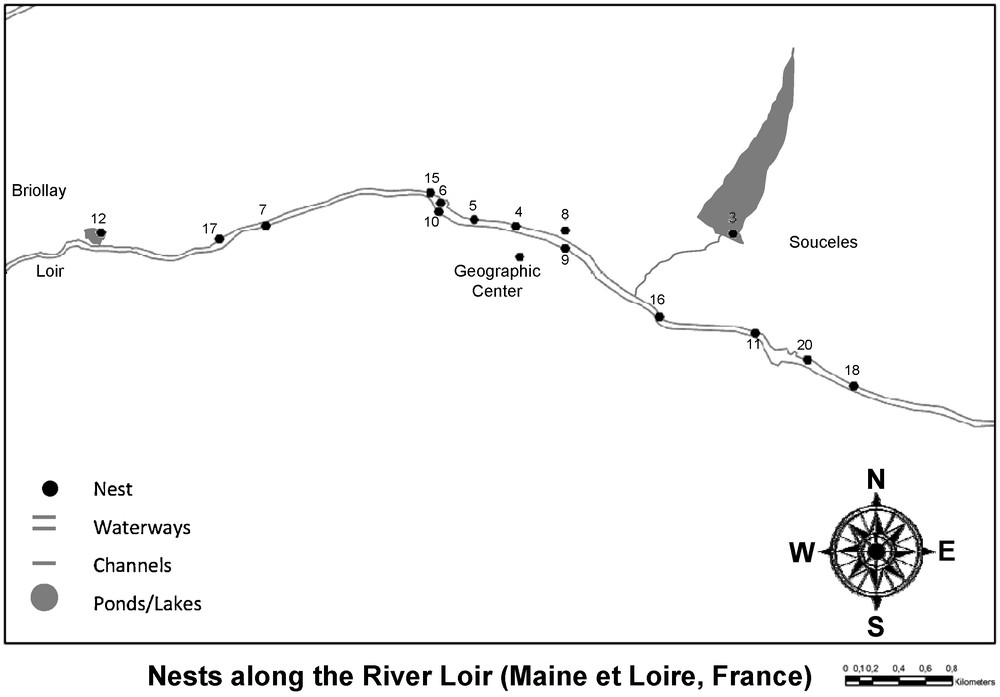
Map of the geographical position of the nests.
To assess the polymorphism of the microsatellites of the loci we used, we also sampled nests from other populations (nests 1, 2, 13, 14, and 19). These populations were along four rivers: Sarthe (47°38′14.01″N, 0°27′47.43″W; 47°38′41.16″N, 0°28′25.48″W), Mayenne (47°32′32.61″N, 0°36′46.05″W), Maine (47°29′46.42″N, 0°32′43.68″W), and Loire (47°25′36.29″N, 0°32′08.62″W; not to be confounded with the Loir river) between 7.9 km and 17.2 km away from the investigated population at the Loir river.
We took blood samples from the right jugular vein and diluted them in 1 mL of PBS containing EDTA (2 mM). The samples were stored at 4 °C until extraction. DNA was extracted using silica columns (QiaQuick 96 Kit, Qiagen) according to the procedure recommended by the manufacturer. After extraction, DNA concentration was measured with a NanoDrop ND-1000 spectrophotometer (peQlab-Biotechnologie) and DNA was subsequently diluted to obtain samples of approximately 50 ng μL-1.
2.3 Microsatellite amplification and polymorphism
We tested the cross-amplification of a set of 29 microsatellite markers by trial and error (Appendix 1 – Supplementary Material). Three of them were isolated in the Tasmanian native hen Gallinula mortierii [21], a species belonging to the same genus as the European moorhen. Therefore, we expected a high probability of successful amplification and polymorphism as these are inversely related to the evolutionary distance between two species [22,23]. We additionally tested 26 microsatellites originally developed for passerines, which were highly polymorphic in the European Coot Fulica atra, another closely related species [24].
