1 Introduction
Natural selection favours a breeding strategy which, in a given environment, is the most likely to ensure the production of the largest number of young that survive to breed, and the survival of parents until they breed again. The decision to breed is triggered by physiological changes set in motion by changes in the environment [1]. In birds, the timing of reproduction is determined by proximate factors and is often initiated before birds return to their breeding grounds [1,2]. Among these proximate factors, the photoperiod, the temperature, and the availability and the quality of the food required for successful breeding are frequently reported [3–6].
The majority of birds lives in a non-uniform environment (spatially and temporarily) and must breed when the conditions are the most favourable. For instance, in temperate and Polar regions, most birds have to synchronize their breeding schedule with the time of the year that will give their offspring the best chances of survival [7]. This restricted period of time dictated by resource availability leads individuals from the same population to breed synchronously. This breeding synchrony can also be associated with different advantages such as the reduction of the impact of predators and the maximisation of chick survival, by timing fledging when prey abundance is highest [8,9], and/or when climatic conditions are most favourable [10].
Among seabirds, penguins are a distinctive group of flightless, long-lived pelagic seabirds. All the 18 species of penguins are grouped within a single family, the Spheniscidae. Within this family, there are six genera and each genus comprises one to eight species (Table 1). Several subspecies of little Eudyptula minor (six) and gentoo penguins Pygoscelis papua (two) have been identified [11,12] but for the purpose of this review, these penguins are considered as a single species. Some penguin species have been studied in detail, especially the Adélie penguin Pygoscelis adeliae (more than 400 papers, see ISI Web of Knowledge), but others, such as the Snares penguin Eudyptes robustus and erect-crested penguin Eudyptes sclateri have not (less than ten publications, see ISI Web of Knowledge). Consequently, there is less information available for some species. All penguins have a similar body shape and structure. The sexes are outwardly similar in all species, though males are usually heavier, at least at the onset of the breeding season, and larger than females (Table 1). They vary considerably in height, from about 40 to 130 cm, and in body mass, from 1 to 37 kg (little and emperor Aptenodytes forsteri penguins, respectively, Table 1). Such a range in body mass is only surpassed by another flightless family: the Struthioniformes, where the smallest species, the Little spotted kiwi Apteryx owenii, measures 40 cm and weighs 1.2 kg compared to the largest living ratite, the African ostrich Struthio camelus, that stands roughly 3 m tall and weighs 160 kg.
Some biological characteristics of the 18 penguin species.
| Scientific name (abbreviation) Common name |
Breeding range (°S) |
IUCN 2012 status | Height (cm) |
Mean body mass (kg) |
Mate fidelity (%) |
Mean egg mass (g) |
Egg formation (d) |
|||
| Male | Female | A-egg | B-egg | A-egg | B-egg | |||||
| Aptenodytes forsteri (Af) Emperor penguin |
66–78 [11,12] |
NT | 100–130 [11,12] |
37.3 [15,16] |
28.8 [15,16] |
15 [31] |
445 [16,36–38] |
21 | ||
| Aptenodytes patagonicus (Ap) King penguin |
45–55 [11,12] |
LC | 85–95 [11,12] |
14.5 [17,18] |
12.9 [17,18] |
29 [17] |
307 [11,17,39] |
20 | ||
| Eudyptes chrysocome (Ec) Northern rockhopper penguin |
37–53 [12] |
VU | 45–58 [11,12] |
3.4 [19,20] |
3.1 [19,20] |
59 [12] |
84 [19,40–43] |
113 [19,40–43] |
16 | 20 |
| Eudyptes chrysolophus (Ech) Macaroni penguin |
46–65 [12] |
VU | 71 [11,12] |
5.0 [12] |
5.2 [12] |
75 [32,33] |
93 [41,44,45] |
149 [41,44,45] |
16 | 21 |
| Eudyptes moseleyi (Em) Southern rockhopper penguin |
37–40 [13] |
EN | 45–58 [11,12] |
3.4 [19,20] |
3.1 [19,20] |
59 [12] |
84 [19,40–43] |
113 [19,40–43] |
16 | 20 |
| Eudyptes pachyrhynchus (Ep) Crested or fiorland penguin |
43–48 [11] |
VU | 55 [11,12] |
4.3 [21,22] |
