1 Introduction
The brown planthopper (BPH), Nilaparvata lugens (Stål) is a major pest of rice and it is widely distributed from tropical to temperate areas of Asia and Australia. This insect is a phloem-feeder and is restricted to cultivated and wild rice as their host plants [1]. It causes “hopperburn” and complete wilting and drying of rice plants [2] and also transmits grassy stunt and ragged stunt viral diseases [3]. Large-scale rice crop damage caused by the pest was reported in the 1970s in several South and South East Asian countries [2]. BPH displays two wing forms in adult stage: long (macropterous) and short (brachypterous). Macropterous adults fly long distances and invade rice-growing areas, whereas brachypterous adults cannot fly long distance [4]. Another species, N. bakeri, closely related to N. lugens, commonly found in rice-growing areas of Asia, has only been found to feed and reproduce on Leersia hexandra and other Leersia species [5].
Bey-Bienko [6] noted that in many organisms’ changes in the ecological and physiological traits of the species are frequently followed by subtle changes in its morphological characteristics. Morphology as the end product of physiological activities is initiated by the genome and modified by the environment. A physiological change at the immature stage would likely result in a morphological change at the adult stage [7]. Morphometrics is the measurement and analysis of form [8], and it has many applications in systematics to analyse morphogenesis and estimate environmental stress on organisms, especially arthropods.
The brown planthopper, N. lugens (Stal), is generally thought to be specific to wild and cultivated rice, but has been found to thrive on a weed grass, Leersia hexandra, that grows abundantly in irrigation canals near rice fields in South East Asia [9,10]. The individuals of this population have strong specificity for the weed host. They fail to survive when caged on rice plants [11,12]. Based on female and male courtship signals, nymphal survival, ovipositional preference, mate choice and hybridization experiments, Claridge et al. [12,13] suggested that rice- and Leersia-infesting populations represented two distinct sympatric biological species. They also found no hybrid in the field, although these two populations produced hybrids in the laboratory. Saxena et al. [14] reported that no significant difference was found between the two host-associated populations of N. lugens in their morphometric study. Saxena and Mujer [15] and Saxena and Barrion [16] studied 18 enzyme systems of rice populations of N. lugens and a population from grass L. hexandra (Swartz) using starch gel electrophoresis, but no diagnostic difference was found. Due to the existence of diagnostic allozyme and molecular markers, Latif et al. [17,18] suggested that brown planthopper with high esterase activity captured from rice and brown planthopper with low esterase activity captured from L. hexandra were closely related sibling species. The rice-infesting population had 94% with high esterase activity while the Leersia-infesting population had 96% with low esterase activity, as reported by Latif et al. [18]. Since previous morphological and host–plant relationship studies were performed between two host-associated populations without esterase activity test of insect, therefore, based on esterase activities, the present studies were undertaken to find out morphological variations and host–plant relationships between two sympatric populations of brown planthopper, one from rice and the other from a weed grass, L. hexandra, and to further resolve the species status of these two sympatric populations.
2 Materials and methods
2.1 Collection of sympatric populations
Two sympatric populations of N. lugens, one from rice and the other from a weed, L. hexandra, were collected from the field in five different locations in Malaysia. The five locations were UPM, Tanjung Karang, Melaka, Perak and Sabah (Table 1). An out-group, N. bakeri, was used in this study, collected from L. hexandra in Cameroon Highland. The insects used in this experiment were from wild populations.
Locations, host plant and population code for 11 populations of Nilaparvata spp.
| Insect Species | Locations | Host–plant species | Population code |
| N. lugens | Universiti Putra Malaysia (UPM), Selangor, Malaysia | Rice | UPM1 |
| N. lugens | UPM, Selangor, Malaysia | L. hexandra | UPM2 |
| N. lugens | Tanjung Karang (Tk), Selangor, Malaysia | Rice | TK1 |
| N. lugens | Tanjung Karang (Tk), Selangor, Malaysia | L. hexandra | TK2 |
| N.lugens | Malim, Melaka (Mk), Malaysia | Rice | MK1 |
| N. lugens | Malim, Melaka(Mk), Malaysia | L. hexandra | MK2 |
| N. lugens | Bander Seberang, Perak (Pk), Malaysia | Rice | PK1 |
| N. lugens | Bander, Seberang, Perak (Pk), Malaysia | L. hexandra | PK2 |
| N. lugens | Tuaran, Sabah (SB), Malaysia | Rice | SB1 |
| N. lugens | Tuaran, Sabah (SB), Malaysia | L. hexandra | SB2 |
| N. bakeri | Cameron Highlands (CH), Pahang, Malaysia | L. hexandra | CH |
2.2 Morphological studies
2.2.1 Morphometric analysis
Seventeen quantitative morphological characters, including characters of the stridulatory organs, were measured for this study. Seven out of 17 characters were scored from 20 macropterus males from each of the 11 populations (Table 2). Dry-mounted specimens were used for hind tibial length, tibial spur length, length of hind tarsus, number of spines on hind tarsus I, number of teeth on tibial spur. To measure the paramere and the aedeagus, the specimens were cleared in a 10% KOH solution, dissected and mounted in glycerol on glass slides. Measurements of parts were made using the 10 × and 20 × objectives of a phase contrast microscope equipped with a linear, graduated ocular micrometer. Counts of the number of teeth on tibial spur and spine on hind tarsus were made under a compound microscope.
