1 Introduction
Fish reproduction is highly relevant to wild population management, including fishing activity and environmental planning. The reproductive success, as measured by both quality and amound of offspring, determines the viability and stability of natural populations [1]. Accordingly, breeding indices, such as sex ratio, stage at first maturity, fecundity, spawning frequency and larval recruitment are commonly used to assess the overall fish reproductive performances [2–5]. Taken all together, these reproductive parameters can provide valuable information on the status of commercially exploited fish stocks and can therefore be used to formulate appropriate management measures of the natural populations. Understanding the different aspects of fish reproduction is also crucial for the sustainable development of aquaculture as it allows developing appropriate breeding methods to control the reproductive cycle for optimal production in the farming of any commercial species. Breeding in fishes is regulated mainly by environmental factors, particularly day-length, temperature and rainfall [6–10]. These most frequently studied factors do not function independently, but interact among themselves to regulate the sexual cycle [11–17]. They may also interfere with other environmental variables, such as salinity, water quality and food availability, which are also known to affect gonadal maturity and fecundity and, hence, the timing of seasonal breeding [7,18–20].
Aquatic environments are affected by the global warming that occurs since more than two decades. The major consequences of this climatic change are the increase of water temperature and the decrease of annual precipitation which may have led to the increase of hyperaline environments in many regions, including tropical areas [21]. Climate change has also other consequences on aquatic ecosytems related to its effects on evaporation rate, dissolved oxygen concentrations, nutrient status and disturbance of biotic/abiotic interactions, such as habitat fragmentation, and species distribution/interactions [22]. Aside from these environmental pressures caused by global warming, aquatic ecosystems are also subjected to anthropogenic alterations, such as drain discharges from domestic and industrial sources that increase the level of pollutants, which can interact with physiological processes and disturb community structures. These natural and anthropogenic interventions may affect reproductive and early life history events and therefore, lead to changes in demography and availability of most aquatic organisms, including fishes [23].
The purpose of this study is to assess the effects of environmental change and anthropogenic forcing on the reproduction of wild black-chinned tilapia Sarotherodon melanotheron (Cichlidae) in Senegalese aquatic ecosystems where global warming has already had observable effects [24]. This marine euryhaline teleost is widely distributed in coastal marine, estuarine and lagoon ecosystems and is known for its ability to tolerate a wide range of environmental factors, including salinity, water temperature and dissolved oxygen [25–28]. Several comparative studies of reproductive traits have been conducted using this species in order to assess the effects of environmental variability on its reproduction in the Saloum Estuary and the neighbour Gambia River [29–31]. These studies have been focused almost exclusively on variations of ambient salinity, which is considered as the environmental factor that has been the most affected by climate change in West-African areas [31–36].
We investigate the effects of climate change on reproduction by analyzing the links of environmental factors with the seasonal variation in gonadosomatic index (GSI) in black-chinned tilapia females from Guiers Lake, Hann Bay, and Saloum Estuary. Given that an accurate identification of the effects of environmental change on reproduction requires appropriately characterized reproductive cycle of the species, we performed histological examination of female gonads for a more accurate maturity classification. We additionally used the percentage of maturity stages in both females and males to validate the spawning periods of S. melanotheron in these three ecosystems.
2 Methods
2.1 Sample collection
Fish were collected on a monthly basis for two consecutive years from two drainage basins in Senegal, namely Guiers Lake and Hann Bay (Fig. 1). Samples were also collected in upstream of the Saloum Estuary, but mainly during the reproductive periods because the reproductive cycle of the species has been previously studied in this area [30]. A detailed geographical description of the sampling areas is available in Guèye et al. [29]. These sites were chosen based on their environmental properties. In the Saloum Estuary, salinity levels have changed drastically over recent decades as a result of climate change [21]. It can be, therefore, used to assess the impact of global warming on reproduction. The salinity values recorded in the estuary during the period of our study varied between 66 and 123 psu. The two other sampling sites Guiers Lake (freshwater) and Hann Bay (seawater) have not undergone salinity variations throughout the year and other environmental variables, such as precipitation would be good indicator for evaluating the effects of climate change. The chrorophyll-a concentration in this ecosystem was particularly lower compared to Guiers Lake and Hann Bay (Table 1). The oxygen (mg L−1) concentrations at these two locations were 6.70 and 4.84 mg L−1, respectively. Finally, the impact of anthropogenic interference would be more apparent at Hann Bay location, which is the most polluted sampling site in this study [37]. Fish were captured using a beach seine net or castnet with small mesh size and were immediately killed by anaesthetization with a lethal dose of 2-phenoxyethanol. They were then sexed and measured for fork length and total body weight (Wb in g). The gonads were dissected from males and females, weighted (Wg in g) and preserved in 95% ethanol.
Map showing the locations (★) in Senegal where Sarotherodon melanotheron samples were collected.