3.8 [21,22] |
Long [11] |
100 [22,46] |
118 [22,46] |
16 | 21 |
| Eudyptes robustus (Er) Snares penguin |
48 [11,12] |
VU | 51–61 [11,12] |
2.6 [23] |
2.5 [23] |
Long [23] |
90 [23] |
117 [23] |
16 | 21 |
| Eudyptes schlegeli (Es) Royal penguin |
54 [11] |
VU | 65–75 [11,12] |
5.7 [24] |
5.3 [24] |
Long [11,12] |
100 [11] |
159 [11] |
16 | 21 |
| Eudyptes sclateri (Esc) Erect-crested penguin |
47–49 [12] |
EN | 67 [11,12] |
6.4 [25] |
5.4 [25] |
85 [47–49] |
151 [47–49] |
16 | 21 | |
| Eudyptula minor (Em) Little blue penguin |
32–47 [11] |
LC | 40–45 [11,12] |
1.2 [11,26] |
1.0 [11,26] |
82 [11,12] |
54 [11] |
53 [11] |
15 | 19 |
| Megadyptes antipodes (Ma) Yellow-eyed penguin |
43–52 [11] |
EN | 66–78 [11,12] |
5.3 [27] |
4.9 [27] |
33 [34] |
138 [34] |
137 [34] |
17 | 21 |
| Pygoscelis adeliae (Pa) Adélie penguin |
54–77 [12] |
NT | 70 [11,12] |
5.6 [28,29] |
4.9 [28,29] |
80 [11,29,35] |
122 [50–52] |
114 [50–52] |
17 | 21 |
| Pygoscelis antarctica (Pan) Chinstrap penguin |
54–64 [12] |
LC | 71–76 [11,12] |
5.0 [11,29] |
4.7 [11,29] |
83 [11,12,29] |
114 [53] |
112 [53] |
16 | 20 |
| Pygoscelis papua (Pp) Gentoo penguin |
46–65 [11,12] |
NT | 75–90 [11,12] |
5.6 [12] |
5.2 [12] |
85 [11,12,29,33] |
129 [43,44,54,55] |
129 [43,44,54,55] |
17 | 21 |
| Spheniscus demersus (Sd) Jackass or African penguin |
24–34 [14] |
EN | 70 [12] |
3.1 [12] |
2.8 [12] |
62 [12] |
107 [56] |
105 [56] |
16 | 20 |
| Spheniscus humboldti (Sh) Humboldt penguin |
5–42 [12] |
VU | 65 [12] |
5.0 [12] |
4.2 [12] |
122 [12] |
122 [12] |
17 | 21 | |
| Spheniscus magellanicus (Sm) Magellanic penguin |
29–54 [12] |
NT | 70 [11,12] |
4.9 [30] |
4.6 [30] |
125 [30,57] |
125 [30,57] |
17 | 21 | |
| Spheniscus mendiculus (Sme) Galápagos penguin |
0–2 [12] |
EN | 53 [12] |
2.1 [12] |
1.9 [12] |
89 [12] |
Penguins are highly adapted for marine life and some species spend as little as 20% of the year on land (Fig. 1A). Nevertheless, this relatively short period on land represents one of the most important parts of their life cycle; during which penguins have to obtain a nest-site and mate, lay egg(s), rear chick(s), and moult. The 18 penguins’ species share some breeding characteristics such as breeding synchrony (except for the Galápagos penguins Spheniscus mendiculus, [72]) for annual reproduction on land, and alternation of sojourns on land and at sea between partners during the chick-rearing period. An important characteristic of all penguin species is their ability to withstand prolonged period of fasting, on land or sea-ice, during breeding. With the exception of the yellow-eyed penguin Megadyptes antipodes, all penguin species breed colonially [73]. In these colonies, birds often breed in high densities, for instance, 1.4 nest.m−2 in Adélie penguins [74] and up to 10 birds.m−2 in huddling emperor penguins [16,75], which highly increases the level of social stimulations and interactions [76]. High densities of breeding birds have led to the evolution of a varied and complex repertoire of visual and vocal displays (e.g. [77]). As penguins are widely distributed in the Southern hemisphere, from the Equator to the Antarctic continent, they are submitted to various ecological constraints that will impact the timing of reproduction. For example, for a high latitude species such as the emperor penguin, two proximate factors controlling the onset of breeding are exogenous factors such as day-length [1,78] and the peak of ocean productivity preceding breeding [79]. For the equatorial Galápagos penguin, the onset of breeding is closely linked to mean sea surface temperature [70]. Therefore, because of the variety of environments where penguins live, the examination of the breeding cycle observed across the Sphenisciformes order can give some insights about how environmental conditions can shape a variety of reproductive strategies. The different aspects of the biology of penguins are relatively sparse in the literature although some books attempted to gather these data (e.g., [11,12,80,81]). Therefore, the aim of the present study is to provide researchers working on seabirds with a concise and clear overview of the breeding cycle of the 18 penguin species.

