1 Introduction
Boswellia papyrifera and Boswellia carterii, commonly known as Arabian incense, are used in the medicine of Ayurveda for curing arthritics [1,2]. Their oil has long been used in perfume industries. Nowadays, their main use occurs in religious ceremonies in many parts of the countries, especially in Middle East regions. Further studies also suggest that boswellic acids exert significant anticancer, antimicrobial and immune-potentiating effects [3,4]. Chemical properties of these plants were well characterized; they proved to contain isoincensole and incensole acetate as their main diterpenic components. These plants belong to a single family, Burseraceae [5].
Apart from its therapeutic values, it causes severe pulmonary changes and decreases the ability of lungs function in exposed animals [6,7]. In recent studies, the toxic effect of Arabian incense on rat liver has proven to significantly decrease the levels of alkaline phosphatase (ALP), alanine aminotransferase (ALT), and aspartate aminotransferase (AST). It also decreased the levels of glutathione (GSH), superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx). However, incense smoke significantly increased lipid peroxidation [8].
Long-term exposure to incense smoke was associated with decreased weight and adverse metabolic changes, i.e. increased triglycerides and decreased HDL-cholesterol concentrations. Exposure to incense was also associated with a transient increase of leptin levels [9]. Studies on B. papyrifera and B. carterii's effect on rat testis evidenced that it also severely affects seminiferous tubules, interstitial cells, and sperm kinetics [10]. Taken all together, these data suggest that incense smoke influences metabolism adversely in rats.
A number of studies have been reported on the pathological and pharmacological effects of these plants on lung, liver, as well as on testis. However, information related to the effects of incense smoke exposure on cauda epididymis and fructose levels is limited. Therefore, we aimed to explore the potential toxic effects of incense exposure on rat cauda epididymal cell types, fructose levels, and to carry out a morphometric analysis.
2 Materials and methods
2.1 Animal stock and test material
Wistar male Albino rats (Rattus norvegicus), aged 7–8 weeks and weighing 210–220 g, were obtained from the Experimental Animal Care Center, King Saud University. They were housed at a temperature of 23 ± 2 °C, exposed for 12–13 h to daylight and fed on a standard rat diet and water ad libitum. The Ethics Committee of King Saud University approved all experiments. B. papyrifera and B. carterii were collected from the Agar wood factory; the stem bark was crushed into small pieces suitably fit to produce smoke.
2.2 Experimental design
After a two-week acclimatization period, rats were randomly divided into three groups, each one consisting of 11 animals. Group I served as control and was kept in fresh air. Group II and Group III were exposed to a B. papyrifera and B. carterii daily dose of ∼ 4 g/kg of body weight for 120 days in especially designed smoking chambers [10,11]. The rats were exposed daily to the smoke emanating from the burning of 4 g of each incense material for 4 months. Smoke exposure durations lasted for 30–40 min/day. The choice of the dose and the duration of incense exposure in this study was based on optimized conditions from our previous studies [8,9]. At the end of the exposure duration, animals were killed by cervical dislocation.
2.3 Measurement of fructose levels
Fructose levels of epididymal fluid and prostate gland were measured by Bauer et al. [12]; briefly, 0.1 mL of an epididymal fluid and prostate gland mixture was diluted in 2.9 mL of distilled water, to which 0.5 mL of 0.3 N barium hydroxide (95 mg barium hydroxide in 2 L of distilled water) were added. After thorough mixing and addition of 0.5 mL of 5.0% (0.175 M) zinc sulfate solution, the mixture was allowed to stand for a few minutes and was centrifuged at 5000 rpm for 10 min. The supernatant was transferred to a fresh tube; 2 mL of it were mixed with 2 mL of 0.1% resorcinol solution and then with 6 mL of 10 N HCl, mixed thoroughly, heated at 90 °C for 10 min and cooled to room temperature. Two milliliters of a 2% working standard, equivalent to 200 mg/100 mL of fructose in the prostate fluid, was similarly processed. The absorbance of our test and standard samples was measured at 490 nm. The average fructose content of fluid was calculated and expressed as mg fructose/100 mL (absorbance of samples/absorbance of standard × 200)
2.4 Histopathological study
Exact parts of cauda epididymis were fixed in 10% buffer formalin and was subsequently preserved in 70% alcohol. Histological sections were made at intervals of 4 μm and stained in hematoxylin and eosin.
