1 Introduction
Clementine tree (Citrus clementina Hort. ex Tan.) was introduced in Algeria in 1902. Citrus cultivation in Central Mitidja and particularly clementine tree is subjected to various stresses related to water availability, technical methods and regulation of pesticide application. Moreover, ageing of orchards accelerated the development of pests, leading to yield decrease. Aphids are the most dangerous pests for clementine trees for two reasons. First, they directly weaken plants as a result of sap harvesting and stings causing a leaf whorl [1]. As a result, the foliar area decreases and photosynthesis is disturbed. The second reason is an indirect weakening due to the injection of Citrus tristeza virus (CTV) by aphids into leaves during stings (which causes plant diseases) and honeydew secretion by aphids, which facilitates fungi development and thus a subsequent production of sooty mould on leaves and fruits [1,2]. Several studies related to aphid populations in Algeria highlighted the necessity to act rapidly against these insect pests [3–6].
Aphids are multivoltine insects with an alternation of an amphisexual generation (with fertile males and females) and generally of several generations with only parthenogenetic females [7]. Fertile females (oviparae) are oviparous, whereas parthenogenetic females are viviparous and thus produce larvae rapidly able to feed and move [8]. At the adult stage, parthenogenetic females are winged or wingless (several morphs). Wingless females play an important role in colonization and reproduction, whereas winged females are involved in population dispersal. Several studies showed that three aphid species are regularly detected on Citrus species: Aphis spiraecola (Patch, 1914), Aphis gossypii (Glover, 1876) and Toxoptera aurantii (Boyer de Fonscolombe, 1841) [3,5,6,9–11].
A. spiraecola is considered as polyphagous, but it colonizes only few botanic genera such as Pyrus, Prunus, Malus, and more particularly Citrus [12–14]. Outside Japan, it has been shown that this aphid is permanently parthenogenetic on Citrus trees [7]. In Italy, this aphid species is more specifically present on clementine, mandarin, and orange trees [11]. A. gossypii is one of the most polyphagous aphid species, despite several host races have been described [7,15]. It is detected on plant species belonging to more than 100 families and considered as a casual pest on Citrus clementina with minor consequences by contrast to the damages on cotton or melon, which are more sensitive to this aphid species [7,16]. As in the previous aphid species, A. gossyppi reproduces continuously by parthenogenesis in the Mediterranean Basin [7]. A. spiraecola and A. gossypii are responsible for an important yield loss on clementine trees in Tunisia (T. aurantii being more sporadic) and populations of A. spiraecola are generally the most abundant on these trees, and particularly in summer [17]. In Japan [13], it was also shown that A. spiraecola population remained significantly more abundant on clementine trees than A. gossypii from mid-June to the end of October. However, this is not always the case, as reported Marroquín et al. [18] on various Citrus species in Spain.
The auxiliary fauna can reduce the proliferation of aphids. Indeed, volatile compounds from honeydew can act as signals of food, prey, host or place for oviposition [19]. Several works showed the effect of Coccinellidae on aphid populations [20–23]. For example, larvae of Coccinella septempunctata are very sensitive to honeydew and adults produce more eggs when honeydew secretion is important [24,25]. Larvae of Cecidomyiidae and Syrphidae are also known as efficient predators and Neuroptera (Chrysopidae family) can affect populations of A. gossypii and Myzus persicae [26–29]. However, the efficiency of the auxiliary fauna depends on climatic conditions and geographical regions as well as on the abundance of aphid populations and food [30].
The aim of this work was to assess some parameters that could explain why and when populations of A. spiraecola are more abundant than A. gossypii on clementine trees. Several hypotheses can be raised: on the origin of these different levels:
- • predation rate by auxiliary fauna;
- • reproduction rate;
- • assimilation of energetic metabolites of the leaves and;
- • sensitivity to several compounds produced by the infested plants.