Historical environmental data of the study areas (adapted from Gueye et al. [29]).
| Variable | Guiers Lake | Hann Bay | Saloum |
| Salinity (psu) | 0 | 37 | 66–123 |
| Water temperature (°C) |
24.9 | 25 | 26.7 |
| Oxygen (mg L−1) | 6.70 | 4.84 | – |
| Transparency (m) | 0.79 | – | 2.5 |
| Conductivity (μs cm−1) |
174 | – | 71 |
| Chrorophyll-a (μg L−1) |
23.8 | 16.83 | 2.3 |
2.2 Determination of sexual maturity stage
Several methods are used for determining the sex and sexual maturity stage of fish gonads. Macroscopic observation methods are usually used to evaluate the maturity stages of both males and females because they are rapid and inexpensive ways to determine the reproductive status. However, for some sexual stages especially in females, macroscopic analysis of gonads cannot provide precise information on the oocyte developmental stage. Such stages rely on characteristics that can only be identified microscopically. In this study, the maturity stage of both males and females was first determined by the macroscopic evaluation of the gonads with Legendre and Écoutin [38] method. The description of each maturity stage in both males and females is available in Table 2. A microscopic evaluation of the maturity stage was carried out in parallel in females to validate the determined sexual stages. The latter classification schema is a seven-stage scale characterized by changes in the type and proportion of germ cells present in the ovaries.
Macroscopic or visual description of female and male gonadal maturity stages in Sarotherodon melanotheron.
| Stage | Description | |
| Female | Male | |
| 1 | Ovaries are poorly differentiated and developed, but translucent. Oocytes are invisible to the naked eye | Gonad is in the shape of a filament slightly larger than undetermined stages |
| 2 | Differentiated ovarian oocytes with very small oocytes | Gonads start to fade and become white, and occupy the third of the abdominal cavity |
| 3 | Ovaries at the beginning of sexual maturation. Oocytes visible to the naked eye. Gonad length generally exceeds half of the abdominal cavity | The gonads are colored pearly white with a width exceeding the third of the abdominal cavity |
| 4 | Advanced maturation of gonads that occupy almost the entire abdominal cavity | The gonads become yellowish white with presence of blood vessels and occupy the entire length of the abdominal cavity |
| 5 | Ovulation stage. Gonads occupy the entire abdominal cavity and exert pressure on the digestive tract. Eggs are ejected at the slightest hand pressure | The gonads have similar characteristics to those observed in stage 4, but the blood vessels are larger and more pronounced |
| 6 | Post-spawning stage. Gonads appear empty, containing a few residual oocytes of large size |
2.3 Histological analysis of female gonads
After anesthesia and fish decapitation, whole female gonads were extracted and immersed in Bouin's liquid for at least three nights at room temperature. They were then rinsed with 70% ethanol, dehydrated using increased concentrations of ethanol (95% and 100%) and butanol for 3 h each. Gonadal tissues were then cleaned with a cleaning agent (Histochoice, MICROM-SARL, Francheville, Lyons, France) and infiltrated with paraffin wax overnight before being finally embedded in fresh paraffin. Transverse sections of 7 μm were cut using a microtome, spread on glass slides, deparafinized and hydrated as follow: gentle heating to melt the paraffin, two toluene, butanol and one 95% ethanol baths for 5 min each, followed by a final rinsing with 70% ethanol for 5 min. The slides were rinsed with water for 5 min and the tissue sections were stained with Masson's trichrome [39]. The tissues sections were examined under a light microscope and images captured with a digital camera and associated imaging software.
2.4 Seasonal progression of sexual maturity
The proportion of each sexual maturity stage was calculated in each ecosystem for both males and females of S. melanotheron. The seasonal variation of sexual maturity stages was then estimated by calculating the monthly cumulated percentages of each stage in the whole samples. The monthly evolution of the mature stages (stages 3, 4 and 5), which are characteristic to spawning periods, was used to validate the reproduction cycle and to determine the gonadosomatic index. The gonadosomatic index (GSI) was calculated for each individual using the standard formula:
The seasonal changes in the percentage of individuals sexually maturing or mature (i.e. with a stage equal or greater than 3) and the seasonal variations of GSI were determined monthly in order to evaluate their relationships with photoperiod, water temperature, rainfall and salinity.
2.5 Environmental data collection
Environmental parameters that could affect the reproduction activity, such as temperature, photoperiod, precipitation and salinity were collected from each sampling site. During each sampling campaign, temperature and salinity measurements were performed in situ in each location with a thermometer (ATAGO) and a refractometer, respectively. Additional data on water temperature were taken from the weather station of the IRD research group UR098-FLAG (Dakar, Senegal) for Guiers Lake and Hann Bay locations. In upstream of the Saloum Estuary, additional temperature data were obtained from experimental fish samplings of the IRD research group UR 070-RAP (Dakar, Senegal). Precipitation data in different ecosystems were obtained from meteorological observations of the Senegalese National Weather Service (http://www.meteo-senegal.net). Photoperiod data were obtained from the French “Bureau des Longitudes” website (www.bdl.fr), which provides photoperiod data in all regions over the world. For each location, sunrise and sunset times corresponding to its latitude and longitude are specified for each day of the year. Changes in sunshine duration over the year correspond to the photoperiod.