2.5 Morphometric analysis
The mean height of epithelium of the elongated principal cells, and the nuclear diameter in cauda epididymidis of all groups were estimated using a computer image analysis system, Leica IM500, coupled with image manager software (version: 5 Release 247). The data were analyzed using the Statistical Package SPSS for Windows, version 16.0. Fifty cells per animal were chosen from the proximal cauda and were measured. This region corresponds to subzone 6A, as classified by Reid and Cleland [13]. In this region, there are principal cells and abundant spherical clear cells. The data were expressed as the mean ± standard error (SEM). The statistical analysis was based on the Student's t-test, taking P < 0.05 as the limit of the significant values.
2.6 Ultra study
Immediately after the removal of cauda epididymis from the dissected rats, the tissues were sliced to a small size (1 mm3) and fixed in 3% buffered glutaraldehyde for 4 h at 4 °C. Tissue specimens were then post-fixed in 1% osmium tetraoxide (OsO4) for 1.30 h. Dehydration of the fixed tissue was performed using ascending grades of ethanol and then the tissue was transferred to a resin via propylene oxide. After impregnation with the pure resin, the tissue specimens were embedded in the same resin mixture [14]. Ultra-thin sections of silver shades (70–85 nm) were cut with an ultra-microtome (Leica, UCT) with a diamond knife (Diatome, Switzerland) and stained with uranyl acetate and lead citrate. Stained tissue sections were observed under TEM with a JEOL 1011 CX apparatus operating at 80 kV.
3 Results
3.1 Body weight and organ weights
No evident changes were noticed in the body and weight of the reproductive organs in the control as well as in treated groups (data not shown).
3.2 Fructose measurements
Plasma fructose of epididymis and prostate gland, which are the major source of energy to the motile sperm were significantly decreased (3-fold) in both treated groups compared to the controls (Table 1), confirming androgen depletion; P < 0.001 is considered as highly significant.
Fructose content (mg/100 mL) of the whole epididymis and of the prostate gland of the control and B. carterii and B. papyrifera treated rats.
| Group | Fructose levels | (Plasma fructose (mg/100 mL) |
| Epididymis | Prostate gland | |
| I – Control | 80.69 ± 2.11 | 103.52 ± 1.43 |
| II – B. papyrifera | 39.15 ± 1.91*** | 43.45 ± 1.31*** |
| III – B. carterii | 29.64 ± 0.32*** | 41.51 ± 1.29*** |
*** P < 0.001.
3.3 Morphometric analysis
Morphometric data of treated groups showed a decrease of the height of the whole epithelium and principle cells, and of the nuclear diameter of the cauda epididymis, as shown in Table 2; the P < 0.001 was considered as highly significant.
Effect of inhalation of B. carterii and B. papyrifera on the epithelial height (μm) and the nuclear diameter (μm) of the cauda epididymis of Albino rats (values are expressed as SEM of five animals).
| Group | 100 × (μm) | ||
| Epithelial height | Nuclear diameter | Principal cell | |
| I – Control | 21.47 ± 0.48 | 9.10 ± 0.29 | 17.13 ± 0.3 |
| II – B. papyrifera | 15.70 ± 0.30*** | 5.31 ± 0.57*** | 10.11 ± 02*** |
| III – B. carterii | 14.55 ± 0.43*** | 4.15 ± 0.19*** | 9.11 ± 0.5*** |
*** P < 0.001.
3.4 Light microscope and ultrastructural changes
The tubules of cauda epididymis of control rats were arranged compactly with very little intertubular connective tissues. The epithelium was low cuboidal and ciliated, along the luminal border. The cells contained prominent spherical to oval nuclei and were found very close to the basement membrane. The interstitium contained numerous interstitial cells, with rounded nuclei and fibroblast-like elements. The pseudostratified tubular epithelium consists of very tall columnar principal cells with long non-motile stereocilia (SC) and small basal cells. The SC were visible and the wide lumen packed with evenly dispersed sperm (Fig. 1A).