One primer of each pair was labeled with a fluorescent dye at the 5′ end. We used a Taq PCR Core Kit (Qiagen) to perform Polymerase chain reactions (PCRs). Each 10 μL volume/sample contained: 1 μL of Qiagen PCR Buffer 10X (TrisCl, KCl, [NH4]2SO4, 15 mM MgCl2, pH 8.7), 0.75 μL of dNTP Mix (10 mM of each), 0.2 μL of each primer at 10 μM, 0.05 μL of Qiagen Taq DNA Polymerase 5U, 1 μL of DNA and H2O. For Pca-8, Tm27 and Tm105 we added 1 μL of MgCl2 (15 mM) and accordingly decreased the amount of H2O. Samples were amplified in a DNA gradient cycler (PTC-200, Peltier Thermal Cycler) according to: 10 min of initial denaturation at 94 °C; 35 cycles of 94 °C for 30 s, corresponding annealing temperature (Ta) for 30 s and 72 °C for 40 s; followed by a final extension of 72 °C for 10 min. 5 μL of the PCR products were controlled under UV light after electrophoresis on a 2.5% TAE-agarose gel stained with ethidium bromide. Microsatellite amplification products were tested for polymorphism on an Abi Prism 3100 Genetic Analyzer (Applied Biosystems) sequencer. We read the runs with GeneMapper v3.7 (Applied Biosystems).
All three Tasmanian hen primers (Tm markers [21]) and two of the 26 passerine primers (8%) successfully amplified and showed consistent interpretable banding patterns and high polymorphism (Appendix 1 and Table 1). The polymorphism of the passerine markers was unexpectedly low compared to the remarkably high polymorphism obtained with the same set of markers in the European Coot [24].
Results from programs Fstat and Microchecker on the loci with interpretable banding patterns. Linkage disequilibrium (LD), null allele frequencies after Chakraborty (Null 1) and Brookfield (Brookfield 1; Null 2), the number of expected (n Ind.het exp) and observed heterozygote individuals (n Ind.het obs), expected (Hexp) and observed heterozygosity (Hobs), Fis, the number of alleles (nalleles), and the number of individuals genotyped at a locus (ngenotyped).
| Locus | LD | Null 1 | Null 2 | n Ind.het obs | n Ind.het exp | H exp | H obs | F is | n alleles |
| Tm105 | No | 0.0829 | 0.0660 | 15.59 | 23 | 0.756 | 0.641 | 0.089 | 13 |
| Mjg1 | No | 0.3492 | 0.2068 | 17.75 | 36 | 0.665 | 0.321 | 0.476 | 9 |
| Tm27 | No | 0.1236 | 0.0934 | 17.28 | 28 | 0.738 | 0.576 | 0.293 | 10 |
| Tm31b | No | 0.0667 | 0.0592 | 4.71 | 10 | 0.914 | 0.823 | 0.056 | 22 |
| Ase50 | No | Na | Na | 0.197 | 0 | 1 | 4 |
The three Tm markers were already used before in a biogeographic study and showed no deviation from Hardy-Weinberg equilibrium. FIS values remained close to zero, indicating a low probability of genotyping errors and null alleles [25]. In our study, we tested for genotyping errors with Fstat v2.9 [26] and Microchecker v2.2.3 [27]. The allele data of the microsatellite loci was checked for linkage disequilibrium using Fstat v2.9 and with the program Microchecker v2.2.3 for genotyping errors resulting from stuttering, or allele drop outs. We did not find any linkage disequilibrium. The results from Microchecker (Table 1) do not support scoring errors due to stuttering, nor large allele drop outs for the Tm-loci, consistent with the one other study using these markers [25]. For the locus Mjg1, however, Microchecker found a shortage of heterozygote genotypes with one allele difference, suggesting genotyping errors due to stuttering. Despite the suggestion of Microchecker, we see a small probability that genotyping errors were made, as the amplification peaks of the electropherograms all showed very similar intensity values and were therefore easily recognizable. The locus Ase50 was not analyzed with Microchecker as it only showed three alleles for our samples. For analyzing reproductive success and strategies in a population, this locus had little value and therefore was excluded from further analyses.