We were interested in the energetics of aphid (measure of total carbohydrates and lipids) as biological markers of survival and reproduction of insects [31]. Through weekly samplings during one year in the field, a first part of this study consisted in the survey of the larvae and adult populations of aphids and their auxiliary fauna. A second part focused on the quantification of several metabolites in clementine tree leaves, which may play a role in the regulation of aphid populations. A low-quality food causes ovary regression and a subsequent lower or delayed egg production [32]. Thus, we measured total soluble carbohydrates and free amino acid contents, sources of energy and nitrogen for aphids, respectively [33]. We focused on proline content as this amino acid has its own variations linked to drought and biotic stresses [34,35]. This compound was shown to have positive effects on most aphid growth and populations, but an excess seems to be a limiting factor for A. gossypii, at least in cotton [36,37]. Tannins were also assayed as they are involved in defense mechanisms against herbivores by disturbing digestion. Indeed, they bind and precipitate mucoproteins of their oral cavity [38,39]. It was shown on various plants that leaves containing high concentrations of tannins are less sensitive to herbivores than leaves with lower tannin content [40]. As for aphids, the consumption of tannin-enriched leaves can decrease fertility, as shown in Aphis craccivora [41].
2 Materials and methods
2.1 Study site and sampling methods
Mitidja is the most extensive sub-littoral plain of Algeria, covering about 1400 km2 with a mean altitude of 50 m ASL. The study was performed in 2010 in the area of Oued-El-Alleug located at 10 km West of Blida in Central Mitidja. The 0.59 ha study site is located at North-East of Oued-El-Alleug with an altitude of 14.76 m, a latitude (DMS) of 36° 33′ 19′′ N and a longitude (DMS) of 2° 47′ 25′′ E.
The Bagnouls and Gaussen ombrothermic diagram for 2001–2010 and for year 2010 indicated a dry period of 8 months from the beginning of April to the middle of October (Fig. 1). Mean rainfalls from 2001 to 2010 were 695.08 mm per year and were 756.56 mm for the year 2010. The coldest month was generally January, whereas August was often the warmest one. The mean of minima for the coldest month was recorded in January 2005 with a value of 1.5 °C, whereas the mean of maxima of the warmest month was recorded in August 2005 with a value of 40 °C (Fig. 1). For 2010, the warmest temperatures were recorded between June and August, with mean values of 34–36 °C.

Bagnouls and Gaussen ombrothermic diagram at Oued-El-Alleug in Central Mitidja. Data were given for the year 2010 and for the period 2001–2010.
The Emberger pluviothermic quotient Q2 [42] was calculated to assess the bioclimatic stage of the site using the following formula: Q2 = 2000 × P/(M2–m2) with P, M, and m corresponding to mean annual rainfalls (mm), highest temperatures of the warmest month (K) and lowest temperatures of the coldest month (K), respectively. The bioclimatic stage of the site was considered as sub-humid with a cool winter, as within the period 2001–2010, the calculated Q2 was 83.17 and 60.035 for 2010.
As other Citrus species, clementine tree has three sap flows during the growing season [43]. In Central Mitidja, the first sap flow occurs from the end of March to the beginning of June. During this period, we observe leaf development, flowering, reproduction, fruit set and the beginning of fruit growth [44,45]. The second sap flow takes place from mid-June to the beginning of August, with high temperatures and precipitation nearly absent. For clementine trees, this period is characterized by a fast growth of fruits, which reach their optimal size. Citrus species need an annual rainfall of 1000 to 2000 mm [46]. During summer, irrigation is necessary and represents approximately 50% of the tree requirements [47]. The third sap flows occurs from the end of August to the end of October and corresponds to autumnal rainfalls. This period facilitates fruit maturation characterized by an accumulation of carbohydrates and secondary metabolites.
Insect (aphids and auxiliary fauna) and leaf samplings were performed weekly from February to November 2010. The field contained 228 trees planted every 6 m and was surrounded by a hedge of Taxodium distichum used as windbreak. According to the transect method [48], it was divided into 3 blocks of 10 trees with blocks separated by 24 m from each other. Control and aphid-infested leaves were harvested on each tree irrespective of its size. According to Djazouli et al. [49], destructive samplings of branches bearing young leaves were done in each cardinal direction (around 25 leaves per direction), at 1.50 m above the ground, so four sub-samplings were considered per tree. Given the size of foliage per tree, this harvesting had a negligible effect on aphid populations. Leaves used for aphid and auxiliary fauna identification and quantification were put in a plastic bag, preventing insects to escape, and stored at 4 °C to stop insect development. Both aphid species and auxiliary fauna were quantified according to the stages of development: egg, larvae (first and second stages were grouped together as well as third and fourth stages), nymph, chrysalis, winged and wingless adults. For both aphid species, wingless females were stored at –20 °C until analyses were carried out. Clementine tree leaves were dried in an oven at 70 °C for 24 h and then at 100 °C for 24 h. They were then roughly grinded and then transformed into fine powder in a blender (Retsch ZM 200, Germany) with a sieve of 0.5 mm porosity.