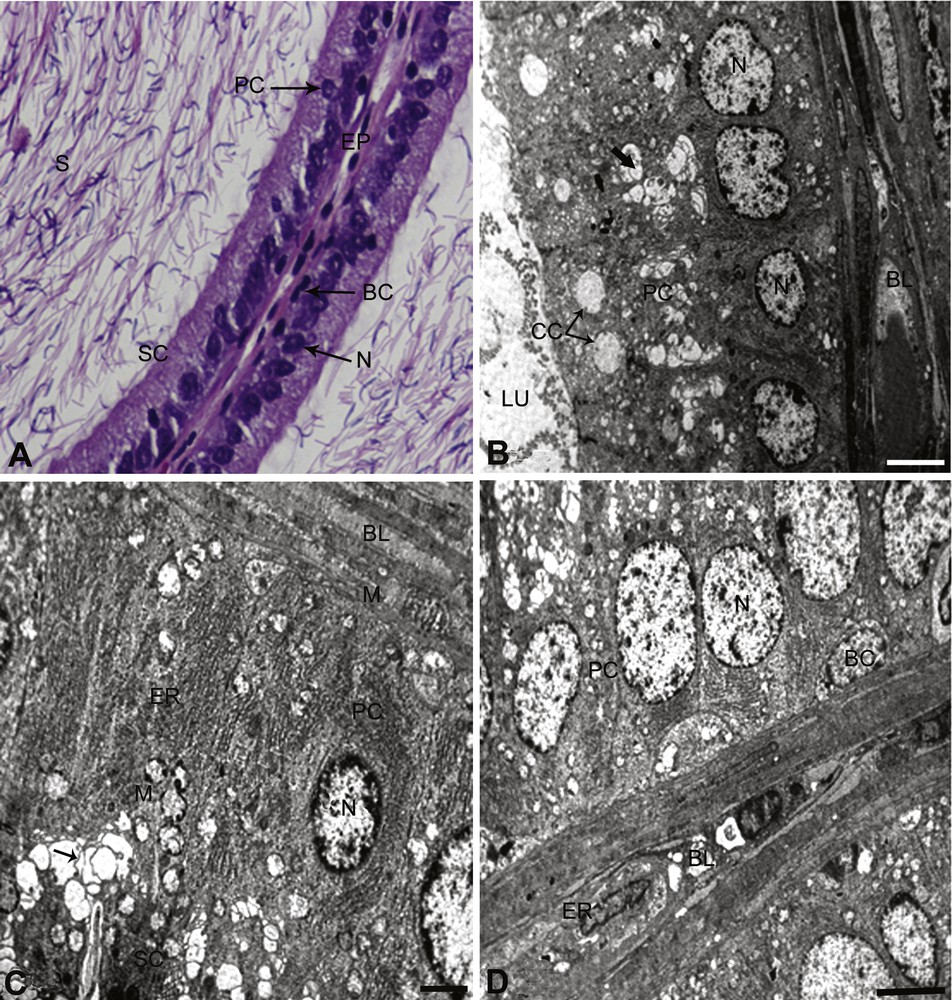
Controls. A: In the cauda epididymis of control rats, the epithelium (EP) exhibits the normal height with intact nuclei (N) and stereocilia (SC). Principle and basal cells appear normal and the lumen was full of sperm (S). × 100. B. Principal cell (PC) and clear cell (CC) with normal Golgi complex (arrow). The nuclear region of the principal cells shows typical features of the cell, and the nucleus (N) is normal. The demarcation between the cells is clear. The principal cell lies above the basal lamina (BL). Few lysosomal bodies are clearly visible. × 5000. C. The principal cell (PC) resting on basal lamina (BL) is present, showing its normal features between two distinct lines. The nucleus (N) is normal lying at the basal lamina. Lysosomal (arrows) and mitochondria (M) are also present. Endoplasmic reticulum (ER) is present in abundant running parallel to PC cells (ER). × 5000. D. The basal cell (BC) is elliptical showing normal in structure. The cell boundary is clearly seen. The nucleus (N) of the basal cell lies parallel to the basal lamina (BL). An endothelial cell is present in the basal lamina (BL) and appears normal. The principal cell (PC) adjacent to basal cell (BC) is normal. × 6000.
