1 Introduction
The genus Alexandrium belongs to one of the most important genera of harmful algal blooms (HABs) producing Paralytic Shellfish Poisoning (PSP) toxins [1]. Alexandrium species have a worldwide distribution, being present in coastal zones, continental shelf waters, in temperate as well as tropical areas [2]. The species A. catenella, A. tamarense and A. fundyense are the most toxic of the genus Alexandrium. Together, these species make up the A. tamarense complex [1,3]. Such species are often observed around the Mediterranean region [2]. In the northern part, A. catenella has first been reported in the Balearic islands in 1983 [4], and later on the French [5], Spanish [6] and Italian [7] coasts. In the southern part, Alexandrium species have been reported in Morocco [8–11], Tunisia [12,13], and Algeria [14].
A. catenella blooms in inorganic nutrient rich [6,15] and nutrient poor [16,17] waters. This is from the viewpoint of inorganic nutrients. The A. tamarense complex has been classified as a « frontal zone taxon » or « mixing-drift » group [18]. and therefore characteristic of areas that are unstable and intermediate between high and low inorganic nutrients. Very little is known generally about dissolved organic nutrients at those sites. It is therefore difficult to find recurrent patterns of bloom development for this mixotrophic species in relation with trophic conditions. Comparative studies of HABs ecosystems have been recognized as a way to deal with such difficulties and could “reveal fundamental processes governing population development” [19].
In this study, we compare A. catenella/tamarense dynamics in two contrasted sites in the Mediterranean. The first one is the relatively eutrophic [14] Bay of Annaba (Algeria) and the second is the Thau lagoon (France) that has recently gone through a period of oligotrophication [20]. We try to relate those blooms to environmental factors in those two coastal sites on both sides of the Mediterranean Sea, and deduce possible causes of their recent development and recurring presence. We study 3 different years over an 18-year period, one being “pre-Alexandrium”, one coinciding with the first A. catenella bloom in Annaba and the last one with a very large A. catenella bloom in Annaba. As the Thau lagoon blooms were connected with oligotrophication, we selected a period over which the same phenomenon appeared to take place in Annaba.
2 Material and methods
2.1 Study sites
The Bay of Annaba is located in the eastern part of Algeria (Fig. 1), between cape Rosa (36°38′N, 8°15′E) and Cape de Garde (36°38 N, 7°16′E), with a maximal depth of 50 m. The main temporary rivers (oueds) are Oued Seybouse (second river in Algeria), Oued Boudjemâa carrying urban and industrial effluents, and Oued Kouba carrying sewage waters. A. catenella has bloomed periodically since 2002 in this bay [14].
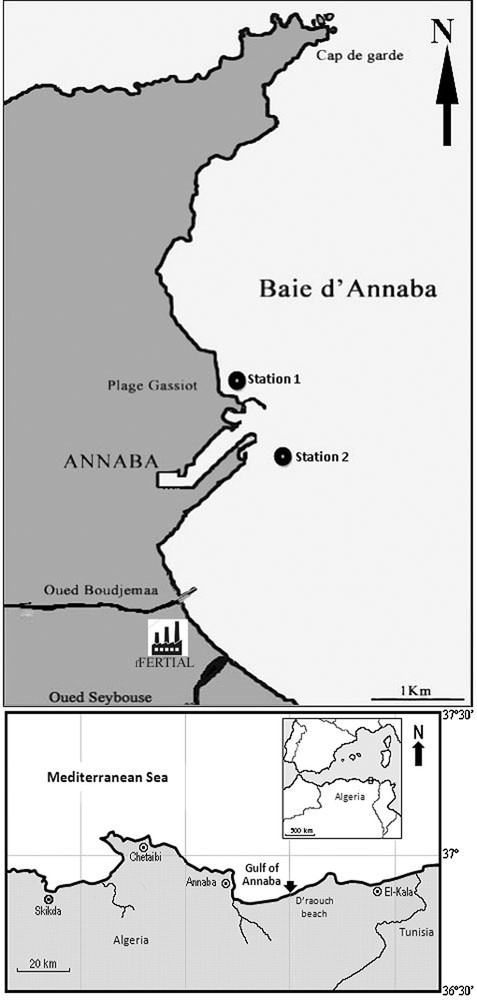
Station positions in Annaba Bay, Algeria.