2.4 Offspring assignment within the Loir population
We used the obtained set of 54 alleles from 4 loci (TM27, TM31b, TM105, MJG1, Appendix 1) to assign parental genotypes to the offspring from the Loir river and to calculate heterozygosity per nest. One individual did not amplify at any of the most informative Tm-loci and was discarded from further analysis. For the remaining chicks (n = 58), we attributed a family status (full-sib, half-sib, unrelated) to each individual and determined the most likely parent with the program colony v2 [28] based on our genetic data. Colony determines whether a pair of chicks is full-sibling, half-sibling, or non-sibling, calculates a probability for the suggested relationship, and suggests the most likely parental pair for each individual and per group (here = nest). Colony allows the consideration of genotyping errors, which neither of the other available parentage programs currently permits. Further, the survey of [29] has shown that Colony generally performed better than other programs in small broods, such as ours. Nest's heterozygosity was measured by determining the proportion of loci that were heterozygous and the number of individuals that were heterozygous for each particular locus, or in other terms the number of heterozygotes at a locus divided by the total number of nest mates. Nest heterozygosity was calculated only for nests with two or more offspring (i.e. 12 nests).
Our set of four polymorphic markers with 54 alleles (Tm27, Tm31b, Tm105, and Mjg1, Table 1) is highly informative for our sample size and can be considered well in the mean of parentage assignments in birds based on microsatellite markers [30–35]. We calculated an assignment success score of 91.80% with the program P-Loci v1 [36].
2.5 Statistical analysis
Our aim was to investigate, on a microspatial scale, whether the structure of the genetic network could be explained by the geographic position of the nests in the Loir population. To investigate the importance of the position of the nest, we determined the geographic center of the population (as the barycenter of all nests). All nests were considered equally, as only one type of habitat (floodplain) was encountered and all nests were connected. Moorhens can easily move along rivers and channels by swimming, walking and flying. We then calculated the distance between each nest and the geographic center (beeline). We also calculated the distance between nests (beeline). We used SAS 9.2 [37] to perform Spearman correlations and Mantel tests. We used both sequential Bonferroni corrections for multiple tests [38,39] and FDR controlling procedures [40,41] to assess the significance of the correlations. The two methods provided the same results. We also employed a Mantel test to correlate two matrices (respectively A and B), a matrix of genetic relationships between each possible pair of offspring (A; full-sib = 2, half-sib = 1, not related = 0) and a matrix of distance between nests (B). The value r (AB) was estimated from 10,000 permutations.
3 Results
In the Loir population, the program Colony determined 24 parental genotypes. The majority of the parental genotypes successfully reproduced with two partners, while higher or lower numbers of partners were relatively rare (three times for one, four, five, or six partners; four times for three partners). In 27 cases, a parental pair did only produce one offspring (71%), nine pairs produced two chicks (24%) and two pairs produced four chicks (5%). Most nests had four (33%) or five parents (25%), while only nest 15 and nest ten had respectively two and three parents. Nests 4, 9, and 18 had six to seven parents. The number of parents contributing to a given nest was not correlated to the distance between this nest and the geographic center of the population (rs = −0.490, P = 0.066).
The Mantel test, examining the genetic relationship (full-sib, half-sib, not related) of each offspring pair with the geographic distance between them, showed a significant, while weak relationship (r[AB] = −0.154; P < 0.001). Our correlations of the nest heterozygosity and number of alleles per nest with the distance to the geographic center revealed a significant negative correlation for the nest heterozygosity, increasing with decreasing distance to the geographic center (nest heterozygosity: rs = −0.669; n = 12; P = 0.021, Fig. 2; number of alleles per nest:rs = −0.483; n = 12; P = 0.115).