Breeding cycle of the penguins. White, dark and dashed bars denote for fasting on land, foraging at sea and feeding, chick rearing, respectively. Lettering stands for species (Table 1). A. Breeding cycle of the 18 penguin species and for both sexes (M stands for males and F for females). B. Relative breeding cycle of the 18 penguin species. Mean courtship period is defined as the shortest time between pair formation and the laying of the first egg. Breeding cycle is defined as the time elapsed from the arrival of the penguins on their breeding site to the fledging of the chick(s). Masquer
Breeding cycle of the penguins. White, dark and dashed bars denote for fasting on land, foraging at sea and feeding, chick rearing, respectively. Lettering stands for species (Table 1). A. Breeding cycle of the 18 penguin species and for both sexes ... Lire la suite
References for each species and periods.
In this review, the breeding cycle of penguins is divided into three stages:
- • the pairing period, when breeders come ashore for courtship and mating;
- • the incubation period, when mates generally take turns to incubate the egg(s);
- • the rearing period, from chick(s) guard to fledging.
We defined the courtship period as the shortest period of time between the early arrival of both sexes on the reproductive site and the laying of the first egg because females may occasionally go to sea before the full clutch is completed (e.g. gentoo penguins, [11], magellanic penguins Spheniscus magellanicus [82]). The body mass of the adult birds considered was the mean body mass at the onset of the breeding period, i.e. at their arrival on the breeding site.
2 Geographical range and population status
Penguins are widely distributed in the Southern hemisphere, mainly between 45 and 60°S (Fig. 2) and four penguin species are endemic to their breeding island: Fiordland (or crested) Eudyptes pachyrhynchus, Galápagos, royal Eudyptes schlegeli and Snares penguins. Penguins represent roughly 90% of bird biomass in the Southern Ocean [12] and Pygoscelis species (chinstrap P. antarctica, Adélie and gentoo penguins) represent 70% of Antarctic avian biomass [83]. Penguins feed in pelagic cold waters, rich in zooplankton and biomass, where they consume approximately two millions tons of carbon per year [84]. However, they occupy a wide variety of habitats while breeding on land, ranging from the burrows in the volcanic rocks of the hot Galápagos Islands (Galápagos penguin), the bushes of south Australia and New-Zealand (little penguin), to the ice of the border of the Antarctic continent (emperor, Adélie, chinstrap and gentoo penguins). This results in a high variability in all aspects of the breeding biology of the different penguins’ species (Fig. 1A). While most species breed annually, some species, such as African (or jackass) and Galápagos penguins, can have no distinct annual breeding season (i.e. breeding season occurs year-round). Because of the short Antarctic summer, breeding is generally much synchronised in Antarctic species, but in temperate climates, it can be spread over a longer period of time. For example, the timing of egg-laying greatly varies between years for the little penguin [85].
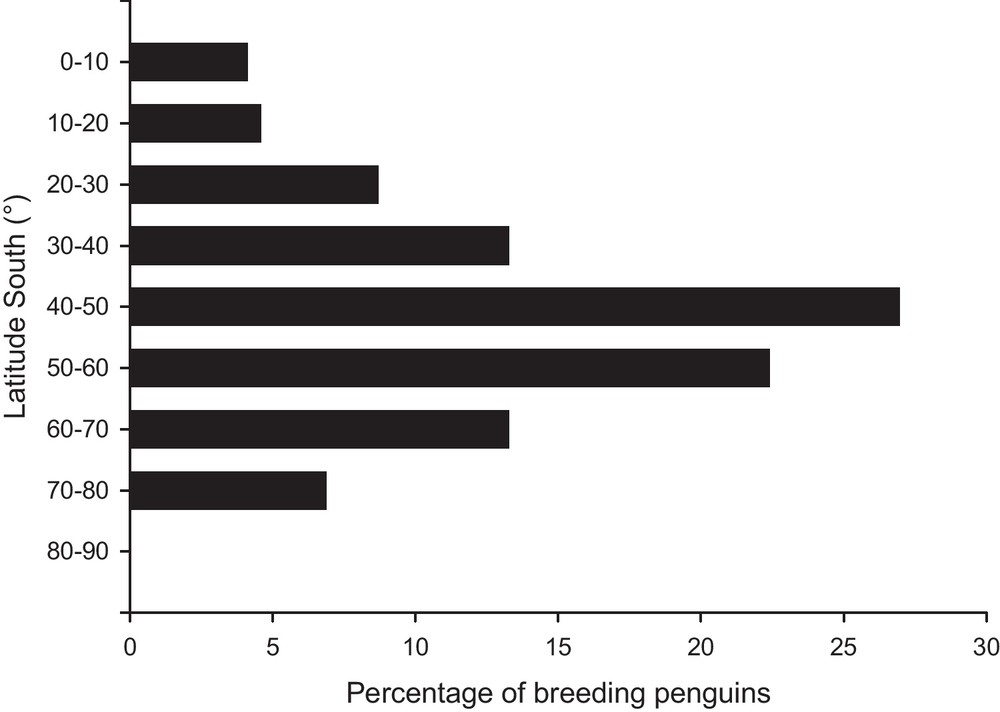
Repartition of the 18 species of breeding penguins (%) according to the Southern latitudinal gradient.