The Thau lagoon is a shallow marine lagoon located on the French Mediterranean coast (43°24’N, 3°36’E) covering 75 km2 (Fig. 2). The mean depth is about 4 m, with a maximum of 10 m. The lagoon is connected to the sea by 3 narrow channels. Three oyster farming areas are located along the northwestern shore. The lagoon represents 10% of French annual oyster production and is the main oyster production center on the Mediterranean. Since 1998, it has experienced recurrent blooms of A. catenella that periodically threaten economic activities [20].
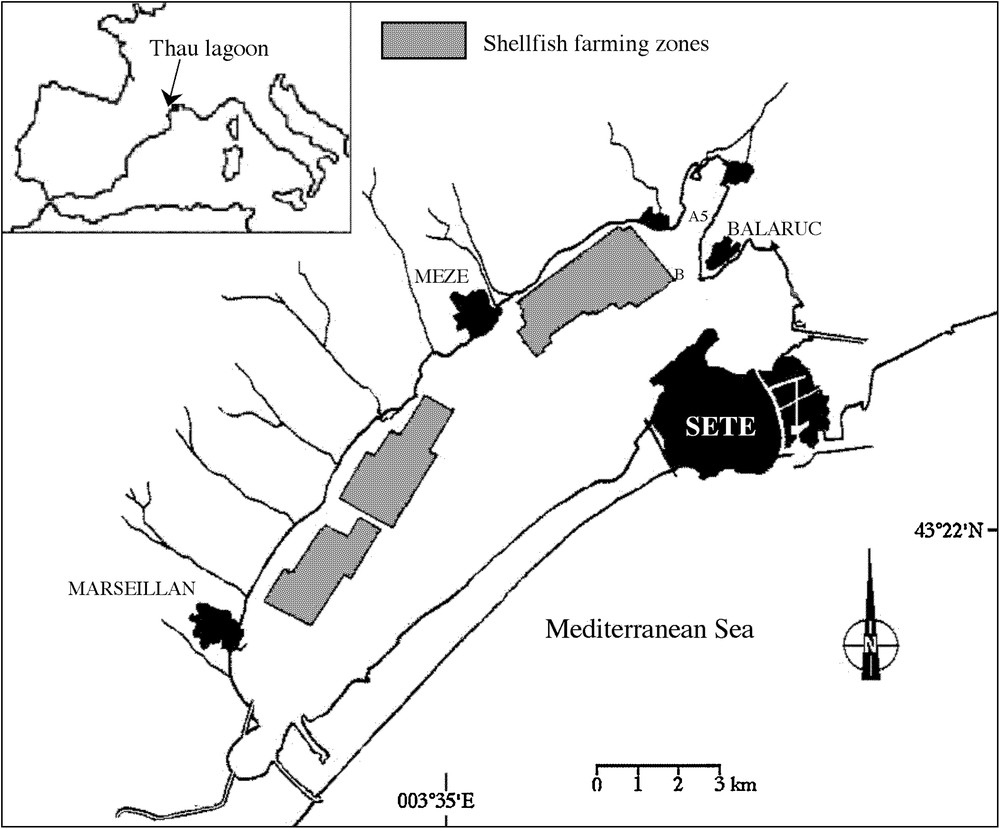
Station positions in Thau lagoon, France.
2.2 Sampling
2.2.1 Sampling stations for Annaba Bay
Station 1 (36°54.073′N, 7°46.929′E) has a depth of 5 m and is sheltered from dominant (NW) winds, with a rocky/sandy bottom.
Station 2 (36°53.976′N, 7°47.111′E) is deeper (13 m) than station 1 and is located in front of the commercial harbor. It is subject to major land influence through input from Oued Seybouse and Oued Boudjemaa and is considered eutrophic relative to station 1. Bottom sediments are silty. The sampling frequency was twice a month.
2.2.2 Sampling stations for Thau lagoon
Station B (43°26.070′N, 3°39.920′E) is located at one of the deepest part of the lagoon (8 m).
Station A5 (43°26.916′N, 3°40.300′E) is located in the Angle Creek and is shallower (2 m) and more sheltered than Station B. The sampling frequency varied from 1 to 8 samples per month for physical variables. For chemical and biological variables, sampling was carried out at least twice a month at station B.