2.2 Energetic reserves of aphids
2.2.1 Lipid content
Extraction and quantification of lipids were performed according to Van Brummelen and Suijfzand [50]. Wingless females (10 per tube) were vigorously mixed for 30 s in 400 μL of a chloroform/methanol/water (1:2:0.8) mixture and the homogenates were centrifuged for 5 min at 14,000 rpm and 4 °C. Pellets were dried with sodium sulphate, washed three times with chloroform (evaporation was done under nitrogen flow) and incubated for 10 min at 100 °C with 300 μL of sulphuric acid. After cooling, the samples were mixed with 2.5 mL of 66.8% (v/v) phosphoric acid containing 0.198% (w/v) vanillin. After 10 min at room temperature, absorbance was read at 540 nm with a spectrophotometer (Jenway 6405, UK). Results were expressed in μg·g−1 fresh weight (FW) using cholesterol as a standard.
2.2.2 Carbohydrate content
Extraction and quantification of carbohydrates were performed according to Windecoen [51]. Wingless females (10 per tube) were vigorously mixed for 30 s in 200 μL water and 100 μL of 15% (w/v) trichloroacetic acid (TCA). Samples were centrifuged for 10 min at 3000 rpm and 4 °C. Supernatants (S1) were stored and pellets were vigorously mixed with 200 μL of 5% (w/v) TCA. The homogenates were centrifuged for 10 min at 3000 rpm and 4 °C and supernatants were pooled with the previous S1 extracts to obtain the total extract (TE). A volume of 250 μL of TE was mixed with 250 μL of 5% (w/v) phenol and 1 mL of sulphuric acid. Samples were then incubated at room temperature for 30 min and the absorbance was read at 490 nm with a spectrophotometer (Jenway 6405, UK). The results were expressed in μg·g−1 FW using glucose as a standard.
2.3 Primary and secondary metabolite contents in clementine tree leaves
2.3.1 Total soluble carbohydrate content
A quantity of 50 mg dry weight (DW) was ground with 1.5 mL of 0.1 M potassium phosphate buffer at pH 7.5 and was then centrifuged for 15 min at 4 °C and 10,000 G [52]. An aliquot (200 μL) of supernatant was mixed with 1 mL of 12.5 N sulphuric acid containing 0.1% (w/v) of anthrone and 0.1% (w/v) of thiourea, and the homogenate was incubated for 10 min at 95 °C. After cooling, the absorbance was read at 625 nm with a spectrophotometer (PharmaSpec UV-1700, Shimadzu, Japan). Results were expressed in μmol·g−1 DW using glucose as a standard.
2.3.2 Total free amino acid content
A quantity of 50 mg DW was ground with 1 mL of 5% (w/v) sulphosalicylic acid and was then centrifuged for 15 min at 4 °C and 10,000 G. An aliquot (200 μL) of supernatant was mixed with 100 μL of 0.2 M citrate buffer, pH 4.6, and 200 μL of a ninhydrin reagent containing 0.003% of ascorbic acid and 0.96% (w/v) ninhydrin in ethylene glycol monomethyl ether. The mixture was heated at 100 °C for 20 min and then cooled on ice. Six hundred microliters of 60% ethanol were added and the mixture was shaken. The absorbance was read at 570 nm using leucine as a standard and the results were expressed in μmol·g−1 DW using leucine as a standard.
2.3.3 Free proline content
A quantity of 50 mg DW was ground with 1 mL of 5% (w/v) sulphosalicylic acid and was then centrifuged for 15 min at 4 °C and 10 000 G. An aliquot (200 μL) of supernatant was mixed with 800 μL of 60% (v/v) acetic acid containing 1% (w/v) of ninhydrin and the homogenate was incubated for 20 min at 95 °C. After cooling, 1 mL of toluene was added and the samples were vigorously mixed for 15 s before an incubation of 4 h in darkness and at room temperature. The upper phase was harvested and its absorbance was read at 520 nm with a spectrophotometer (PharmaSpec UV-1700, Shimadzu, Japan). Results were expressed in μmol·g−1 DW using proline as a standard.