Studies on different morphological characters between rice and Leersia-infesting N. lugens from 11 locations.
| Location | Host plant | Length of tibia (mm) | Length of tarsus (mm) | Length of tibial spur (mm) | No. of tibial spur teeth | No. of spine of tarsus I | Length of paramere (mm) | Length of aedeagus (mm) |
| UPM1 | Rice | 0.809 (± 0.003) | 0.862 (± 0.009) | 0.462 (± 0.008) | 30.2 (± 0.07) | 2.6 (± 0.01) | 0.454 (± 0.009) | 0.552 (± 0.011) |
| UPM2 | Leersia | 0.800 (± 0.004) | 0.823 (± 0.008) | 0.439 (± 0.005) | 31.2 (± 0.06) | 2.8 (± 0.03) | 0.427 (± 0.010) | 0.509 (± 0.013) |
| TK1 | rice | 0.818 (± 0.007) | 0.854 (± 0.007) | 0.464 (± 0.007) | 30.6 (± 0.08) | 2.6 (± 0.01) | 0.443 (± 0.007) | 0.531 (± 0.010) |
| TK2 | Leersia | 0.805 (± 0.004) | 0.824 (± 0.009) | 0.423 (± 0.006) | 31.9 (± 0.10) | 3.0 (± 0.03) | 0.417 (± 0.010) | 0.514 (± 0.009) |
| MK1 | Rice | 0.817 (± 0.006) | 0.852 (± 0.008) | 0.452 (± 0.005) | 30.6 (± 0.11) | 2.1 (± 0.04) | 0.451 (± 0.010) | 0.531 (± 0.008) |
| MK2 | Leersia | 0.804 (± 0.004) | 0.822 (± 0.007) | 0.435 (± 0.007) | 32.2 (± 0.12) | 1.9 (± 0.05) | 0.424 (± 0.007) | 0.516 (± 0.007) |
| PK1 | Rice | 0.818 (± 0.004) | 0.852 (± 0.005) | 0.474 (± 0.004) | 30.5 (± 0.10) | 2.2 (± 0.03) | 0.450 (± 0.011) | 0.535 (± 0.009) |
| PK2 | Leersia | 0.809 (± 0.005) | 0.828 (± 0.006) | 0.431 (± 0.007) | 31.6 (± 0.07) | 1.8 (± 0.02) | 0.428 (± 0.008) | 0.518 (± 0.006) |
| SB1 | Rice | 0.813 (± 0.003) | 0.851 (± 0.004) | 0.482 (± 0.009) | 30.8 (± 0.10) | 2.8 (± 0.04) | 0.447 (± 0.010) | 0.532 (± 0.007) |
| SB2 | Leersia | 0.805 (± 0.004) | 0.821 (± 0.008) | 0.439 (± 0.006) | 32.0 (± 0.11) | 2.2 (± 0.04) | 0.424 (± 0.006) | 0.514 (± 0.008) |
| CH | Leersia | 0.849 (± 0.007) | 0.873 (± 0.006) | 0.386 (± 0.009) | 28.4 (± 0.10) | 1.8 (± 0.03) | 0.470 (± 009) | 0.479 (± 0.010) |
Acoustic courtship signals are emitted by the specialised stridulatory organs of BPH males and females; these are located at the junction of the metathorax and abdomen on each side of the body. Each organ comprises a sclerotized meta-coxata and a petal-like abdominal sclerite extended in front of the third strenopleuron (a sclerite in the lateral wall of the thorax, just above the base of the middle leg). For the stridulatory organ, a total of 10 characters were measured (Table 3). Twenty macropterus males and females were collected from each site of seven locations. Insects were heated at 50 °C in 95% ethyl alcohol on a hot plate for 10 min. Then, the specimens were macerated in 10% KOH solution for 15 min and washed with 95% ethyl alcohol. Left and right stridulatory organs (only the petal-like abdominal sclerite) were oriented and mounted on glass microslides and examined using oil emersion. Measurements were made with the 100 × objective of a phase contrast microscope fitted with a linear graduated ocular micrometer. Calibrated micrometer units were converted into microns.
Studies on different characters of stridulatory organs between rice and Leersia-infesting N. lugens from 11 locations.
| Location | Host–plant | Sclerite length (μ) | Sclerite width (μ) | No. of chitinous scales on sclerite | Chitinous Scale length (μ) | Chitinous Scale width (μ) | |||||
| M | F | M | F | M | F | M | F | M | F | ||
| UPM1 | Rice | 101.8 (± 0.21) | 140.0 (± 0.32) | 53.9 (± 0.14) | 66.5 (± 0.22) | 111.5 (± 0.25) | 122.9 (± 0.30) | 11.3 (± 0.10) | 12.5 (± 0.11) | 6.1 (± 0.10) | 7.0 (± 0.12) |
| UPM2 | Leersia | 110.8 (± 0.20) | 130.0 (± 0.33) | 48.2 (± 0.16) | 57.7 (± 0.23) | 98.5 (± 0.27) | 121.1 (± 0.32) | 9.5 (± 0.12) | 10.6 (± 0.12) | 5.2 (± 0.09) | 5.8 (± 0.13) |
| TK1 | Rice | 101.9 (± 0.22) | 139.8 (± 0.35) | 54.0 (± 0.17) | 66.4 (± 0.23) | 112.9 (± 0.26) | 122.6 (± 0.28) | 11.1 (± 0.11) | 12.8 (± 0.14) | 6.4 (± 0.12) | 7.2 (± 0.15) |
| TK2 | Leersia | 111.1 (± 0.24) | 129.5 (± 0.31) | 48.1 (± 0.15) | 58.0 (± 0.21) | 98.4 (± 0.25) | 121.4 (± 0.29) | 9.6 (± 0.10) | 10.4 (± 0.13) | 5.2 (± 0.14) | 5.7 (± 0.12) |
| MK1 | Rice | 100.0 (± 0.26) | 141.4 (± 0.33) | 53.2 (± 0.18) | 65.5 (± 0.20) | 110.7 (± 0.24) | 122.8 (± 0.33) | 11.8 (± 0.09) | 12.3 (± 0.15) | 6.2 (± 0.13) | 7.3 (± 0.14) |
| MK2 | Leersia | 110.5 (± 0.27) | 130.5 (± 0.30) | 48.0 (± 0.16) | 57.6 (± 0.23) | 99.1 (± 0.28) | 121.2 (± 0.31) | 9.7 (± 0.12) | 10.2 (± 0.13) | 5.3 (± 0.10) | 5.9 (± 0.11) |