2.3 Physical variables
In Annaba, surface temperature and salinity were measured with a WTW 191 multiparameter probe (precision 0.1 °C for temperature and 0.05 for salinity).
For Thau, the Ifremer observation network provided records of surface water temperature and salinity (monthly means).
2.4 Nutrients
At both sites, samples for ammonium determination were immediately fixed and measured at the laboratory [21]. Samples for the other nutrients were kept in the cold and dark, then frozen at −20 °C until analysis. Nitrate [22], nitrite [23] and soluble reactive phosphorus (SRP) [24] were measured after thawing at room temperature. Detection limits were 0.05, 0.01, 0.05 and 0.02 μM for nitrate, nitrite, ammonium and SRP respectively.
2.5 Biological variables
2.5.1 Chlorophyll
In Annaba, chlorophyll a was measured by spectrophotometry [25]. In Thau lagoon, Chlorophyll a was estimated from 90% acetone extracts and fluorimetry [26] or spectrofluorometry [27].
2.5.2 Microphytoplankton
In Annaba, for the qualitative survey of phytoplankton, horizontal net tows were carried out at 50 cm below the surface, with a 20 μm mesh size net. During Alexandrium blooms and for the quantitative study, bucket sampling was used, and 40 l were filtered on a 20 μm mesh size net. Samples were then resuspended in a 100 ml volume, then subsamples of 1 or 5 ml were used depending on the cell density. Samples were immediately fixed with buffered formaldehyde (5% final concentration). Identification was by electron microscopy in 2002 [14] and by optical microscopy in 2010, using morphological criteria. Both 2 cell and 4 cell chains were observed.
In Thau lagoon, at least one liter of seawater was taken with a sampling bottle. Additions of 0.2 to 0.4 ml Lugol's solution per 100 ml was carried out for quantitative analysis. A counting chamber was filled with 10 ml of fixed sample for sedimentation [28].
Phytoplankton cells greater than 10 μm equivalent cell diameter were counted by optical microscopy.
2.6 Statistical analyses
Variables were compared by Kruskal-Wallis one-way analysis of variance by ranks and Dunn's multiple comparison tests.
3 Results
In 1992, A. catenella/tamarense had not yet been recorded either in Thau lagoon [5] or in Algeria [14].
3.1 Alexandrium catenella/tamarense blooms
A. catenella was first observed in Annaba in March 2002 and in Thau in July 1995. Temporal changes of cell densities during 2002 are shown for station 2 in Annaba and stations A5 and B at Thau (Fig. 3). Highest monthly mean cell densities (117000 cells L−1) in Annaba were recorded in March with a secondary peak in December (200 cells L−1). In Thau, monthly mean cell densities were generally higher at A5 (maximum of 66,700 cells L−1 in May) than at station B (maximum of 5400 cells L−1 also in May), with a secondary peak in August (9600 cells L−1).

Changes in monthly mean Alexandrium catenella/tamarense cell densities during 2002. Diamonds: Thau station A5; squares: Thau station B; triangles: Annaba station 2. Detection limits: 100 cells L−1.
In 2010, 2 stations were sampled in Annaba (Fig. 4) with higher cell densities at Station 2 (up to 681000 cells L−1 in May). At station 1, monthly mean cell densities never exceeded 73,000 cells L−1 (also in May). For individual dates, a record peak of 1.38 × 106 cells L−1 was recorded in May 2010 at the surface of station 2. This was the highest cell density ever recorded so far in Annaba.