Penguins exclusively rely on marine food sources that are spatially and temporarily unpredictable. Penguins make use of wide geographical areas in the ocean while foraging and during migrations. Penguins, which are central place foragers during the breeding season, are thus particularly sensitive to variations in ecosystem structure and processes. Of the 18 penguins’ species, 13 are considered endangered or threatened (Table 1) and some species are now at their lowest recorded populations: Galápagos, yellow-eyed, and Fiordland have less than 3,000 pairs; Humboldt (Spheniscus humboldti), Snares and African have less than 30,000 pairs. Even abundant species like the macaroni (Eudyptes chrysolophus) and the rockhopper (Eudyptes chrysocome and E. moseleyi) are in steep decline [86]. On the contrary, the global population trend is stable for Snares and Adélie penguins [86] or is increasing for gentoo [87,88] and king penguins [86]. The status of the emperor penguin might change in the near future. Indeed, thanks to satellite images showing faecal stains on ice, Fretwell and Trathan [89] discovered ten new emperor penguins colonies [90]. Around 80% of the threatened species live on islands, increasing their vulnerability to threats such as introduced predators. Many penguin species face the same four key threats: global climate change, marine pollution, fisheries mismanagement, and introduced mammalian predators [91].
3 Relative importance of each phase of the breeding cycle: focus on pairing period
Among the 18 penguin species, the duration of the breeding season ranges from 4 to 15 months (Fig. 1A). It is especially long for the two largest penguins (who need more time for their chick to grow) and particularly for the king penguin Aptenodytes patagonicus. Emperor penguins’ cycle lasts 9 months including a 1.5-month pairing period that is long relative to other penguin species (Fig. 1A; [79]). Indeed, while the courtship period ranges from less than 1 week in little, gentoo, Jackass or African Spheniscus demersus and magellanic penguins, it lasts up to 6 weeks in emperor penguins (Fig. 1A). The ratio courtship-breeding duration accounts for 16% in emperor penguin (1.5 month vs. 9 months) whereas it accounts for only 3% in its closest relative, the king penguin (1.5 week vs. 15 months). Considering the long duration of the total breeding cycle in emperor penguins, this ratio is, however, almost of the same magnitude (14–18%) as with other species breeding in Antarctica (Adélie, chinstrap and gentoo penguins; Fig. 1B). Contrary to the emperor penguin, the king penguin does not exhibit a long courtship period but an extended chick-rearing period (84% of the breeding cycle while it represents about 57% in other species; Fig. 1B). In king penguins, breeding overlaps two years (Fig. 1A) which consequently results in a maximum of two chicks being reared within a three year period.
4 Fidelity, sex ratio, and courtship
All penguins are monogamous, mating with only a single partner each year. In addition, most penguin species are territorial and show a moderate to high inter-annual fidelity in breeding (59–89%; Table 1), partners reuniting from year to year on their nest site. Pair bonds can therefore be long-lasting (Table 1) with many birds returning to meet their previous partners at the same breeding site each year. Two Aptenodytes species and especially the emperor penguin, however, show a low inter-annual fidelity (15%; Table 1), due to the fact that they do not use a nest, and incubate their egg on their feet [92,93]. Partners cannot therefore reunite themselves from year to year on the nest site and one could hypothesize that these penguins need more time than other species to find and reunite with their previous mate. King penguins, however, show a low inter-annual fidelity (29%; Table 1) despite being highly territorial and occupying distinct nest sites. These birds could easily reunite with their partners provided they return on time but it does not seem to be the case for 70% of them.