| PK1 | Rice | 102.3 (± 0.19) | 140.8 (± 0.32) | 53.4 (± 0.13) | 65.9 (± 0.26) | 111.9 (± 0.26) | 122.5 (± 0.34) | 11.6 (± 0.13) | 12.7 (± 0.11) | 6.5 (± 0.08) | 7.2 (± 0.10) |
| PK2 | Leersia | 110.8 (± 0.24) | 131.0 (± 0.36) | 47.9 (± 0.13) | 57.5 (± 0.24) | 97.9 (± 0.24) | 120.9 (± 0.36) | 9.4 (± 0.14) | 10.5 (± 0.10) | 5.1 (± 0.11) | 5.8 (± 0.16) |
| SB1 | Rice | 99.90 (± 0.26) | 140.5 (± 0.34) | 52.8 (± 0.16) | 65.8 (± 0.22) | 111.8 (± 0.26) | 122.7 (± 0.38) | 11.5 (± 0.13) | 12.4 (± 0.12) | 6.3 (± 0.12) | 7.1 (± 0.14) |
| SB2 | Leersia | 109.8 (± 0.23) | 132.0 (± 0.30) | 47.8 (± 0.14) | 57.5 (± 0.21) | 98.9 (± 0.23) | 120.9 (± 0.40) | 9.5 (± 0.14) | 10.3 (± 0.10) | 5.2 (± 0.13) | 5.6 (± 0.11) |
| CH | Leersia | 112.5 (± 0.25) | 142.5 (± 0.34) | 55.5 (± 0.15) | 67.8 (± 0.24) | 113.5 (± 0.27) | 123.8 (± 0.38) | 12.4 (± 0.11) | 12.8 (± 0.14) | 6.8 (± 0.11) | 7.5 (± 0.08) |
The morphometric data of BPH with high esterase activity for rice population and low esterase activity for Leersia population were only analyzed using Jaccard's similarity coefficient [19] and run on NTSYS-pc Versions 2.1 [20]. These similarity coefficients were used to produce a dendrogram for which the UPGMA algorithm and SAHN clustering (unweighted pair group method using arithmetic average) were employed for depicting the genetic relationships.
2.2.2 Scanning electron microscopy (SEM)
Scanning electron microscopy was done to find out fine-structural differences of selected characters between two sympatric populations. As the host of two insect populations was different, there might be chances to get structural differences. The genitalia are also some important characters that distinguished insects, species especially the parameres and aedeagus for males. Nilaparvata species could be distinguished from other species of Delphacidae by the presence of number and shape of spines on the basal segment of the hind tarsus. Considering all the things, ten newly emerged macropterous males each of N. lugens from rice and Leersia were collected from the UPM experimental farm. The head, tibial spur, spine on the basal segment of the hind tarsus, hind tarsal claw, paramere, aedeagus, and labium were separated from the adult insects and treated with 10% NaOH for 10–15 min at 70° C. Then, the different parts of the insect were washed with distilled water. The specimens were pre-fixed in 2.5% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4) for three times, 10 min each and post-fixed in 2% osmium tetra-oxide in the same buffer. Before immersion in osmium tetra-oxide, the specimens were rinsed thrice with the same buffer. The fixed specimens were dehydrated with a seven-graded ethanol series, viz. 30, 50, 70, 90, 99, 99.9, and 100% for 5, 10, 15, 20, 25, 30, and 60 min, respectively. After dehydration, the specimens were transferred into a specimen basket and put into a critical point dryer for about 30 min. The specimens were mounted on SEM stubs with double adhesive tape, coated with gold ions (20 nm) in an ion sputter counter (JFC-1100) and observed under a SEM at 10–15 kV accelerating voltages [21].
2.3 Simple filter paper test for esterase activities
The insects used for morphometric and morphological studies were tested for esterase activity by a simple filter paper test [22]. Since allozymes and molecular markers can only detect two host-associated populations, one from rice with high esterase activity and the other from Leersia with low esterase activity [17,18]. So, insects with high esterase activities captured from rice and insects with low esterase activities captured from L. hexandra were considered for this experiment.
2.4 Host–plant relationship studies
Brown planthopper adults were collected from L. hexandra, growing in irrigation canals of the experimental farm of Universiti Putra Malaysia (UPM) and rice-infesting from the paddy field of the same farm. All the plants used in host–plant relationship studies were insect-free. All adults or nymphs from rice and Leersia-infesting population were used in the following experiments derived from insects with high and low esterase activities, respectively. In previous reports, no esterase activity test was performed on insect for host–plant relationship studies.