2.6 Statistical analyses
The relationship between each environmental factor and the GSI of female individuals was assessed by the linear regression. The rainfall profiles per decade were compared by using one-way ANOVA test. These tests were performed using the R statistical language [40]. For all the tests, a probability of less than 5% as fiducial level of significance was used.
3 Results
3.1 Precipitation
Fig. 2 represents the cumulative precipitation per decade recorded at Keur Momar Sarr, Dakar and Kaolack from 1962 to 2001. The average precipitation in these locations was respectively, 376.3, 518.8 and 718.4 mm during the first per decade (1962–71). During the last decade (1992–2001), the average precipitation at Keur Momar Sarr, Dakar and Kaolack was 269, 334.3 and 559.3 mm, respectively. These results indicate an overall decrease (one-way ANOVA; P < 0.05) in annual precipitation over our study area since the early 1970s.

Rainfall recorded at the Keur Momar Sarr, Dakar and Kaolack meteorological stations (one-way ANOVA test on data per decade from 1962 to 2001; Keur Momar Sarr: F = 4.636, P = 0.008; Dakar: F = 3.384, P = 0.028; Kaolack: F = 6.578, P = 0.001).
3.2 Maturity and spawning season
A total of 901, 574 and 862 individuals, respectively, from Guiers Lake Hann Bay and Saloum Estuary were analysed in this study. Seven maturity stages (Fig. 3) were determined in S. melanotheron females according to the structure of the gonadal tissue and the advancement and arrangement of different types of germ cells in the gonads. A detailed description of these different maturity stages is available in Table 3. The proportions of maturity stages indicated a high prevalence of sexual stages 3, 4 and 5 in both males and females from January to August (Fig. 4) and from January to October at Guiers Lake and Hann Bay locations (Fig. 5). The presence of stages 4 and 5 females was higher in March, June and July at Guiers Lake location with two peaks of sexual activity, one which occurred in March and the other less marked in June (Fig. 4A). Stages 4 and 5 were not observed in November and December, the period characterized by high presence of stage 6 (Fig. 4A). This indicates that sexual rest in females occurs between November and December. Although stages 4 and 5 were observed in males throughout the year, their proportion was lower from October to December, the period that coincides with sexual rest of females in this ecosystem (Fig. 4B).
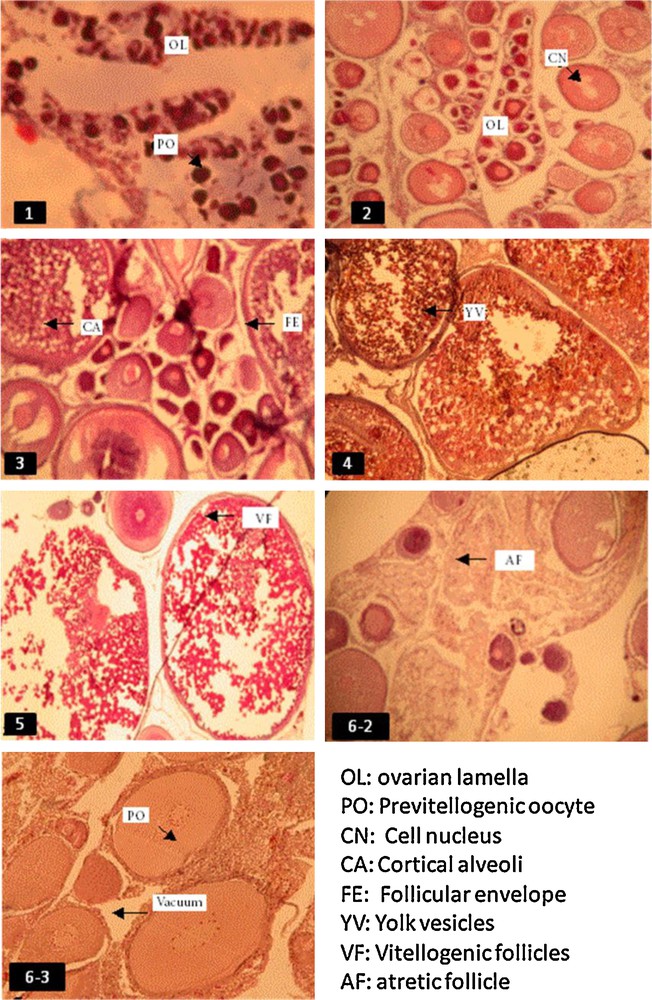
Histological section of Sarotherodon melanotheron ovaries showing oocytes at different stages of development. 1: Immature; 2: early maturation; 3: advanced maturation; 4: pre-spawning; 5: spawning active; 6-2: early post-spawning; 6-3: advanced post-spawning. Colour available on the web.