The ultrastructural study showed that the principal cells were present along the entire length of the cauda epididymis. These cells have a round or elliptical nucleus containing granular chromatin. The multi-vesicular bodies contain amorphous material. The Golgi complex was composed of fenestrated cisternae. The supranuclear region of the cell showed well-developed mitochondrial cristae and endoplasmic reticulum, arranged in the form of whorls (Fig. 1B–D). The clear cells were generally found in between and at the upper part of the principal cells. They contained ovoid nuclei placed slightly above the basal position and contained granular chromatin material. The cytoplasm abounded with lipid droplets. Micropinocytotic vesicles were prominent (Fig. 1B). The basal cells were elliptical and nuclei were elongated and flattened against the basement membrane (Fig. 1D). The basal lamina and other endothelial cells are normal, as shown in Fig. 1B–D. B. papyrifera- and B. carterii-exposed rats showed a reduction in epithelial heights and nuclear diameters (Figs. 2A–D and 3A–D). The nuclei were pycnotic and the height of stereocilia was reduced. The lumen was devoid of sperm and filled with lymphocytes and debris of degenerated sperm. The basement membrane was thin and disrupted. The cell showed vacuolization and the cell debris was evident due to cytolysis. Few cells exhibited signs of degeneration.
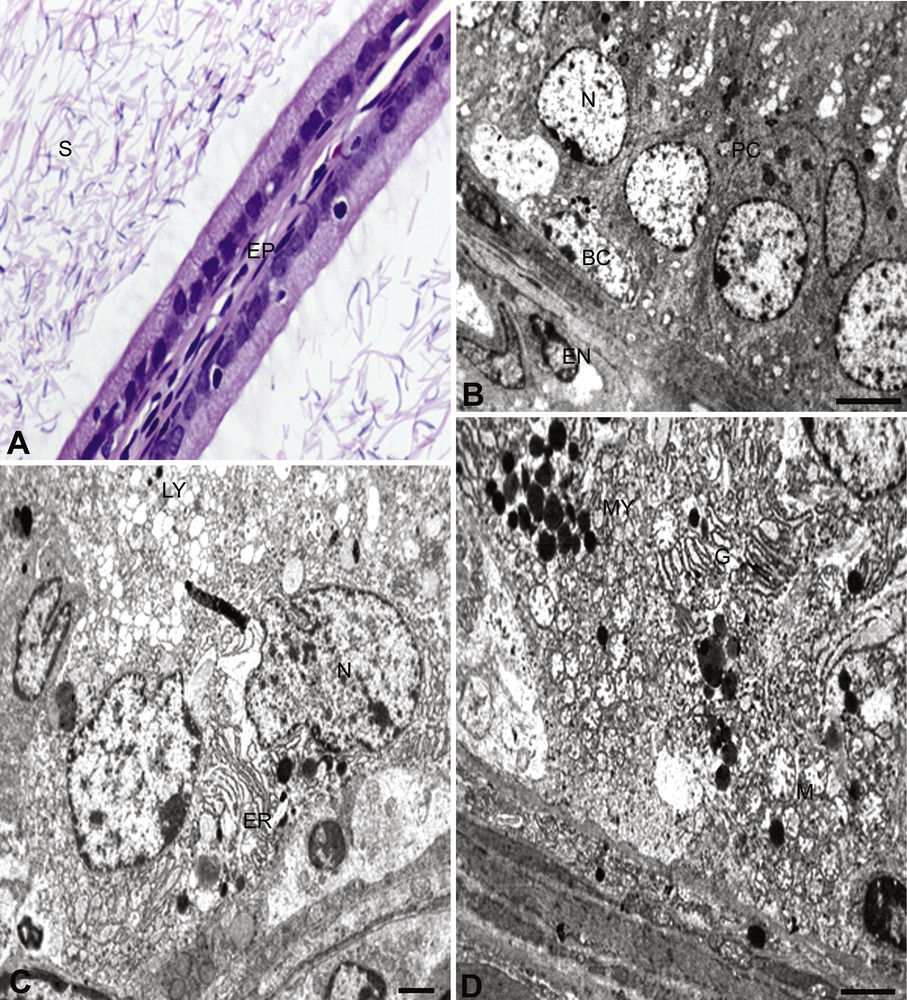
(B. papyrifera). A. In B. papyrifera exposed rats; the epithelium (EP) is disturbed and reduced stereocilia are seen. The lumen is devoid of sperm, with disruption in sperm (S). × 100. B. Basal cell (BC) and principal cell (PC), showing an abnormal pattern. The nucleus (N) of the principal cell shows reduced and scattered chromatin material. The nucleus of the basal cell (BC) shows reduced nuclear chromatin material laying at the disrupted basal lamina. × 6000. C. Principal cell shows intended nucleus (N) in the plane of the section. The cell is totally disturbed and cell debris is seen prominently. The endoplasmic reticulum (ER) is more visible. The lysosomal bodies (LY) are increased, with no distinct cell boundaries. × 6000. D. Cross-section of principal and basal cells. The multi-vesicular bodies (MV) and lysosomal bodies are increased along with the Golgi apparatus (G). There is an accumulation of a large quantity of cell debris with disturbance in structural boundaries. A complete degeneration of mitochondria (M) was taking place. × 6000.