2.4.1 Nymphal development and survival of N. lugens on rice and L. hexandra
First instar nymphs (7 days after hatching) of N. lugens from the rice and Leersia-infesting populations were placed in test tubes (8′′ × 1′′) containing either a 35-day-old rice seedling and/or a single Leersia cutting. One insect was put in a test tube. Each population was replicated 25 times. The bottom part of each test tube (approx. 1 inch) was filled with rice cultivation soil (pH (H2O): 5.8, 0.9% organic matter, total C and N: 20.5 and 2.2 g kg−1). One millilitre of a solution of hyponex fertilizer (in mg L−1 as follows: N [100], P [200], K [100], Mg [10], Mn [0.02], B [0.1]) was added before planting to each test tube to support the plant growth during the duration of the experiment. Earlier rice seedling and Leersia cuttings were raised in the nursery, where all the plants were freed from insects. The rice-infesting population was tested on rice (cv.MR 84) and L. hexandra, and the Leersia-infesting population on rice and L. hexandra. The following data were recorded: (a) % mortality, (b) % insects reaching the adult stage, (c) mean weight of adult females that were produced, (d) days required to reach the adult stage, (e) duration (days) of each nymphal instar of each population. The experiment was laid out in a Complete Randomised Design (CRD).
2.4.2 Ovipositional preference of N. lugens from rice and Leersia
Ovipositional preference means that both populations of BPH have choice. To determine if the two populations of N. lugens exhibit any ovipositional preferences, gravid females of each population were given a choice among rice, L. hexandra and Ischaemum timorense (a weed grass abundantly grows in the field and sometimes brown planthopper observe on it). Individual gravid rice or Leersia-infesting female (same age) was released in a test tube (8′′ × 1′′) containing a rice seedling, a stem cutting of L. hexandra and a stem cutting from I. timorense. The females were allowed to oviposit for 72 h. The number of hatched nymphs was recorded daily from each host plant, until no more nymphs appeared; then, the plants were removed from the test tube and the unhatched eggs were counted separately. The plants were maintained for 15 days. The experiment was replicated 15 times and the experimental design used was a Complete Randomised Design (CRD).
2.4.3 Ovipositional response and egg hatchability of N. lugens of two different hosts
Ovipositional response means that both populations of BPH have no choice in this experiment. The experiment was conducted to determine ovipositional response and egg hatchability of two sympatric populations on non-host plants. The gravid female has no choice for oviposition in three different hosts. Individual gravid rice or Leersia-infesting females was released in the test tube (8′′ × 1′′) containing rice seedling or stem cuttings of L. hexandra or I. timorense. The females were allowed to oviposit for 72 h. The plants were maintained for 15 days and the number of emerging nymphs was recorded. At the end of nymph emergence, the plants were removed from the test tube and the unhatched eggs on the plants were counted. The experiment was replicated 15 times and was followed by the Complete Randomized Design (CRD).
2.4.4 Adult life span and fecundity of N. lugens from rice and Leersia
The life span of newly emerged brachypterous and macropterous males and females, and the fecundity of the females were determined on rice and Leersia plants. The potted plants were arranged in a Complete Randomized Design in a water-filled plastic tray, covered with Mylar film cages. Each pot received two plants of each species. The plants were infested at a rate of one pair of rice or Leersia-infesting N. lugens males and females per pot. There were ten replications in each treatment, each pot representing a replication. The survival of males and females was recorded daily up to 30 days after infestation. Nymphs emerging on the plants were counted as a measure of lifetime fecundity.
3 Results
3.1 Morphological studies
3.1.1 Morphometric analysis
All the characters showed clear differences in means between two sympatric populations of brown planthopper (Table 2). Irrespective of locations and hosts, rice-infesting insects with high esterase activities had higher average values of hind tibial length, tibial spur length, length of hind tarsus, length of paramere and length of aedeagus compared to Leersia-infesting population with low esterase activities. Leersia-infesting population showed higher number of spines on hind tarsus I and number of teeth on tibial spur. No overlapping existed between the two sympatric populations of N. lugens from each location in the morphometric study.
In the stridulatory organ, average values of width of petal-like sclerite, number of chitinous scale on sclerite, chitinous scale length and chitinous scale width in both males and females of the rice-infesting population of N. lugens with high esterase activities was higher compared to Leersia-infesting population, with low esterase activities for each location. Sclerite length of males of rice-infesting populations were always lower than that of Leersia-infesting populations (Table 3).
The UPGMA analysis resolved the individuals into three main clusters at coefficient level 1.00. The UPM1, TK1, MK1, PK1, and SB1 populations (all rice-infesting populations; Table 1) were clustered into one group, and the Leersia-infesting populations (UPM2, TK2, MK2, PK2, and SB2; Table 1) were clustered into another group. The out-group population, CH (N. bakeri), was distant from the N. lugens populations but more similar to rice insects than to the Leersia insects (Fig. 1).