Histological description of gonadal maturity stages of Sarotherodon melanotheron females.
| Stage | Name | Description |
| 1 | Immature | Homogeneous oocyte cytoplasm. Ovaries contain oocytes with homogeneous cytoplasm, each containing a large spherical nucleus barely visible. The nucleus contains several nucleoli stuck to the nuclear membrane. The follicle membrane is barely visible |
| 2 | Early maturation | Heterogeneous oocyte cytoplasm. Some follicles begin vitellogenesis. The nucleoli are clearly visible. The membrane of the follicle cells is clearly visible |
| 3 | Advanced maturation | Presence of large number of vitellogenic ovarian follicles. Presence of numerous cortical alveoli and yolk sac in the oocyte cytoplasm. The follicular epithelium forms a continue crown surrounding the oocyte and pressed up against the oocyte |
| 4 | Pre-spawning | Mature ovarian follicles. The cortical alveoli begin migration toward the nucleus. The nucleoli detach from the nuclear envelope and begin their migration towards the centre of the nucleus |
| 5 | Spawning active | The follicles are mature and ready to ovulate. The nucleoli are completely detached from the nuclear membrane |
| 6-2 | Early post-spawning | Ovarian follicular degeneration. Follicles are eliminated from the ovary |
| 6-3 | Advanced post-spawning | The oogonia begin to differentiate into pre-vitellogenic oocytes that increasingly colonize the ovarian lamellae |

Seasonal evolution of sexual maturity stages of Sarotherodon melanotheron females (A) and males (B) in Guiers Lake. The analysis was conducted in fish collected on a monthly basis during two consecutive years (February 2003–August 2004). The number of fish analysed per month varied between 21 and 63 individuals for females and 19 to 52 for males.
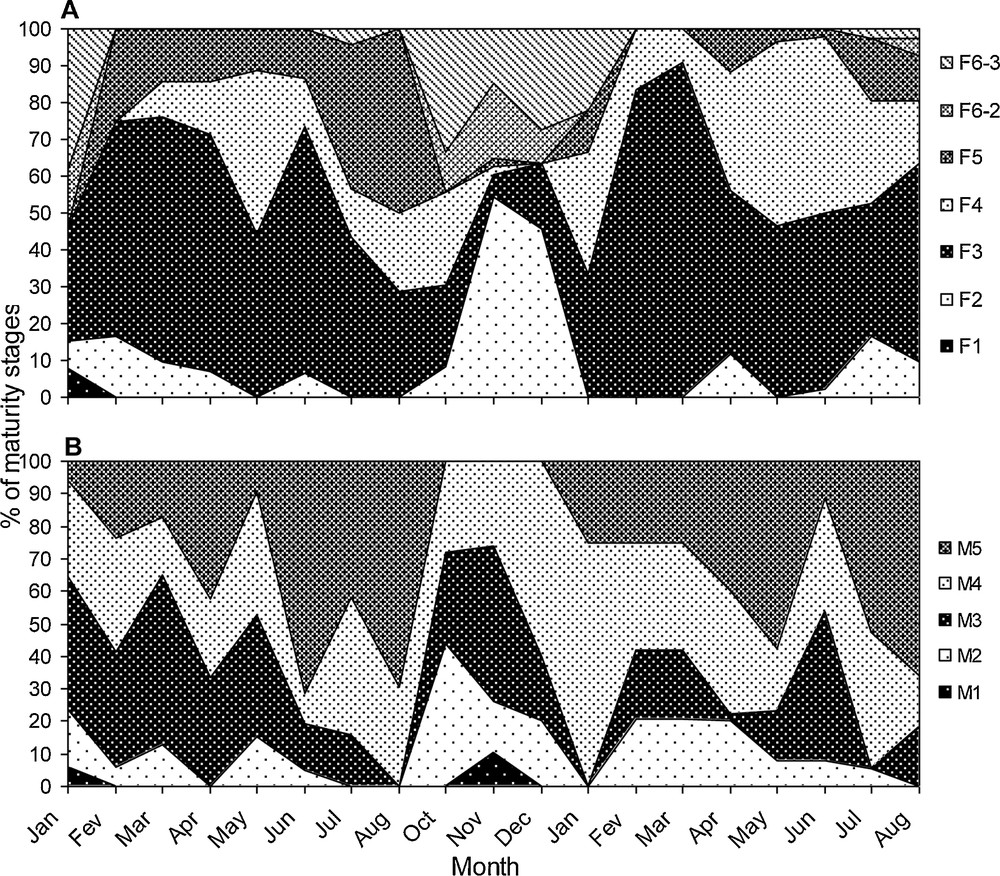
Seasonal evolution of sexual maturity stages of Sarotherodon melanotheron females (A) and males (B) in Hann Bay. The analysis was conducted in fish collected on a monthly basis in two consecutive years (January 2003–August 2004). The number of fish analysed per month varied between 17 and 45 individuals for females and 12 to 38 for males.