2.3.4 Condensed tannin content
A quantity of 100 mg DW was ground with 5 mL of 70% (v/v) acetone containing 0.1% (w/v) of sodium metabisulphite [53]. The samples were sonicated twice for 10 min and then centrifuged for 12 min at 4 °C and 3000 G. The supernatant was stored at 4 °C and the pellet was mixed with 2.5 mL of the previous solution of acetone/sodium metabisulphite, sonicated twice for 10 min and centrifuged as previously described. This second supernatant was pooled with the first and corresponded to the total extract. An aliquot (250 μL) of the total extract was mixed with 1.25 mL of butanol/chlorhydric acid (95:5) and incubated for 1 h at 95 °C. After cooling, the absorbance was read at 550 nm with a spectrophotometer (PharmaSpec UV-1700, Shimadzu, Japan). The results were expressed in μmol·g−1 DW using cyanidin as a standard.
2.4 Statistical analyses
All experiments were made in triplicate. For each parameter, the mean values weekly measured on safe and infested leaves were compared using a Wilcoxon test. In order to assess temporal variations between the different parameters (plant biochemical compounds, aphid energetic markers, aphid and auxiliary fauna abundance), temporal statistics (lag and cross-correlation) were computed using PAST version 2.17 [54]. The lag between the fluctuations of two parameters corresponded to the best cross-correlation, estimated by the P-value. In order to predict aphid abundances from linear combinations of quantitative variables, analyses were conducted using standardized multiple regression with SYSTAT version 7.0 [55] Due to the lag between several parameters, all weekly variations were synchronized as if all the parameters were varying at the same time.
3 Results
3.1 Variations in aphid populations and auxiliary fauna
The measurements indicate that A. spiraecola populations were more important than A. gossypii ones for all stages, and more particularly for the wingless adults (Table 1). The mean number was 232 for A. spiraecola against 144 for A. gossypii. The most important difference was observed from the end of April to the middle of May (Fig. 2A). Tests of cross-correlation show that there was no lag between A. spiraecola and A. gossypii whatever the stage (larvae, winged and wingless adults) (P < 3·10−7, Table 1).
Abundance of each stage of development of A. spiraecola and A. gossypii.
| n | Mean | Median | Wilcoxon | Monte-Carlo | Lag (week) | P-value | |
| Larvae L1 + L2 |
|||||||
| A. spiraecola | 27 | 241 | 222 | 6.44·10−5 | 2.00·10−5 | 0 | 2.7·10−7 |
| A. gossypii | 161 | 120 | |||||
| Wingless adults | |||||||
| A. spiraecola | 27 | 232 | 143 | 5.71·10−5 | 1.00·10−5 | 0 | 1.2·10−7 |
| A. gossypii | 145 | 77 | |||||
| Winged adults | |||||||
| A. spiraecola | 27 | 84 | 39 | 0.0447 | 0.0430 | 0 | 7.7·10−8 |
| A. gossypii | 73 | 36 |
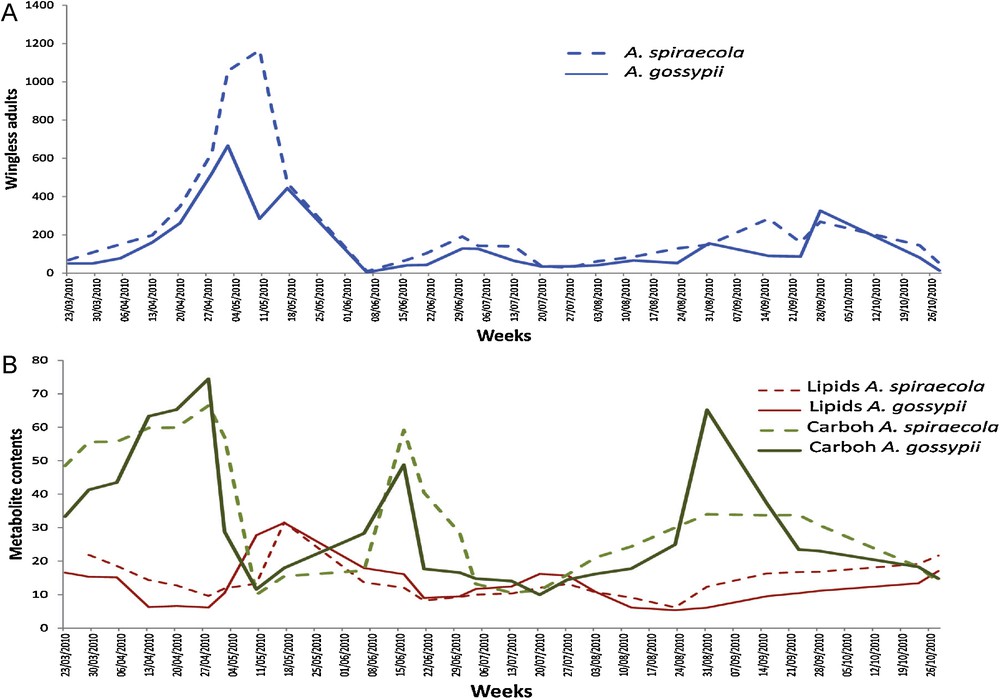
(Color online.) Abundances of population of wingless females (A) and energetic metabolite contents (B) in A. spiraecola and A. gossypii. Lipid and carbohydrate (Carboh) contents were expressed in μg·g−1 FW. Results for total carbohydrate contents were multiplied by a factor 1000 in the figure.