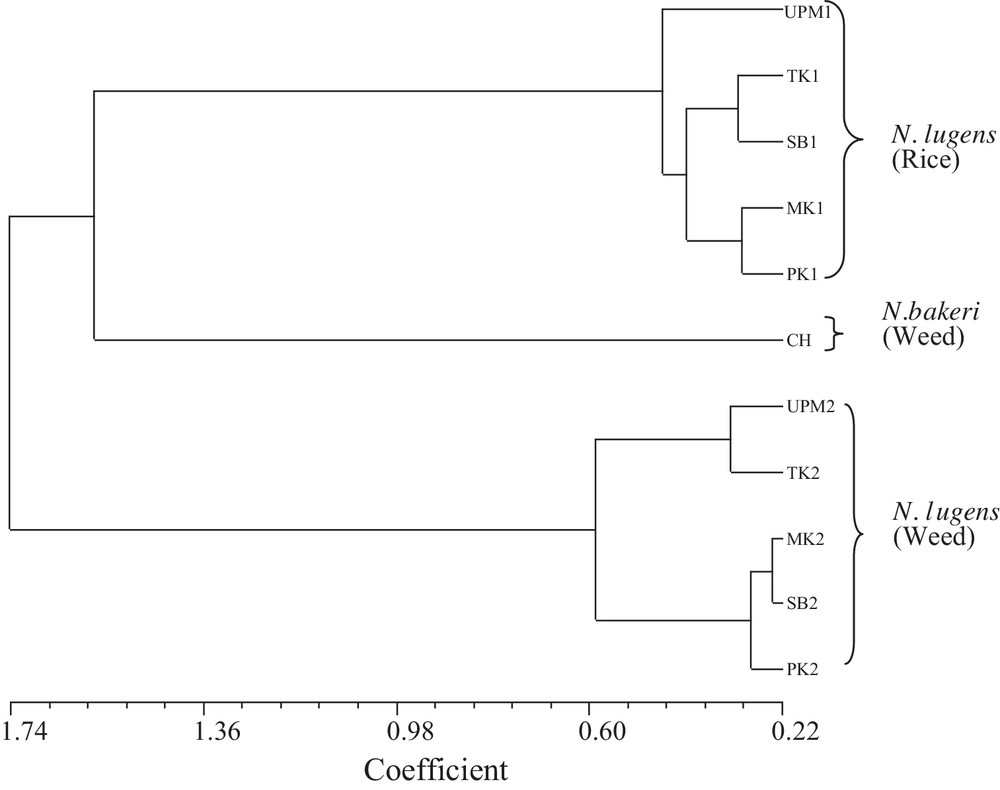
UPGMA tree of 11 populations of Nilaparvata spp. based on quantitative genetic distance. (UPM1, TK1, MK1, PK1, and SB1 = rice-infesting populations of N. lugens; UPM2, TK2, MK2, PK2, and SB2 = Leersia-infesting populations of N. lugens; CH = N. bakeri).
Quantitative genetic distances were computed between all individuals of 11 populations based on 17 quantitative characters. Among the rice-infesting populations, genetic distances ranged from 0.298 to 0.504, while Leersia-infesting population ranged from 0.240 to 0.726. The genetic distances also varied between two sympatric populations of N. lugens, one from rice and the other from L. hexandra, and ranged from 1.543 to 1.703. The distance between rice-infesting populations of N. lugens and Leersia-infesting populations of N. bakeri (an out-group) varied from 1.447 to 1.729, while the Leersia-infesting populations of N. lugens and Leersia-infesting populations of N. bakeri varied from 2.255 to 2.398 (Table 4).
Quantitative genetic distance of 17 traits in 11 populations of Nilaparvata spp.
| UPM1 | UPM2 | TK1 | TK2 | MK1 | MK2 | PK1 | PK2 | SB1 | SB2 | CH | |
| UPM1 | 0.000 | ||||||||||
| UPM2 | 1.627 | 0.000 | |||||||||
| TK1 | 0.420 | 1.551 | 0.000 | ||||||||
| TK2 | 1.703 | 0.320 | 1.618 | 0.000 | |||||||
| MK1 | 0.502 | 1.612 | 0.418 | 1.693 | 0.000 | ||||||
| MK2 | 1.682 | 0.580 | 1.600 | 0.651 | 1.563 | 0.000 | |||||
| PK1 | 0.458 | 1.629 | 0.308 | 1.724 | 0.298 | 1.607 | 0.000 | ||||
| PK2 | 1.682 | 0.618 | 1.614 | 0.726 | 1.570 | 0.254 | 1.614 | 0.000 | |||
| SB1 | 0.443 | 1.543 | 0.306 | 1.618 | 0.504 | 1.622 | 0.404 | 1.656 | 0.000 | ||
| SB2 | 1.686 | 0.428 | 1.601 | 0.521 | 1.598 | 0.240 | 1.629 | 0.310 | 1.607 | 0.000 | |
| CH | 1.668 | 2.331 | 1.529 | 2.398 | 1.447 | 2.317 | 1.510 | 2.255 | 1.729 | 2.361 | 0.000 |
The two-dimensional graphical view of Principal Coordinate Analysis (PCoA) showed the spatial distribution of the different populations of BPH across the two principal axes. The population CH was placed far from the centroid of the cluster, while the rice and Leersia-infesting populations of BPH formed two different clusters that were also placed comparatively less far away from the centroid (Fig. 2). The results indicated that the population placed far from the centroid was more genetically divergent, while the populations placed comparatively around the centroid possessed a more similar genetic background. However, the centroid may be defined as the vector representing the middle point of the cluster, which contained at least one number for each variable. The connecting line between each population and the centroid represented eigenvectors for the respective population.