Higher proportions of females at maturity stages 4 and 5 were observed between May and August in Hann Bay, suggesting intensive sexual activity during this period (Fig. 5A). By contrast, the period extending from October to December was characterized by a small number of these two stages and by a high presence of stages 6-2 and 6-3, indicating a lower reproductive activity in S. melanotheron females during these three months in this ecosystem. In males, sexual maturity stages 4 and 5 were observed over the year, but their proportion was lower in November and December (Fig. 5B). These results are indicative of a continued sexual activity throughout the year in S. melanotheron males, with a decrease in its intensity during the sexual rest of females (from November to December).
Upstream of the Saloum Estuary, the analysis of the evolution of maturity stages throughout the year indicated that stages 4 and 5 are present in females only between June and August (Fig. 6A). Higher proportions of these two stages were observed in July, suggesting that the peak of sexual activity occurs in this month. These results confirm that the reproductive period of S. melanotheron females in upstream part of the Saloum estuary is limited to these three months with a probable extension until September. None of these sexual stages were observed from October to May, indicating a strong slowdown or absence of sexual activity in S. melanotheron females during this period. In males, stages 4 are higher in June but appeared in May (Fig. 6B), which suggests an earlier sexual maturation compared to females.
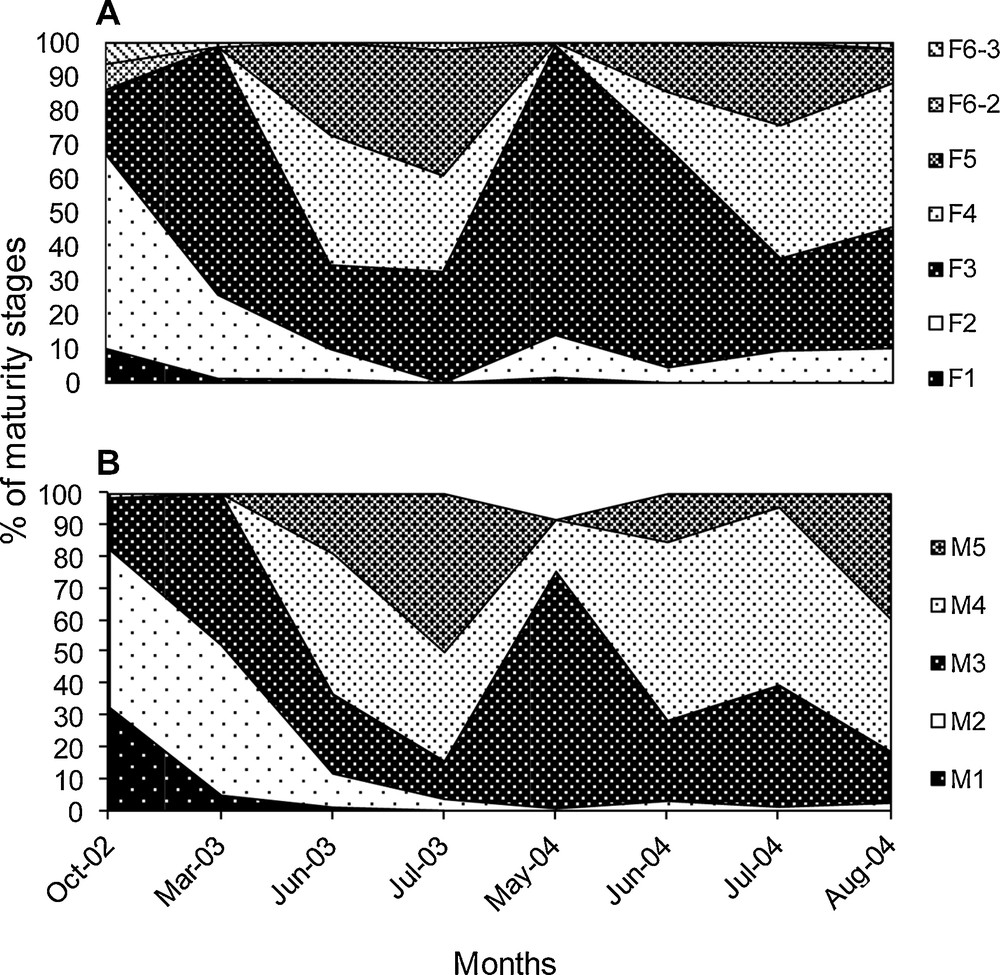
Seasonal evolution of sexual maturity stages of Sarotherodon melanotheron females (A) and males (B) in upstream of the Saloum Estuary. The analysis was conducted in fish sampled mainly during the spawning season.