A male-biased sex ratio appears to be a characteristic of breeding populations of several penguin species such as yellow-eyed, gentoo, little, Adélie, macaroni penguins [11]. In Adélie and macaroni penguins, males return first [11] to previous year's nest-site to maximize their chances of reuniting with their mates from the previous year. This suggests that the primary “aim” of male penguins returning to the breeding colony would be to retain their old nest-sites and, only secondarily, to reunite with their previous mates. Because in many species there are more males in the population than females, male-male competition for nest-sites, rather than for mates, is an important determinant of breeding opportunity. In contrast, at least for the Pointe Géologie colony (Adélie Land, 140°E-67°S), sex ratio favours female emperor penguins that outnumber males by about 10% [31,92–94]. This might be due to a higher mortality of male breeders after their long winter fast [95]. Because of this unbalanced sex ratio and because mate fidelity is particularly low in this species, competition between females is high to find a male and the earlier a female returns at the onset of the breeding cycle, the higher the probability she will get a mate [31], and the lower the probability that her previous mate will already be paired with another female. Furthermore, the number of male partners available per unpaired female decreases as time passes. Consequently, an early arrival enhances the likelihood for the females to preserve their breeding status by finding an unpaired mate. Unbalanced sex ratio in emperor penguins probably also explains occurrence of polygynous trios (one male with two females) but these groups are temporary, one female usually ejecting the other after a few hours [16,31].
5 From yolk development to fledging
The period of yolk development that precedes ovulation is given by the following equation for non-procellariiform seabirds: log t = 0.396 + 0.283 log egg mass [96] where t stands for time (day) and egg mass is expressed in grams. Once the yolk is fully developed, it is retained within the ovarian follicle for about 6 days before ovulation [97]. The albumen and shell are then added over about 24 hours, following ovulation. In species with two eggs [98–101], development of the second egg (B-egg) is initiated 4 days after the first one (A-egg). Thus, in order to calculate the time elapsed from yolk formation until egg-laying, we added 7 (6+1) and 11 days (4+6+1) for the A-egg and the B-egg, respectively (Table 1).
Incubation refers to the process by which birds lay their egg(s), and to the development of the embryo within the egg. The most vital factors of incubation are the temperature required for embryo development over a specific period, humidity (which could be problematic in dry environments like the Galápagos Islands or Antarctica), and egg rotation rate [102]. If the air is too dry, the egg will lose too much water, which can make hatching difficult or impossible. As incubation progresses, an avian egg becomes lighter, and the air space within the egg becomes larger owing to the evaporation from the egg. In most penguin species, incubation is divided differently between parents with the male and the female taking turns incubating the egg(s). The emperor penguin represents an exception as in this species, only the male incubates.
Compared with other birds, penguins lay small eggs (and small egg clutches) relative to their body weight (2.9 ± 0.9%, n = 16, mean ± SD, Table 1), and eggs of smaller penguin species are proportionately larger (Table 1). The majority of penguins lays two white eggs (except genus Aptenodytes laying only one egg), weighing from 55 to 445 g (little and emperor penguins, respectively, Table 1). Within the genus Eudyptes, the second egg or B-egg is 20 to 78% larger than the first one (Table 1) and consequently the first chick is smaller than the second one and generally fails to survive [11]. Erect-crested penguins are obligate brood reducers [46] and exhibit the most extreme egg-size dimorphism of any bird: the second egg is up to 100% the size of the first and is the only one to be incubated. It remains unclear why these birds should lay two eggs but only ever rear one chick. The total time necessary for yolk deposition is proportional to female's body mass [97] but full clutch synthesis has the same duration among the 18 penguin species, ca. 3 weeks (Table 1; 20.6 ± 0.7 days, n = 16, mean ± SD). By subtracting the duration of the full clutch completion from the duration of the pairing period, we can report that egg formation begins while females are still at sea (in 13 species), while they arrive at the breeding site (in one species), and while they already are on the breeding site (in three species; Fig. 3). Among the latter, emperor penguins’ egg formation begins late, three weeks after their arrival on the colony. Thus, egg formation is not responsible for the long duration of the pairing period, representing only one half of the courtship duration.