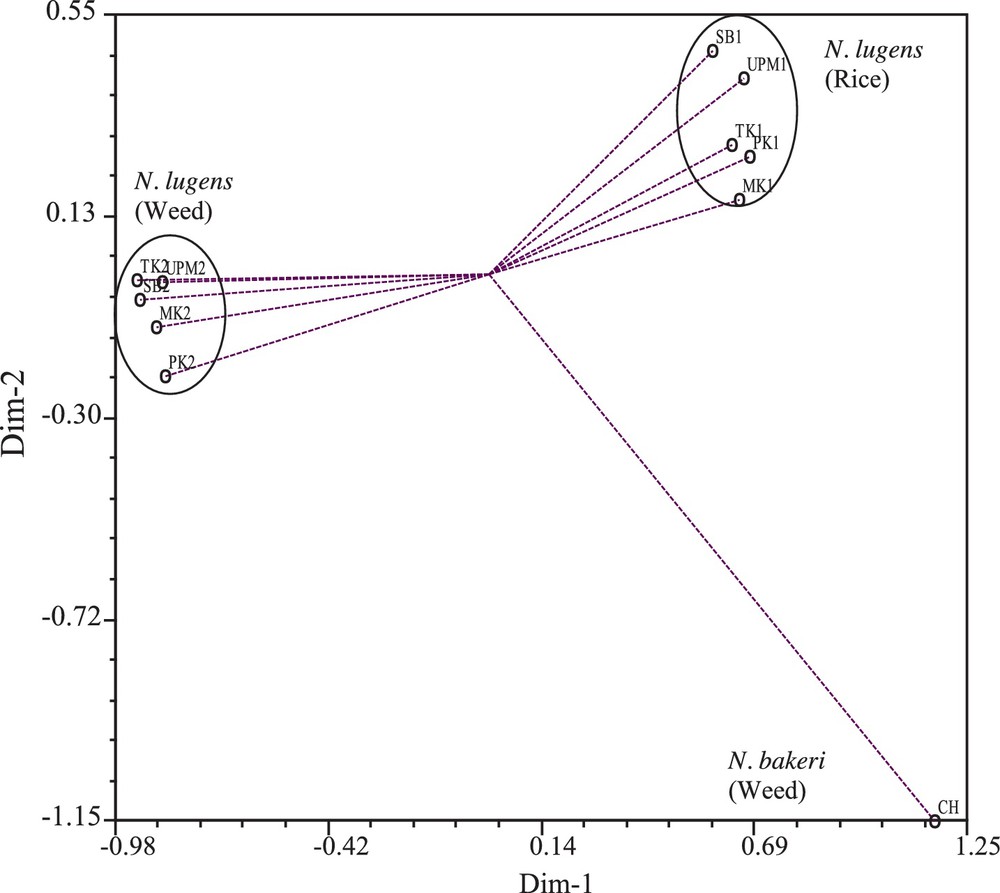
Two-dimensional view of Principal Coordinate Analysis (PCo A) with 17 quantitative characters over 11 population of BPH complex (UPM1, TK1, MK1, PK1, and SB1 = rice-infesting populations; UPM2, TK2, MK2, PK2, and SB2 = Leersia-infesting populations of N. lugens; CH = N. bakeri).
3.1.2 Scanning electron microscopy (SEM)
The head, tibial spur, spine on the basal segment of the hind tarsus, hind tarsal claw, paramere, aedeagus, and labium were mostly identical between two sympatric populations. Some of electron micrographs were shown for clarity. The size and shape of the tibial spur and spines on the basal segment of hind tarsus were similar in both rice- and Leersia-infesting populations of N. lugens (Supplementary data, Fig. 1S). Some variations were observed in the frequency of the number of spines on the hind tarsus between the two populations, but they were not population specific. Parameres were distinctively shaped and aedeagus were slender and upturned (Supplementary data, Fig. 2S). Supplementary data, Fig. S2 and S3 showed that both parameres and aedeagus were morphologically indistinguishable between the two sympatric populations of N. lugens. The number of spines in aedeagus remained essentially the same in both rice- and Leersia-infesting populations of N. lugens (Supplementary data, Fig. S3). Arrangement, size and shape of spines varied among the individuals, but were not population specific. An enlarged view of both labial tips (Supplementary data, Fig. S4) showed that sensory fields were symmetrical and that stylet groove was well defined. Sensilla were present on either side of the stylet groove. There was no significant variation observed between the two sympatric populations of N. lugens.
3.2 Host–plant relationship studies
3.2.1 Survival and nymphal development of N. lugens on both rice and L. hexandra
The rice population did not survive on Leersia, and the Leersia-infesting population did not survive on rice (Table 5). The mean weight of ten emerged adult females was not significantly different (P < 0.05) from one population to the other, and the same was true for the total duration of nymphal development (Table 5).
Survival of first instar nymphs of N. lugens from rice- and weed-associated populations tested on L. hexandra and rice.
| Population of origin | Host plant tested | No. of individuals/replicate | Survival (%) | Mean weight. of ten females (mg) | Nymphal period (days) |
| Rice-infesting | Rice | 25 | 82a | 2.15a | 13–16 |
| Leersia | 25 | 0b | – | – | |
| Leersia-infesting | Leersia | 25 | 80a | 2.01a | 14–17 |
| Rice | 25 | 0b | – | – |
The duration of each nymphal instar for the two populations did not differ statistically on their respective host plants, but differed significantly when reared on non-host plants and their duration of nymphal period from one instar to another instar was prolonged. Days of survival of the first- to the fifth-instar (survival from one stage to the next) nymphs of rice-infesting population on their respective host (rice) were 2.91, 2.29, 2.37, 2.63, 3.83d, respectively, while Leersia-infesting nymphs on their respective host (L. hexandra) were 3.00, 2.38, 2.45, 2.68, 3.85d, respectively. Rice-infesting nymphs died after 3.93d in the first instar and Leersia-infesting nymphs died after 8.28d (4.12 days at first instar and 4.16 days at 2nd instar), respectively, when reared on non-host plants (Table 6).
Duration (days) of each nymphal instar of the rice and Leersia-infesting populations reared on both rice and L. hexandra.
| Population origin | Host plants tested | Nymphal instars | ||||
| I | II | III | IV | V | ||
| Rice-infesting | Rice | 2.91b | 2.29b | 2.37a | 2.63a | 3.83a |
| L. hexandra | 3.93a | – | – | – | – | |
| Leersia-infesting | L. hexandra | 3.00b | 2.38b | 2.45a | 2.68a | 3.85a |
| Rice | 4.12a | 4.16a | – | – | – |
3.2.2 Ovipositional preference and response of N. lugens from two different hosts
In the choice experiment (ovipositional preference), gravid females from each population laid significantly more eggs on the host species from which originally they were collected, although each population laid eggs on non-hosts plants (Table 7). In the case of the rice-infesting population, the highest number of nymph emerged and the highest percentage of egg were hatched on the rice plant, followed by L. hexandra and I. timorense, while the Leersia-infesting population showed the highest number of nymphs and the highest percentage of egg hatch on Leersia plants followed by rice and I. timorense.