As for auxiliary fauna, populations were more abundant from mid-April to mid-May and from end of August to end of September (data not shown). The samplings revealed that Coccinellidae and Cecidomyidae were much more frequent than Chrysopidae populations (Table 2). Cecidomyidae larvae were the most abundant ones, with a population approximately twice higher than Coccinellidae larvae. Results show significant correlations between each stage of development of both aphid populations and the abundance of predator larvae, with higher Pearson coefficients for Coccinellidae than Cecidomyidae (Table 3).
Mean abundance of aphidophages on clementine tree leaves.
| Stage | Mean abundance | |
| Coccinellidae | Larvae | 11.7 ± 2.3 a |
| Adults | 2.1 ± 0.9 b | |
| Cecidomyidae | Larvae | 21.1 ± 3.4 c |
| Chrysopidae | Eggs | 0.3 ± 0.2 b |
| Larvae | 0.7 ± 0.3 b | |
| Adults | 0.5 ± 0.3 b |
Correlations between Aphis spiraecola and Aphis gossypii stages and Coccinellidae and Cecidomyidae larvae.
| Aphis species | Stages | Coccinellidae larvae | Cecidomyidae larvae | ||
| R | P-value | R | P-value | ||
| A. spiraecola | Wingless | 0.792 | 6.61·10−8 | 0.573 | 6.07·10−4 |
| Winged | 0.677 | 2.06·10−5 | 0.613 | 1.93·10−4 | |
| Larvae L1 + L2 | 0.673 | 2.42·10−5 | 0.501 | 3.46·10−4 | |
| Larvae L3 + L4 | 0.818 | 1.10·10−8 | 0.556 | 9.48·10−4 | |
| A. gossypii | Wingless | 0.795 | 5.45·10−8 | 0.589 | 3.91·10−4 |
| Winged | 0.839 | 2.07·10−9 | 0.693 | 1.11·10−5 | |
| Larvae L1 + L2 | 0.748 | 8.81·10−7 | 0.437 | 1.23·10−5 | |
| Larvae L3 + L4 | 0.831 | 3.84·10−9 | 0.649 | 5.76·10−5 |
3.2 Variations in leaf metabolites of clementine tree
Results show several peaks of the soluble carbohydrate content throughout the year and particularly during the first sap flow (Fig. 3A). During this stage, the first increase was observed at the beginning of spring (29 March 2010), followed by the most important peak in April and a lower one in May. These peaks correspond to several stages of plant phenology: leaf maturation, flowering and beginning of fructification. After an important decrease, the carbohydrate content grew up during the second and third sap flows, during fruit growth and maturation. The variations in the carbohydrate content were globally similar and synchronized between control and aphid-infested leaves, but with generally higher values in control than in infested leaves (283 and 255 μmol·g−1 DW respectively) (Table 4).