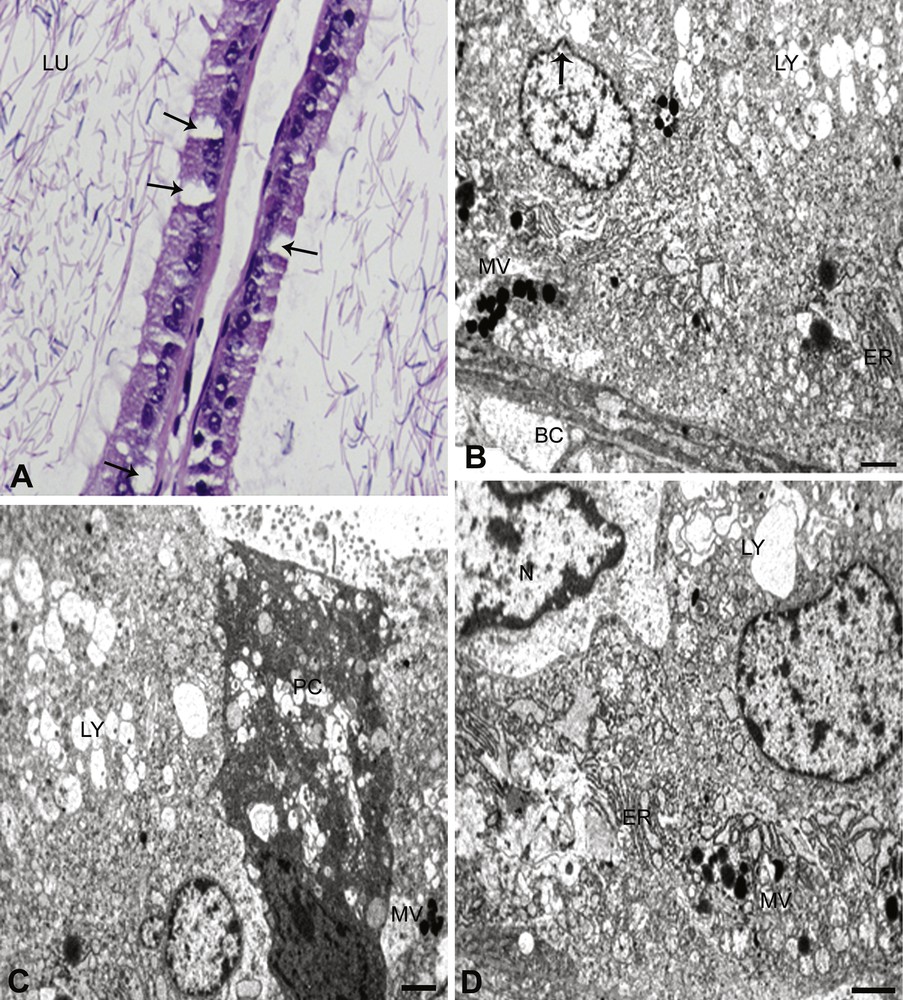
(B. carterii). A. In B. carterii exposed rats, the disrupted tubules show vacuolization in the cells with condensed nuclei (arrows). The lumen (LU) is devoid of sperm, with disruption of interstitium. The epithelium is completely disturbed and reduced stereocilia are seen. × 100. B. Principal and basal cells (BC), showing abnormal structures. The nucleus of the principal cell with reduced chromatin material can be seen, and there is a clear bulging of the nuclear envelope (arrow). The lysosomal bodies (LY) show a complete degeneration and disruption of mitochondrial cristae. The nucleus of the basal cell (BC) shows no nuclear chromatin material laying at the disrupted basal lamina with few multi-vesicular bodies and endoplasmic reticulum (ER) (MV). × 6000. C. Principal cell (PC) shows the absence of nucleus with missing other organelles in this section. The cell is totally disturbed and cell debris is seen prominently. The multi-vesicular bodies (MV) and lysosomal bodies (LY) are seen, with lot of fuzzy materials. × 6000. D. The principal cell nucleus (N) is highly indented. The basal lamina is disturbed (not shown in the figure) and few multi-vesicular (MV) bodies, ER and lysosomal bodies (LY) were observed on the surface of the basal lamina. × 6000.