Number of nymph emerged in ovipositional preference and response tested for individually mated females of rice and Leersia-infesting populations of N. lugens on L hexandra, rice plant (MR 84) and I. timorense.
| Population of origin | Host plants tested | Total no. of nymph emerged | Egg hatchability (%) |
| Ovipositional preference (Choice test) | |||
| Rice | Rice | 442a | 86a |
| L. hexanrda | 114b | 73b | |
| I. timorense | 24c | 62c | |
| Leersia | L. hexandra | 403a | 82a |
| Rice | 52b | 70b | |
| I. timorense | 31c | 58c | |
| Ovipositional response (No choice test) | |||
| Rice | Rice (MR 84) | 518a | 88a |
| L. hexanrda | 179b | 84a | |
| I. timorense | 58c | 60c | |
| Leersia | L. hexandra | 467a | 87a |
| Rice | 118b | 76b | |
| I. timorense | 82c | 62c |
In the ovipositional response (no choice test), the Leersia-infesting population laid more eggs on Leersia hexandra, compared to rice and I. timorense, while the rice-infesting population laid more eggs on the rice host (Table 7). Oviposition decreased when rice plants and I. timorense were offered to the Leersia-infesting population or when L. hexandra and I. timorense were offered to the rice-infesting population. For both populations, the percentage of egg hatch was higher in their respective host plants compared to other non-host plants.
3.2.3 Adult life span and fecundity of rice and weed-associated populations of N. lugens
This was also a no choice test and the life span was studied for both sympatric populations. Survival of males and females from rice and weed-infesting populations of N. lugens was significantly higher (P < 0.05) on their respective host plants compared to non-host plants. In the case of the rice-infesting population, brachypterous and macropterous males survived on rice plants for 22.4 and 24.5 days, respectively, while females survived for 25.3 and 27.6 days, respectively. Similar trends were observed for brachypterous and macropterous males and females of the Leersia-infesting population. For both populations, the life span of macropterous insects was higher in comparison with brachypterous insects. All newly emerged adults from each host–plant species had died within 3 days when caged on the alternating host–plant species. Fecundity was higher in both brachypterous and macropterous rice- or Leersia-infesting females on their respective host plants compared to their non-host plants. Females of N. lugens of rice- and Leersia-infesting populations laid eggs on L. hexandra and rice, respectively, but in smaller amounts (Table 8). With respect to wing formation, brachypterous females of both populations laid more eggs compared to macropterous females.
Life span and fecundity of rice and weed-associated adults of N. lugens on rice (MR84) and L. hexandra.
| N. lugens | Host plant tested | Life span (days) | Fecundity (no. of eggs laid/female) | |
| Male | Female | |||
| Brachypterous | ||||
| Rice-infesting | Rice (MR84) | 22.4a | 25.3a | 478 |
| L. hexandra | 2.8b | 2.4b | 10 | |
| Leersia-infesting | L. hexandra | 19.2a | 22.4a | 352 |
| Rice (MR84) | 2.4b | 2.3b | 8 | |
| Macropterous | ||||
| Rice-infesting | Rice (MR84) | 24.5a | 27.6a | 430 |
| L. hexandra | 2.9b | 2.6b | 11 | |
| Leersia-infesting | L. hexandra | 21.7a | 24.2a | 303 |
| Rice (MR84) | 2.5b | 2.4b | 3 |
4 Discussion
The results from cluster and principal coordinate analyses revealed the morphological relationships between two sympatric populations with high and low esterase activity. Based on 11 populations of Nilaparvata spp., the average morphometric data of 17 traits of rice- and Leersia-infesting population of N. lugens and N. bakeri clearly showed that the rice-infesting populations of N. lugens formed a group, while Leersia-infesting populations formed another distinct group. It seems that the genetic structures of two sympatric populations are different. However, without esterase activity test, Saxena et al. [14] reported that no significant difference was found between the two host-associated populations of N. lugens in their morphometric study. But in our findings based on esterase activity, we found distinct differences between the two populations. Insects with high esterase activities that were generally caught from rice, and insects with low esterase activities that were generally caught from Leersia were analysed across our study. Similar morphological studies also reported by Blackman et al. [23] in Rubus-feeding aphids of the genus Amphorophora, i.e. A. rubi which feeds on Rubus caesius, R. fruticosus aggr., and other Rubus species and A. ideai, which feeds on R. idaeus and transmits raspberry viruses, are indistinguishable using a range of enzymes. However, both species can be separated using canonical variant analysis of eight morphological characters. Conversely, Fernando and Walter [24] reported that two populations of A. lingnanensis, the first one from California red scale and the other one from white louse scale, were reproductively isolated in the field, although no consistent anatomical difference was found between them. Both host-associated populations are two independent species.