3.3 Correlations between environmental factors and GSI
Table 4 shows the relationship between the evolution of gonadosomatic index (GSI) with photoperiod, temperature, rainfall and salinity. Correlations of GSI with these environmental parameters varied quite remarkably between ecosystems. There were significant positive correlations between the GSI and the photoperiod in all locations. By contrast, the GSI and water temperature were not significantly correlated in any habitat. There was no significant correlation between GSI and precipitation at Guiers Lake location. Significant positive correlations were observed between GSI and precipitation in upstream of the Saloum Estuary. The GSI was also not significantly correlated with salinity in upstream of the Saloum Estuary (Table 4), as might be expected from the fact that salinity levels in this habitat are highly dependent on annual precipitations and evaporations.
Relationships between environmental factors (photoperiod, temperature rainfall and salinity) and the reproductive activity of Sarotherodon melanotheron.
| Parameters/Fecundity | Guiers Lake | Hann Bay | Saloum Estuary |
| Photoperiod | 0.500* | 0.725* | 0.653* |
| Temperature | 0.345 | 0.075 | 0.345 |
| Rainfall | 0.134 | 0.459* | 0.500* |
| Salinity | 0.034 |
* Significant at probability level 0.05
3.4 Environmental effects on reproductive cycle
The intensity of reproduction was considerably lower or inexistent in Hann Bay and Guiers Lake during periods of short day-length (from September to April) and higher during long day-length from May to August (Fig. 7). In upstream of the Saloum Estuary, the highest GSI values were recorded during periods of long day-length (Fig. 7). In Hann Bay, the reproduction occurred predominantly from May to August when water temperatures were higher (Fig. 8), although fish spawn also during periods of low temperatures (from January to April). At Guiers Lake location, fish spawn at both low and high temperature (Fig. 8). In Saloum Estuary, the production occurred predominantly between June and September and there is no clear relationship between the GSI and temperature (Fig. 8). In Guiers Lake and Hann Bay, the breeding season of S. melanotheron occurs predominantly during the dry season (Fig. 9). In upstream of the Saloum Estuary, however, the reproduction seems to occur exclusively during the rainy season. In this location, the higher GSI values were observed in June and July (Fig. 10) when salinities in the estuary were high.
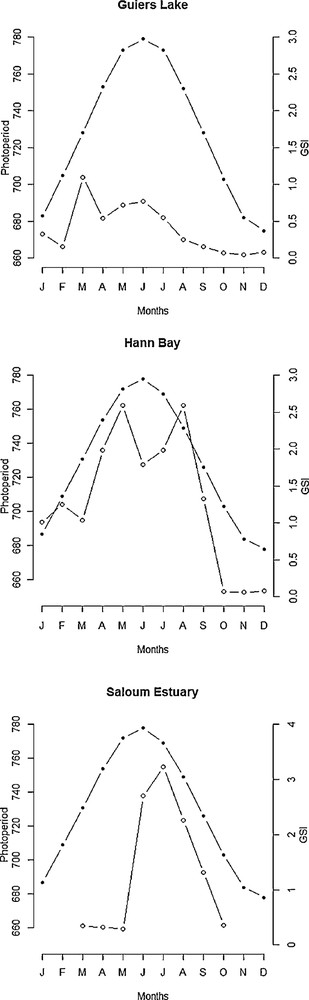
Seasonal variations in reproductive activity in black-chinned tilapia females in relation to photoperiod in Guiers Lake, Hann Bay and Saloum Estuary (shaded circles = photoperiod, empty circles = GSI).
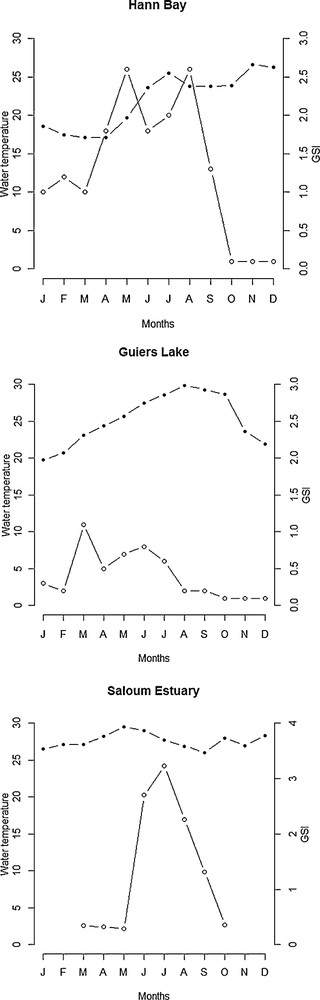
Seasonal variations in reproductive activity of S. melanotheron females in relation to water temperature in Guiers Lake, Hann Bay and Saloum Estuary (shaded circles = water temperature in °C, empty circles = GSI).