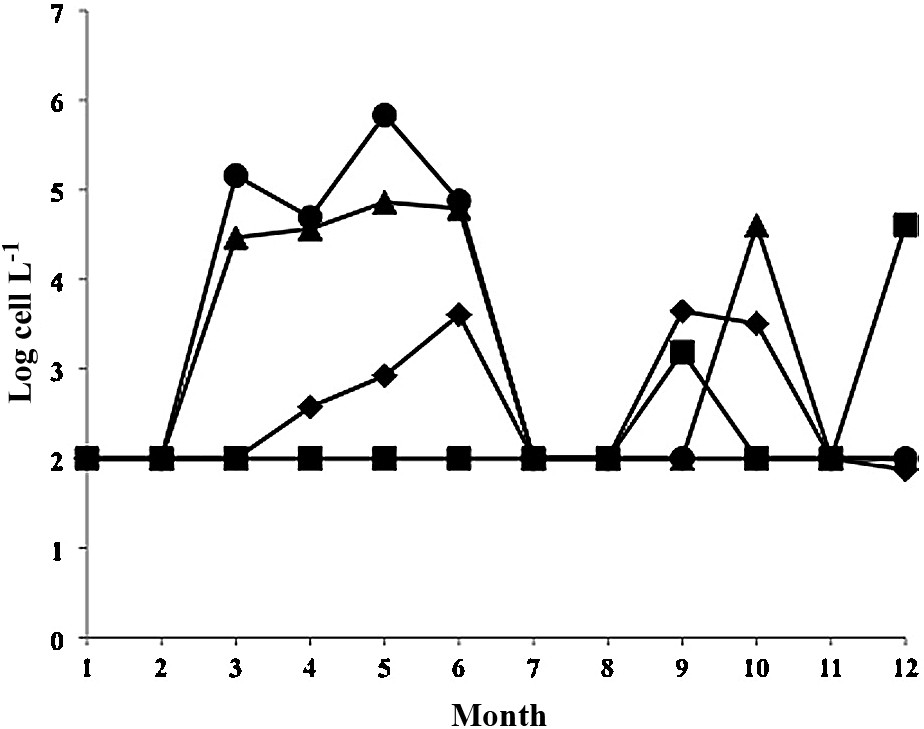
Changes in monthly mean Alexandrium catenella/tamarense cell densities during 2010. Diamonds: Thau station A5; Squares: Thau station B; triangles: Annaba station 2; circles: Annaba station 1. Detection limits: 100 cells L−1.
In Thau, during the same year, no bloom developed in the spring, but cell densities reached a maximum of 4400 cells L−1 in September at station A5 and 1500 cells L−1 also in September at station B.
In 2002, there appeared to be no synchrony in blooms between sites (Fig. 3), but in 2010, there was a bimodal distribution at both sites, with maxima in May–June and September–October (Fig. 4).
3.2 Environmental factors
Table 1 summarizes the ranges of physical and chemical variables at both sites. Sea surface temperatures were higher in Annaba than in Thau. The range of salinities was larger in Thau than in Annaba, with maximal values being greater in Thau lagoon. Maximal nitrate, ammonium and SRP concentrations were observed in Annaba, and were sometimes greater than in Thau by an order of magnitude, such as for ammonium. Concerning phytoplankton biomass, the range of Chl a values was about the same.
General physical and chemical features at both sites. Range of values (monthly means) over several years (1992, 2002 and 2010 for Annaba; 1992 to 2010 for Thau).
| Site | SST (°C) | S | NO3 (μM) | NH4 (μM) | SRP (μM) | Chl a (μg/liter) |
| Annaba | 14.1–28.6 | 35.3–38.1 | U-65 | U-110 | U-43 | 0.8–32.8 |
| Thau | 5.0–25.6 | 29.5–39.2 | U-20 | U-10 | U-10 | 0.3–37.4 |
Table 2 summarizes the changes in environmental parameters occurring over an 18-year period. The year 1992 can be considered as a “pre-Alexandrium” period for both sites. No significant changes in either annual mean SST, salinity (not shown) or ammonium occurred over this period at either site. In Annaba, the most spectacular changes were due to nitrate concentrations that decreased by a factor of 50 (significant difference with p < 0.001) and specifically SRP concentrations that decreased by a factor of 250 (significant difference with p < 0.001), from a mean annual value of 17.4 μM in 1992 to 0.07 μM in 2010. In Thau, the most dramatic decrease in SRP occurred between 1972 and 1992 (from about 10 to 1 μM in summer and from 3 μM to undetectable levels in winter). Between 1992 and 2010, the decrease was significant (p < 0.05).