Time elapsed from arrival at the breeding site (dashed line) and onset of yolk formation for the 17 penguin species among the 18 ones. Lettering stands for penguin species (Table 1).
Incubation ranges from ca. 35 to 65 days (Eudyptes and Pygoscelis species, emperor penguins, respectively, Fig. 1B). It is an energetically demanding process, especially in male emperor penguins that can lose up to 45% of their initial body weight during this time. Some species begin incubation with the first egg, causing the young to hatch asynchronously; others begin after laying the second egg, thus decreasing hatching asynchrony between siblings [103].
Both parents are involved in parental care. From the moment the egg is hatched, one parent cares for the newly hatched chick(s) while the other forages for food. Penguin chicks are semi-altricial, i.e. they need parental care (food, warmth, protection) before becoming independent. At the start of the rearing period, the chicks either sit on their parents feet (emperor and king penguins) or under their bellies, to be kept warm and dry. Young are guarded (guarding stage) by both parents for varying periods of time before forming crèches. Both parents feed the chicks by regurgitation. Nestlings beg for food by pecking adult's bill and/or by singing. Guarding stage may be affected by environmental conditions [102] and when they are not being protected by the adult, the chicks form crèches to keep warm and stay protected. As the chicks grow, their feeding requirements quickly increase, making it difficult for just one of the parents to obtain enough food. Eventually, the chicks are large enough so that both parents can go to sea to gather food for their chick simultaneously. When parental provisioning is low, alloparental feeding (feeding of offspring by adults other than their own parents) sometimes occurred in little [104], emperor [105], king [106] and Adélie penguins [74]. In most species, the chicks gather together in crèches to provide protection both from predators and from the elements. In some species, such as king penguins, crèches can be large with many hundreds of chicks tightly packed together. In other species, such as African penguins, the crèches are smaller (with up to ten chicks coming together) and less dense. Age at fledging ranges from one to eleven months (Fig. 1A) and fledglings are a bit smaller and lighter than adults, except in emperor penguins for which body mass of fledglings is about half of adults’ [16]. While they are still on their breeding grounds, chicks have to moult to adorn themselves with a waterproof plumage, which will allow them in turn to go hunting offshore and acquire their food independently. Adults also have to moult like other birds and all penguins replace their feathers each year on their breeding grounds, except for the emperor penguins for which moulting often takes place far away from their breeding grounds [107–109]. Although other birds lose some feathers individually at a time and replace them over a few months, penguin moulting is fast, taking only two (e.g., Pygoscelis antarctica, [110]) to five weeks (e.g., genus Aptenodytes, [16,111,112]). Moulting is extremely important to penguins as they need to maintain their feathers in perfect condition at all times to insulate their body from environmental conditions at sea or on land. Adults generally moult after the breeding season. Once their chicks have moulted into their own juvenile plumage the adults return to sea for a few weeks to build up their own fat reserves and then come back ashore to moult. During the moult, penguins are no longer waterproof and cannot enter the sea, they can lose up to 50% of their body mass, they are not well insulated and they are vulnerable to predation. Therefore, this is a critical period of time for penguins during which they have to face the elements and starve until their new set of feathers is ready.
6 Genus Aptenodytes
Birds of the genus Aptenodytes (A. forsteri and A. patagonicus) are bigger and taller than other penguin species (Table 1). They do not build a nest and only lay one large egg (on average 445 and 307 g, respectively; Table 1), which is kept on the top of the incubating parents’ feet at least for 54 days (Fig. 1B). As for other penguins, parents recognise their chick by voice and young also recognise parents by call [11].
The breeding cycle of the genus Aptenodytes largely differs from the breeding cycle of most of the other penguin species. In most penguin species, it takes from 8 to 15 weeks to raise a chick to the juvenile stage (Fig. 1B) but it can take 10 to 13 months for king penguins to fledge their chick [18]. Because of this long chick-rearing period, king penguins only produce two chicks every three years. As a result, 12-month-old chicks cohabit with incubating birds in king penguin colonies. Every year, emperor penguins manage to raise their large chicks more quickly (five months), using a different strategy. Indeed the chicks moult into juvenile plumage while they are still much smaller than their parents. The juveniles then continue to grow out at sea.