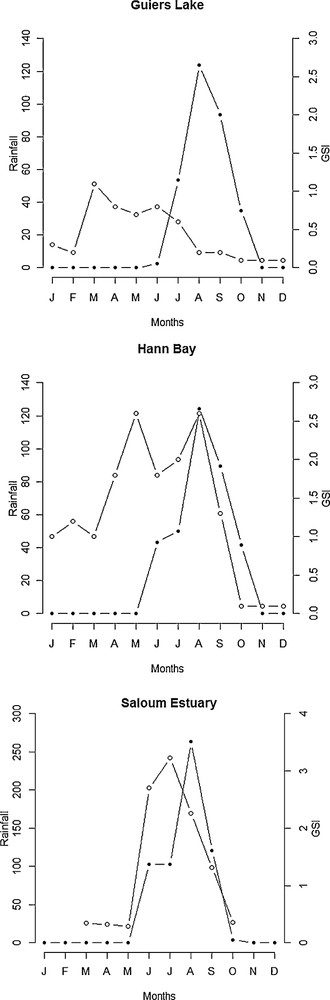
Seasonal variations in rainfall and reproductive activity in black-chinned tilapia females from Guiers Lake, Hann Bay and Saloum Estuary (shaded circles = rainfall in mm, empty circles = GSI).
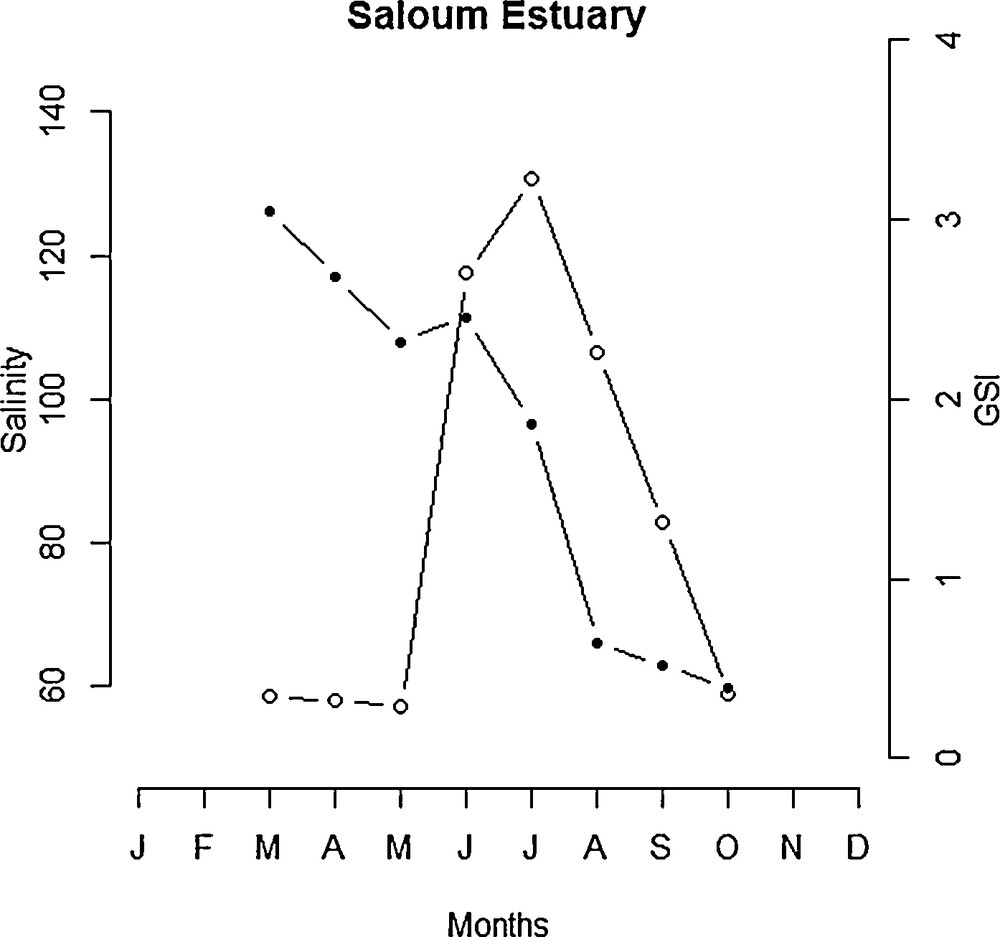
Seasonal variations in ambient salinity and reproductive activity in black-chinned tilapia females from upstream of the Saloum Estuary (shaded circles = salinity in psu, empty circles = GSI).
4 Discussion
The results of this study have significant implication on understanding the impact of environmental variability on timing and intensity of the reproduction cycle of the black-chinned tilapia, S. melanotheron in Senegalese and Gambian coastal marine, estuarine and freshwater ecosystems. In this area, the effects of climate change together with human activities has resulted in drought and intense evaporation, which after 30 years have led to hypersaline conditions in some estuaries, such as the Saloum and Casamance estuaries [21,41]. While salinity increase is considered as the major consequence of global warming that affects reproductive traits of the species in these areas [29,30,42], little is known about the relative contributions of other environmental stimuli, such as precipitation and/or pollutants. Moreover, there is limited information on how the environmental factors prevailing in these habitats interact to influence the reproduction of the species. The present study merely suggests that seasonal variations in day-length and water temperature affect sexual maturity in both males and females of S. melanotheron at Guiers Lake and Hann Bay. The results indicate also that precipitation accounted for a substantial proportion of temporal variation in sexual activity of black-chinned tilapia males and females from Hann Bay. Likewise, precipitation significantly correlated with sexual maturity in the Saloum Estuary, suggesting that fish reproduction in this area is linked to annual precipitations.