Changes in environmental factors at two coastal sites North and South of the Mediterranean for 3 selected years over an 18-year period. Seasonal distributions are available at http://www.st.nmfs.noaa.gov/nauplius/media/time-series/site__mediterranean-thau-lagoon-phy/copepodite/index.html for Thau and in [34] and [14] for Annaba respectively in 1992 and 2002.
| Year | SST (°C) | Nitrate (μM) | Ammonium (μM) | Soluble reactive P (μM) | Chlorophyll a (μg/liter) | ||||||||||
| Mean | SD | n | Mean | SD | n | Mean | SD | n | Mean | SD | n | Mean | SD | n | |
| Annaba | |||||||||||||||
| 1992 | 21.6 | 4.7 | 8 | 25.8 | 20.5 | 8 | 49.9 | 37.7 | 8 | 17.4 | 14 | 8 | 10.3 | 9.5 | 6 |
| 2002 | 19.5 | 4.1 | 12 | 1.9 | 3.0 | 12 | 11.6 | 15.3 | 12 | 1.2 | 1.8 | 12 | 9.0 | 11.3 | 11 |
| 2010 | 20.7 | 4.8 | 11 | 0.5 | 0.9 | 12 | 23.6 | 24.3 | 11 | 0.07 | 0.13 | 11 | 2.4 | 1.4 | 11 |
| Thau | |||||||||||||||
| 1992 | 18.4 | 7.1 | 12 | 0.5 | 0.5 | 11 | 1.4 | 3.1 | 10 | 1.2 | 1.2 | 10 | 1.5 | 0.6 | 9 |
| 2002 | 15.9 | 5.6 | 12 | 0.2 | 0.3 | 3 | 1.1 | 0.7 | 10 | 0.5 | 0.2 | 10 | 3.0 | 1.5 | 12 |
| 2010 | 15.1 | 6.9 | 12 | 0.2 | 0.1 | 3 | 0.7 | 0.6 | 3 | 0.2 | 0.1 | 3 | 2.2 | 1.1 | 11 |
4 Discussion
Although nitrate decreased dramatically between 1992 and 2010 in Annaba, nitrogen limitation is unlikely because ammonium levels remained high (Table 2). The decrease in SRP was due to the discontinuation of phosphogypsum outputs from a local fertilizer plant in Annaba (M. Retima, pers. com.), and from implementation of sewage treatment plants in Thau [29]. Concerning A. catenella ability to use low SRP levels, laboratory studies show that the half-saturation constant for SRP uptake was variable in cultures, depending on the growth rate, but could reach values as low as 0.03 μM for strain ACT03 and 0.01 μM for strain TL01 [30]. This indicates that A. catenella could be a very strong competitor at the low SRP concentrations recently observed in Annaba Bay. An alternative to its periodic dominance is dissolved organic phosphorus use. Alkaline phosphatase activity was induced in A. catenella at SRP levels between 0.4 and 1 μM [30] that are intermediate values between those observed in 2002 and 2010 in Annaba Bay.
Thus it seems very likely that the appearance of blooms of A. catenella in Annaba Bay is due to SRP reaching very low levels where other members of the phytoplankton community cannot compete for the acquisition of this limiting resource.
This situation is similar to that experienced in Thau lagoon [20] where SRP reached undetectable winter levels in 1992–1993 and A. catenella was first reported in 1995.
A parallel can also be made with the Seto Inland Sea (Japan) where SRP decreased from 0.7 to 0.2 μM between 1978 and 1984 [31]. A. catenella vegetative cells were first reported in this area in 1982 [32], so that A. catenella probably started to bloom when SRP reached levels between 0.7 and 0.2 μM. In the Gulf of Olbia (Sardinia, Italy), the first A. catenella bloom was reported in 1999 [33] under conditions of SRP below 1 μM that were lower than observed in 1992–1993, a situation also similar to the results presented here.
5 Conclusions
This inter-site comparison between the Northern and Southern coasts of the Mediterranean has revealed that one of the causes of the development of A. catenella/tamarense is the onset of inorganic phosphorus limitation in both environments that allowed this organism to develop periodically and produce recurrent blooms. No other environmental variable could be related to the emergence of this organism. Such an explanation is an alternative to the general concept that eutrophication leads to harmful algal blooms and may require a revision of coastal water management policies.
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.
Acknowledgements
We wish to thank the anonymous referee and Ian Jenkinson for useful comments.