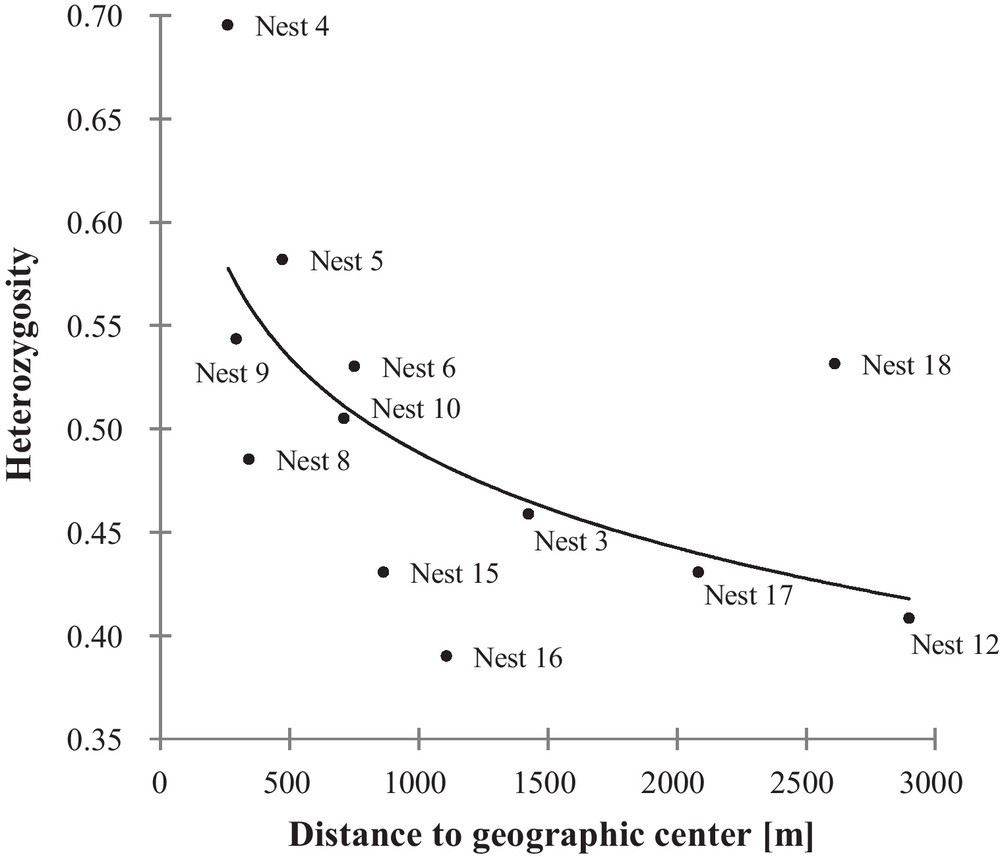
Correlation between the heterozygosity per nest and the distance to the geographic center of the Loir population.
4 Discussion
We used genetic information to investigate mating strategies in a low-density population of the Common moorhen, a socially monogamous water bird. We found a surprisingly low level of genetic monogamy. Our data suggests three main reproductive strategies:
- • a parental pair is monogamous and has chicks in one nest only;
- • parents are monogamous, but females lay eggs in several nests;
- • parents are polygamous, changing nests and partners often.
We found some indication that social interactions of adults increased towards the center of the population. We also found that nest heterozygosity was highest close to the geographic center of the population. Our analysis reveals that in the geographic center of the population the number of parents contributing to the offspring of one nest may increase and nest mates were less related in the population center. As a consequence, we assume that the microspatial structure had an impact on the mating strategies of the moorhen. Within our population, a given chick was on average related with six other chicks (full-sibs and half-sibs), two of them being full-sibs. Full-sibs and half-sibs did not necessarily share the same nest, resulting in low relatedness among offspring in some nests and a low relatedness between the social parents and the offspring.
Our analysis suggests that monogamy with brood in one nest was a rare strategy in the population studied (only found once). On the contrary, monogamy with offspring in several nests, and polygamy where offspring resulted from pairings with several partners and in several nests, were two common strategies, leading to a high frequency of brood parasitism. Our results are consistent with previous findings reporting that between 4 and 9% of eggs [17] and 40% of the nests [42] were parasitic in two high-density populations. That we find a similar pattern in a low-density population suggests that parasitism occurrence may be density-independent in this species, also reported for the Ruddy duck Oxyura jamaicensis [43]. In moorhens, parasitism was suggested to have evolved in response to predation, as laying in many nests may increase the likelihood that one offspring survives to independence [15].