Seven maturity stages were established based on the structure of the gonadal tissue, its developmental stage as well as on the arrangement of germ cells in the gonads. Those include stages 6-2 and 6-3 that are very difficult to identify at the macroscopic level. These results not only confirmed the six macroscopic stages previously determined but are also consistent with those reported by Legendre and Écoutin [38], who have identified seven macroscopic sexual stages in S. melanotheron from Ivory Coast ecosystems. The combination of these two methods has enabled to accurately classify the maturity stages of S. melanotheron, thereby allowed to better clarify the reproductive cycle of the species over the studied area, which was crucial for properly evaluating the impacts of environmental factors.
Although significant correlations between photoperiod and reproductive activity were found in all three studied ecosystems, the pattern of this relationship differed between locations. The reproductive activity was higher during long day-length in Guiers Lake, Hann Bay and upstream of the Saloum Estuary. Interestingly, this period coincides with the rainy season when precipitation was higher, resulting in low salinities in the estuary. This suggests that precipitation may also interfere with reproduction of S. melanotheron in this ecosystem probably indirectly through its effects on salinity levels. This finding is consistent with results of Panfili et al. [30] who have observed that the reproduction of S. melanotheron in the Saloum Estuary starts just before the beginning of the rainy season and extends until August. This conclusion is also supported by more recent observations [29] that the relative fecundity of the species in the Saloum Estuary was higher during the wet season in comparison to the dry season. Indeed, the salinity levels in the Saloum Estuary are related to loss via evaporation but more importantly to freshwater inputs, which essentially come from precipitations [24,43]. The salinity increases during the first months following the end of the rainy season, because of intense evaporation [34], to become maximal at the end of the dry season. During the short rainy season, by contrast, salinity in the estuary may decrease considerably due to inflow of freshwater from precipitation [31]. Therefore, seasonal variation in the reproductive activity of S. melanotheron population may have been affected by change in salinity regimes in the estuary. Aside from its effects on ambient salinity, precipitation may also affect S. melanotheron spawning through the stimulation of food resources and prey availability. It has been demonstrated that precipitation can promote nutrient increase that stimulate mangrove growth and its associated fauna, including invertebrates and microorganisms, which are considered as the main food resource of fish in estuarine ecosystems [44].
The reasons why S. melanotheron reproductive activity is correlated with rainfall in Hann Bay where the salinity is constant throughout the year are not very clear. Nonetheless, one possible explanation could be that the absence of freshwater inflow which characterizes the dry season in this area contributed to increased concentrations of some pollutants, which at extreme levels, may lead to reduced water quality and therefore affect the ability of individuals to reproduce properly. In fact, Hann Bay has been affected for several years by industrial and domestic pollutant discharges, and natural eutrophication caused by increased nutrient concentrations [45,46]. Periodic upwellings of cold and nutrient-rich waters occur quite frequently in this bay. The resulting nutrient-enrichment increases the concentrations of macroscopic algae (Ulvales) and natural phytoplankton, leading to episodes of algal blooms [45]. The decomposition process of dead algae absorbs oxygen from water and may therefore induce anoxic conditions. The solid pollutants from industrial and domestic recharges can also damage fish gills and interfere with respiration. Taken all together, these factors represent serious environmental stressors, which may be a limiting factor for individual reproduction in this area. In rainy season, by contrast, the increased freshwater inputs from precipitation may help to dilute pollutants and therefore relieve fish from these stressors. Therefore, the highest spawning activity during the wet season may be attributed to high increase of the dilution of contaminants by precipitations.
Our study showed that water temperature has little or no indirect influence on the reproduction of S. melanotheron in the studied area, as evidenced by the absence of significant correlation between temperature and sexual activity. These results do not coincide with those of Baroiller and Toguyeni [6] and Duponchelle et al. [9] who have reported that water temperature regulates breeding cycle of tilapias. It has been demonstrated that water temperature induces the production of gonadal hormones, which stimulate or inhibit certain stages of gametogenesis or spawning [7,18–20]. This allows fish to spawn at specific times that ensure that the larvae have sufficient prey availability to feed and grow.
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.
Acknowledgements
We thank Omar Sadio for his help during the sampling. This study was financially supported by IRD: Research Unit 070-RAP and Department support and training (DSF). The authors thank also the two anonymous reviewers for their comments that have helped improve this manuscript.