Although qualitative morphological criteria are mostly convenient and useful for diagnostic purposes at the generic or species levels, the use of such criteria becomes difficult when dealing with sibling or cryptic species. These inter-specific taxa may, however, be revealed through statistical analyses of fine-structural evaluations of groups of specimens from various sources [25]. In the stridulatory organ that produces courtship signals, the rice-infesting population of N. lugens possessed shorter and wider sclerites than that of the Leersia-infesting population. The number, length and width of chitinous scales both in males and females in rice-infesting populations of N. lugens differed distinctly from Leersia-infesting populations. Based on the morphological characters of stridulatory organs, it was indicated that BPH with high esterase activity caught on rice were totally different from BPH with low esterase activity usually captured on Leersia. Since the acoustic signals differed in the two sympatric populations of N. lugens, our results based on esterase activities were consistent with the results of Saxena and Barrion [26]. The main disadvantage of morphometrics is that the method alone cannot easily distinguish between environmental and genetic contributions to the phenotype; therefore, it cannot directly establish the biological validity of a species, i.e., whether populations are reproductively isolated [27]. Our previous studies based on esterase activities showed that heterozygote deficiency was observed between two sympatric populations in nature, i.e. both populations are reproductively isolated based on allozymes analysis [18].
Saxena and Barrion [16] reported that karyoytpe, idiogram, nuclear organelles, and chromosomes with nucleolus organizing region showed clear differences between rice- and Leersia-infesting populations. Species differentiation in early stages of a species formation may not be associated with substantial genetic change [28,29]. Many ecologists have accepted that the evolutionary processes are common in animals with specialized food habits [30,31]. But, in our investigation, different population structures in N. lugens might have developed due to two reasons:
Therefore, this specialized food habit and physiological change might lead to changes in morphology at the adult stage.
Individual N. lugens derived from one host did not thrive on the other host; they suffered a significant reduction in survival and nymphal development, ovipositional preferences and ovipositional response. In nymphal development and survival experiment, we used a first instar nymph for a better adaptation of the nymph on the host and non-host plant, while previous authors used 4–5th instars nymphs or newly emerged adults. Our results corroborate those of Khan et al. [32]. Based on adult life span and fecundity, the two populations were found to be different from each other. Host plant is a very vague term. It is important at least to differentiate between plants on which adults will alight and possibly feed, plants on which eggs may be laid and plants on which immature individuals will develop from the first instar to reproductive adults in the case of N. personata [33]. The quantity of food ingested and assimilated by rice and weed-infesting individuals of N. lugens was significantly higher on their respective hosts. Ingestion and assimilation of food were significantly reduced when individuals derived from one host were caged on the other [1]. So, host plant differentiation played an important role in speciation. Our findings based on esterase activities, survival and nymphal development, ovipositional preferences, ovipositional response, egg hatchability and adult life span were in agreement with results of Heinrichs and Medrano [11], Saxena and Pathak [34], Domingo et al. [9] and Claridge et al. [12], according to which two sympatric populations of N. lugens showed strong preferences for their own host plants and very low preference for the other host plant. In host–plant relationship studies, we used insects or nymph from known esterase activities, while previous authors did not perform any esterase activity test in their experiment. Egg hatchability also influenced the establishment of those insects that lay eggs inside the plant tissue, as reported by Saxena and Pathak [35]. All species of leafhopper and plant hoppers, as far as it is known, are herbivores. Not only do the nymphs and adults generally feed on the host plant, but also the eggs are usually laid within its tissues. Thus, the relationship with the host is very close. In most groups so far studied, a tendency towards host–plant specificity had been shown [36,37]. In terms of host–plant relationship, populations of N. lugens of rice differed from the populations of L. hexandra. This implies that the two populations are two different biological species. In many other cases, different host-associated forms have very low gene flow and might better be characterized as sibling species, such as the Enchenopa tree hoppers and certain other species allied closely to Rhagoletis pomonella [38,39]. Based on Mayr's biological species concept, Frolov et al. [40] hypothesized that the two Ostrinia taxa primarily based on host–plant type found in France may constitute two different species, O. nubilalis and O. scapulalis. Malausa et al. [41] suggested that there was a low level of gene flow or that heterozygote deficiency existed between the two Ostrinia taxa. Heterozygote deficiency also existed between two host-associated populations of brown planthopper [18]. Sibling species are more likely to be common in nature than anticipated under the isolation concept, because individuals themselves define or specify their genetic affiliations through their mating behaviours in nature [42]. The issue of sibling species has not been fully resolved under the isolation concept [43], mainly because of the emphasis on isolating mechanisms and because taxonomic concepts of species are still conflated with genetic concept of species.
These two host-associated populations of brown planthopper were reproductively isolated [12] and heterozygote deficiency or low gene flow existed in nature [18]. Therefore, our morphological and host–plant relationship studies clearly indicated that BPH with high esterase activity usually captured from rice plant and those with low esterase activity, usually captured from L. hexandra in Malaysia, represent two distinct closely related sibling species, which supported the results as reported by Latif et al. [17].
5 Conclusion
A series of experiments were conducted on morphological and host–plant relationship studies to differentiate two sympatric populations of brown planthopper (BPH), N. lugens, one from rice (O. sativa) and the other from L. hexandra, a weed grass. A UPGMA dendrogram using seventeen quantitative morphological characters, including stridulatory organs (courtship signal-producing organs) between two sympatric populations of N. Lugens, one from rice with high esterase activities and the other from L. hexandra with low esterase activities, revealed that both populations are separated from each other. Rice plants were most suitable for the establishment of the rice-infesting population, and L. hexandra was for the Leersia-infesting population. Brown planthopper derived from rice did not survive on the L. hexandra and, as a result, significant reduction was found in survival and nymphal development, ovipositional preferences and responses. Morphological and host–plant relationship studies indicate that rice-associated population with high esterase activities and L. hexandra-associated population with low esterase activities are closely related species.
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.
Acknowledgements
The authors greatly acknowledge the RUGS project (01-02-12-2033RU), Universiti Putra Malaysia for providing research facilities and financial support for these research activities.