In our population, all social pairs seemed to be socially monogamous (A.L., pers. obs.). We never have observed social trios or nest sharing in contrast to earlier studies [9,16], but the density in our population was low. However, our genetic data suggest that these socially monogamous pairs performed extrapair copulations at high frequency in both sexes. In most of the nests, three or more parents contributed to offspring production. To our knowledge, this is the first evidence of extrapair copulations in the Common moorhen. Our results contrast with previous work by McRae and Burke [17], which may have suffered from a biased sampling effort. The authors stated themselves that they did not intend to assess extrapair copulations, but only parasitism level, and hence did not adopt the necessary sampling design. Altogether, parasitism and extrapair copulations reduce relatedness between parents and offspring and between offspring of a given nest. Uncertainty of relatedness between parents and offspring may explain the prevalence of parental aggression in this species. Moorhen parents are known to reprimand their young by grabbing them at the neck and shaking them vigorously [44,45]. This behavior was explained by the parental willingness to favor small chicks over bigger chicks [46]. However, Shizuka and Lyon [47] observed parental behavior of American coot (Fulica Americana). They first showed that coots are able to recognize conspecific brood parasitic chicks, and then linked parental aggression towards particular chicks (including vigorous pecking and drowning attempts) to brood parasitism. Because parental aggression resulted in lower survival prospects of parasitic chicks, Shizuka and Lyon [47] suggested that parental aggression reduces the costs of brood parasitism by rejecting some parasitic chicks. In the Common moorhen, behavioral observations of parental attacks, in the light of parent–offspring genetic relatedness, would provide an insightful comprehension of parent–offspring interactions in this species.
Helping at the nest is a form of cooperative breeding in which individuals help rearing young which are not their own. In moorhens, the offspring of the first brood, still reproductively immature, remain on their parents’ territories and help their parents to raise subsequent broods [44,48] (A.L. pers. obs.). While such young individuals do not give up breeding opportunities, cooperation is thought to impose a cost on individuals expressing this behavior since they perform egg incubation, feed the young and defend them against conspecifics and predators [44,48]. Explaining the evolution and maintenance of cooperation is still a fundamental challenge in evolutionary biology [49,50]. The best theoretical models are based on genetic relatedness such that individuals are more likely to cooperate with more genetically similar individuals [50]. For example, the Bell miner (Manorina melanophrys) helpers discriminate kin from non-kin based on offspring vocalizations, to better provision the young of closer genetic relatedness [51]. In the moorhen, parasitism and high levels of extrapair copulations are likely to reduce the relatedness between chicks of the first and second broods. Cooperation between offspring is therefore surprising in the moorhen, and it would be of great interest to investigate whether cooperative juveniles are able to discriminate between their sibs and preferentially help more genetically similar sibs, as predicted by the kin-selection theory [52]. This may explain why not all juveniles help and why the amount of help provided by cooperative individuals varies, as observed by Gibbons [53]. Here, a future analysis of the social interactions and the genetic relatedness may shed new light on this from an evolutionary point of view very interesting situation.
We conclude that the mating strategies of the Common moorhen are influenced by the microspatial structure, with nests in the center of a population containing chicks of more than two parents leading to mixed genetic relationships between chicks. Generally, our analysis suggests that the mating strategies of the Common moorhen are highly variable, despite the social monogamy of the species. These mixed strategies result in a high variation of the genetic composition of nests, explaining observed patterns of parent–offspring and offspring–offspring relationships.
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.
Acknowledgements
We would like to thank several of our colleagues for their support, help in the lab and in discussing the content of this article. For that we are indebted to Murielle Richard, Audrey Chaput-Bardy, Nicole Stetefeld, Walter Durka, Simone Lampa, Kristin Schirmer, Klaus Henle, Susanne Zajitschek and Jukka Palo. Financial support was provided by the Helmholtz Centre for Environmental Research-UFZ. M. Richard and the Laboratoire d’écologie kindly provided the 26 passerine primers and J.C. Buchan kindly provided the sequences of the Gallinula primers.