The emperor penguin also seems to be an exception among penguins as they begin their breeding cycle when other Antarctic birds have finished theirs. Each year, from late December to March (i.e. late summer), emperor penguins disperse into the oceans, travelling and foraging into the waters surrounding the Antarctic continent [109,113]. In March-April, as winter approaches and fast-ice extent grows, all mature emperor penguins move south towards their colonial breeding areas at the border of the Antarctic continent. The breeding cycle of emperors stands in contrast with that of other penguin species (except for the king penguin) by its long duration and by the fact that it takes place in the midst of the severe Antarctic winter, whereas other penguins breed during the short and milder summer season. Indeed, the emperor penguin is one of the few birds for which gonadal growth is coincident with short days, when birds are still at sea. Gonadal steroids are several fold above basal level at the time of arrival on the breeding area suggesting that environmental cues, especially decreasing daylength, decreasing air temperature, and sea-ice formation, stimulate gonadal development and reproduction [1]. Before breeding, emperor penguins forage far away from their breeding grounds [109,114,115]. In the Southern Ocean, a period of high productivity occurs during summer, from October to April, and is followed by a period of low productivity during winter, from May to September [116–118]. Because emperor penguins breed in winter, they have to anticipate their breeding season by accumulating body reserves during high ocean productivity in the previous summer [79]. To our knowledge, this breeding strategy is unique among animals. Furthermore, emperors breed on sea-ice in a few favourable zones that may be a hundred kilometres distant from the open sea or polynias where they exclusively feed [119]. As a consequence of the distance from the feeding grounds, and because breeding activity competes with feeding, female and male emperors fast for as long as 1.5 and 4 months, respectively [16,31]. For females, the breeding fast comprises only the courtship period, since they leave their single egg to their mate as soon as it is laid and then go back to sea for building up their reserves. For males, the period of fasting includes the courtship and the whole incubation period. To face the austral winter, emperor penguins have to exploit in an optimal way their limited body fuels in order to succeed in their breeding [16,120,121]. This is possible only thanks to their huddling behaviour, which allows them to decrease energy expenditure [120,121].
7 Which environmental changes might affect the breeding cycle of the different penguin species?
The Earth's climate is undergoing rapid warming, which is driving shifts in the distribution and phenology of many plants and animals [122,123]. Among animals, penguins are adapted to live in extreme environments (Fig. 2), but, because each species is restricted to a limited latitudinal range (Table 1), they can be highly sensitive to climate change [124]. Environmental changes are not uniform across regions, with resource increasing in the subantarctic areas and decreasing in Antarctica [123]. Quantifying changes in breeding phenology is important for understanding how populations respond to these changes, especially those resulting from human activities [123].
7.1 Climatic changes and resource availability
Detecting and predicting how populations respond to environmental variability are crucial challenges in management and conservation research. This is particularly true for populations at high latitudes, many of which demonstrate changes in population dynamics associated with global warming [125]. Some seabird populations of the Southern Ocean have been responding to climate change for the last three decades and demographic models suggest that projected warming will cause dramatic population changes over the next century [114]. In the Antarctic ecosystem, population dynamics of top predators like penguins may yield important information about how the environment is changing [126]. The phenotypic plasticity of penguins may allow them to continue to exploit their transformed ecological niche and maintain their current distributional ranges. For instance, penguins may vary the timing of breeding in response to changes in environmental conditions [127]. However, palaeoecological records suggest that penguins are more likely to respond by dispersal rather than adaptation [124]. Thus shift in species distribution is likely to be one of the major possible adaptations to changing environmental conditions [114]. This is exemplified by the distributional range of chinstrap [124,126,128], gentoo and Adélie penguins [124,129,130] that has shifted southwards around the Antarctic Peninsula.
However, as each species is limited to a specific latitudinal range, a latitudinal shift may be very limited. Thus, emperor penguins’ colonies north of 70°S are projected to decrease or disappear, and limited growth might occur south of 73°S [131]. These population trends are likely to be related to sea ice conditions [132]. For example, at Pointe Géologie (Adélie Land), distance to the fast-ice edge and its extent are major determinants of emperor penguin breeding success [132]. Therefore, the increased frequency of warm events associated with projected decreases in sea ice extent is likely to reduce population viability [133,134].
Other physical factors than sea-ice can also affect penguin populations. For instance, sea surface temperature consistently drives the foraging behaviour of king penguins, and, according to climate models, the projected warming of surface waters could lead to a gradual southward shift of their more profitable foraging zones [114]. Such a shift would negatively affect the king penguin population, unless penguins develop alternative foraging strategies [114] as to modify their timing of breeding [127].