The most obvious changes in principal cells exposed to B. papyrifera and B. carterii were the decreased number of coated micropinocytotic vesicles, invaginations of luminal surface, disruption in mitochondrial cristae and Golgi apparatus. The rough and smooth varieties of endoplasmic reticulum also exhibit the changes in the structure (Figs. 2C and 3D). Furthermore, the multi-vesicular bodies were increased and contained a homo- or heterogeneous materials. The principal cells reflected the change in terms of vesicular elements and lysosomal bodies. The nuclear region of the cell was highly indented (Figs. 2C and 3D). Micropinocytotic vesicles were rarely seen in the clear cells and a slight decrease in the number of multi-vesicular bodies (Figs. 2A and 3D). The number of cytoplasmic vacuoles was reduced and the density of their flocculent content showed an increase. Cytoplasmic vacuoles and micropinocytotic vesicles were much reduced in the exposed groups. Decreased lipid droplets were also observed in cells. Autophagic bodies, containing remnants of cellular organelles, become prominent in the perinuclear region of the cells, indicating an enhancement of the autophagic process (Figs. 2C and 3B–D). The multi-vesicular bodies were increased and contained some heterogeneous material in the supranuclear cytoplasm of cell. Lysosomal bodies increased either in the basal cytoplasm or in the supranuclear cytoplasm or in both. Furthermore, the basal cells showed the absence of the scattered spherical electron-dense granules in the cytoplasm. Disruption of mitochondrial cristae and Golgi apparatus were also evident in this region (Figs. 2A and 3A).
4 Discussion
Epididymis is an important component of the male reproductive tract that is highly androgen-dependent and plays a vital role in male fertility [15]. Epididymis provides a suitable environment for morphological and biochemical changes in spermatozoa [16]. It performs both secretory and absorptive functions. Androgen deficiency causes a marked reduction in the tubular diameter, a general regression of epididymal epithelium, a sudden decline in spermatozoa, in cauda epididymis and changes in the composition of epididymal plasma [17]. In this study, we evaluated the toxicity of B. papyrifera and B. carterii smoke exposure on cauda epididymis; we had already established in our recent studies the changes in testis and sperm parameters in male Albino rats [10].
Fructose has been reported to be a source of energy for the motility of the gametes and act as a secondary indicator for androgen levels [18,19]. Patel et al. [20] demonstrated a positive correlation between seminal levels of fructose and percentage of motile sperm. In this study, the decreased levels of fructose in epididymal plasma and prostate gland directly affect the sperm motility and may be androgens as these organs are androgen-dependent.
Many studies, such as cigarettes exposure on rats showed significant reductions in epididymal sperm content, motility and in fertility in vivo and in vitro. In addition, smoking leads to a secretory dysfunction of the Leydig cells, deficiency in sperm maturation and spermatogenesis [21,22]. It is still not clear whether incense smoke affects fertility or not.
Cauda epididymis treated with benzene extract of Ocimum sanctum leaves showed a reduction in epithelial height and nuclear diameter of epithelial cells. Cells showed vacuolization with signs of degeneration. The ultrastructural study also revealed that cauda epididymis was affected, particularly the principal, clear, and basal cells. Furthermore, a decrease in the size of lipid droplets, mitochondria, Golgi complex, endoplasmic reticulum, and an accumulation of lysosomal bodies [23] has been observed. Our study strongly supports these findings; we observed similar changes in the epididymis. Vitamin E deficiency affects the structural differentiation of epithelial cell of the epididymis [24]; exposure of rats to Gossypol has a direct effect on the epididymis, provoking an exfoliation of the epididymal epithelial cells, increased phagocytosis of sperm in the cauda, and/or accelerated transit through the cauda region [25]. Taken together, these data confirm that they target the epididymis, disturbing both the structure and function of this organ and presumably disrupting sperm maturation. We observed exfoliation, degeneration of cells and other similar changes in our studies. Gossypol administered to male rats caused hypertrophy of the cauda epididymal epithelium. The principal cells lost most of their microvilli and formed apical blebs, which appeared to produce the dense secretory material, which was found in the lumen [26]. The epithelium and principal cells were dramatically changed in our studies, which supports this hypothesis.