The Antarctic Peninsula is among the fastest-warming areas on the Earth, with 5–6 °C increases in mean winter air temperatures and associated decreases in winter sea-ice cover [135]. These perturbations have affected the ecosystem profoundly [135]. To respond to these climatic changes, varying the timing of reproduction in response to local environmental conditions is a key factor influencing reproductive success [127]. For example, clutch initiation and hatching dates of royal, Adélie and gentoo penguins occur earlier with warmer temperatures [123,127]. However, these behavioural adjustments may not be sufficient to prevent populations from declining. The “sea-ice hypothesis” proposing that ice-obligate species directly decline because of sea-ice reduction, does not appear to be sufficient to explain why populations of both ice-loving and ice-avoiding penguins have declined significantly [135]. Some researchers argue in favour of an alternative, more robust hypothesis, that attributes both increases and decreases in penguin populations to changes in the abundance of their main prey, Antarctic krill [135]. Indeed, decline of chinstrap penguin populations has been suggested as being related to climate change through a reduction in sea-ice extent during winter and a consequent decline in the abundance of krill in summer during the breeding season [126].
Climate changes can also have more subtle consequences on the foraging behaviour of penguins. For instance, mixed water regimes resulting from storms, result in the dispersion of prey items in the water column. This lack of prey stratification has been described as resulting in reduced foraging efficiency and poor breeding success in the little penguin [136]. Mixed water regimes are currently unusual during the breeding period of little penguins, but are expected to become more frequent due to climate change and may therefore represent an important threat for this species [136].
7.2 Tourism
Antarctica now fuels one of the fastest growing tourism markets in the world with over 30,000 visitors annually travelling to the continent [137]. Increasing ecotourism activity has led to concerns about the effects of ecotourism on wildlife populations. While some species of penguins habituate to human visits, others exhibit negative effects due to disturbance [138]. Behavioural, physiological, and reproductive parameters might thereby be affected. For example, human presence at the nest site is physiologically stressful for breeding Magellanic penguins that are not accustomed to seeing humans [139]. Indeed, Magellanic penguins in visited areas have higher corticosterone stress responses than penguins in undisturbed areas [138,140]. Moreover, birds exposed to moderate levels of disturbance do not show evidence of habituation over a period of a few years [139]. However, penguins may habituate to Humans, as birds that have been exposed to very high levels of human visitation do not respond anymore to human presence as a stressor. Furthermore, Magellanic chicks from tourist-visited colonies do not flee anymore when approached by humans [140], and breeding success is not affected by visitation levels in this species [138]. However, penguin species differ in their sensitivity to human presence. For instance, in contrast to Magellanic and Adélie penguins, yellow-eyed and gentoo penguins show significantly lower breeding success at sites exposed to unregulated tourism compared to areas visited infrequently [130,141]. This may be attributed to the presence of people on beaches that delays post-foraging landing by penguins provisioning their chicks, which may in turn affect the amount of food delivered to the young. Indeed, yellow-eyed chicks from nesting areas with high numbers of tourists have significantly lower fledging weights than chicks from areas with no tourist visitors [142]. Taking into account that the probability of survival is positively associated with mass at fledging, lower fledging weights may have long-term population consequences [142].
8 Conclusion
The present article shows that breeding strategies are diverse and differ between penguin species. However, breeding behaviour can also exhibit some plasticity within each penguin species and particularly when environmental conditions vary (e.g. [102]). More studies simultaneously conducted (1) in several penguin species breeding in the same location and (2) on the same species in different locations/environmental conditions would be useful to highlight how environmental conditions influence breeding strategies in penguins and how penguins can adapt to environmental perturbations.
Many penguin species face the same threats [91]. Marine and coastal ecosystems are undergoing unprecedented alterations in their processes and structure. Penguins are sensitive species impacted by these phenomena. As top predators, they are key constituents of marine ecosystems, and are indicators of the oceanic and coastal ecosystem health. Larger scale ecosystem-based conservation planning and more focused local efforts are needed for the successful conservation of many penguin species.
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.
Acknowledgements
We thank Drs C.-A. Bost, A. Chiaradia, P. Dee Boersma, C. Hull, M. Massaro, K. Pütz, Y. Ropert-Coudert, A. Steinfurth and P. Trathan for providing data on the different species of penguins. We also thank Dr. S. Gallon for her help in revising the language. The manuscript benefited from critical comments by anonymous reviewers.