The leaves of Andrographis paniculata and the shoot extract of Bambusa arundinacea reduce the weight and the epithelial height of epididymis and exhibit severe effects on the epididymal spermatozoa in rats [27]. The leaf extract of Vinca rosea causes severe damage to the histoarchitecture of cauda epididymis, reduces the weight and the epithelial height of epididymis [28,29]. The present findings evidence the same changes as those exposed above with these two plants.
Azadirachta indica leaves affect the height of the epithelium and the diameter of the nuclei. In addition, sperm was packed at the center of the lumen, with evident intertubular fibrosis and damaged stereocilia [30,31]. Various extracts of Hibiscus rosasinesis affect the internal milieu of the epididymis, causing the change in the spermatozoa in treated Albino mice [32]. An aqueous extract of papaya seed affects the microenvironment and sperm metabolism of cauda epididymis of Albino rats [33]. It was demonstrated that nicotine, a tobacco extract, reduces the diameter and the epithelial cell height of cauda epididymis of male Albino rats [34]. These changes were common to our observations. Therefore, in the present study, the disorganized epithelium of cauda epididymis and cell vacuolization with signs of degeneration was due to the exposure of B. papyrifera and B. carterii, suggesting that these plants possess potential toxins in their bark wood. Epididymis as a whole and the principal cells in particular are androgen-dependent; androgen withdrawal is known to cause extensive changes in the principal cells [35]. In present study, the direct action of B. papyrifera and B. carterii on the principal cells cannot be excluded. Therefore, it is possible that B. papyrifera and B. carterii exposure may have caused pathological changes in the principal cells.
In this study, principal, clear and basal cells underwent ultrastructural changes following the treatment of B. papyrifera and B. carterii; among the significant changes observed in the principal cells, were a decrease in the number of micropinocytotic vesicles and a reduction in the size of mitochondria and the Golgi apparatus. Similar observations were reported by Ahmed et al. [23]. The present study further indicates that in response to the smoke inhalation of B. papyrifera and B. carterii, clear cells undergo hypertrophy, hyperplasia and hyper activity in an attempt to remove the cell debris. Similar observations were noticed in cauda epididymis of rats treated with vincristine [23,36]. In clear cells of B. papyrifera and B. carterii exposed rats, organelles, like cytoplasmic vacuoles, electron-dense secretory granules and mitochondria, exhibited a reduction in their number. Also, the lipid droplets in the cells of the treated rats were smaller as compared with those in control rats. Furthermore, there was a reduction in the number of micropinocytotic vesicles and multi-vesicular bodies. These findings indicate that the endocytic function of the clear cells was affected following the intake of B. papyrifera and B. carterii.
The available cytological evidences does not support that the basal cells are involved either in secretion or in absorption. This conclusion was drawn from the observations that the basal cells are numerically less, their location is far away from the lumen and they do not have complex cellular machinery for secretion or absorption [37]. But Veri et al. and Regadera et al. [38,39] have proposed a hypothesis that the basal cells were involved in a scavenging role in a local immune defense mechanism, in which antigenic products derived from sperm degeneration were phagocytized. The role of basal cells seems to be imperfectly understood, although these cells are present in all mammalian epididymis; in contrast, recent studies show that basal cells play an important role in the renewal of the epithelium or in the secretion of recognition molecules of the sperm membrane [37]. Ultrastructural changes indicate that the principal and clear cells are affected, thus, altering the composition of the epididymal fluid. This hypothesis was supported by the findings of earlier studies [40].
It is concluded that the changes were due to the exposure of B. papyrifera and B. carterii smoke through the respiratory system. However, these conclusions are based on the pathological studies where the rats were forced to inhale the smoke of B. papyrifera and B. carterii. A careful further study is indeed required to correlate its exact mechanism on target organs, including its molecular changes on sperm, acquisition of their fertilizing capacity, mobility, recognition sites in ova, as well as the masking of these sites.
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through the research project NoRGP-VPP-164. The authors also wish to thank Transmission Electron Microscope Unit College of Science for electron microphotographs.