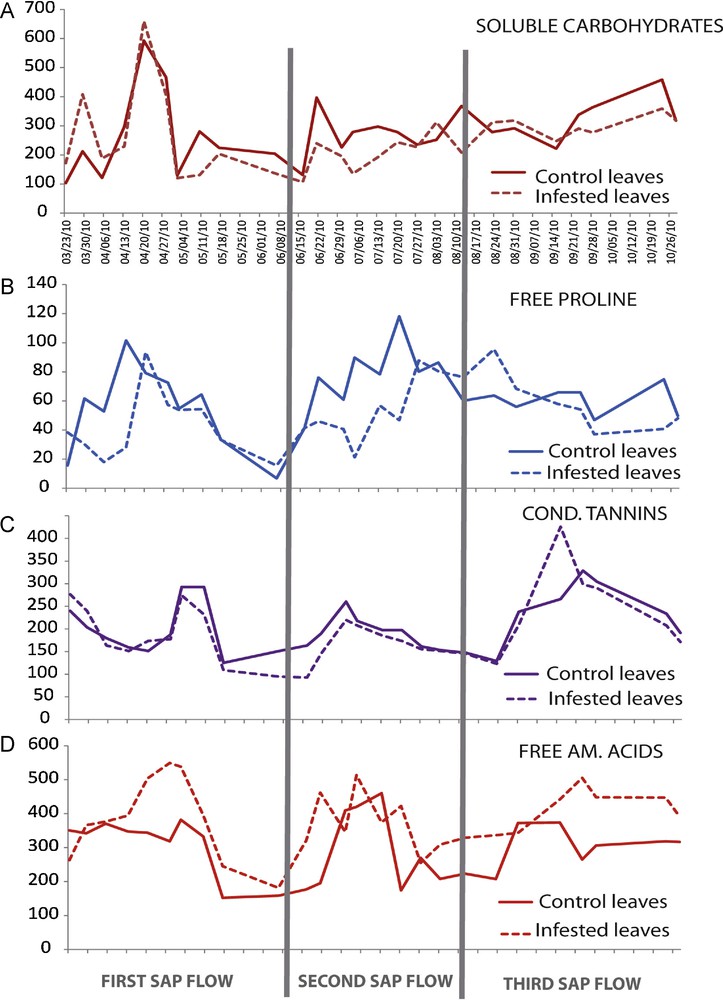
(Color online.) Variations in trophic and defence metabolites in control and aphid-infested leaves. A) Total soluble carbohydrate contents, B) total free proline content, C) condensed tannin contents, D) total soluble amino acids contents. All results were expressed in μmol·g−1 DW.
Mean content of metabolites in control and aphid-infested leaves of clementine tree.
| Lag (weeks) | Cross-correlation probability | Mean content (μmol.g−1 DW) | Wilcoxon | ||
| C | IL | ||||
| Total soluble carbohydrates | 0 | 1.93·10−5 | 283.3 | 255.2 | 8.64·10−2 |
| Total free amino acids | +1 | 1.31·10−2 | 310.9 | 387.4 | 2.61·10−3 |
| Free proline | +1 | 1.92·10−3 | 63.7 | 50.9 | 3.95·10−2 |
| Condensed tannins | 0 | 4.00·10−7 | 206.3 | 195.9 | 1.42·10−2 |
Our results show three peaks of total free amino acid content in leaves: the first during spring (28 April 2010), the second during summer (4 July 2010) and the last during autumn (23 September 2010) (Fig. 3B). The total amino acid concentration in infested leaves was consistently higher compared to control leaves with 387 against 310 μmol·g−1 DW, respectively (Table 4), and with a delay of one week compared to control leaves.
There were also three peaks of proline in leaves: the first during spring (13 April 2010), the second during summer (20 July 2010) and the third (at a lower extent) during autumn (23 October 2010) (Fig. 3C). Proline content in infested leaves was significantly lower compared to control leaves with 50.9 μmol·g−1 DW against 63.7, respectively, and a delay of one week was recorded in infested leaves compared to control leaves (Table 4). Cross-correlation tests showed that the fluctuations of proline and amino acid contents were uncoupled.
As for condensed tannin production, we observed four peaks: two during the first sap flow (23 March 2010 and 2–10 May 2010), the third one during the second sap flow (30 June 2010) and the last one during the third sap flow (23 September 2010) (Fig. 3D). Variations in tannin contents were globally similar in control and infested leaves and synchronized. However, the mean content was significantly higher in control than in infested leaves, with 206.3 and 195.9 μmol·g−1 DW, respectively (Table 4). There is a synchronicity (Fig. 4A) between the fluctuations of condensed tannin and amino acid contents on one hand (P = 0.035), and between proline and total soluble carbohydrate contents on the other hand (P = 0.018).
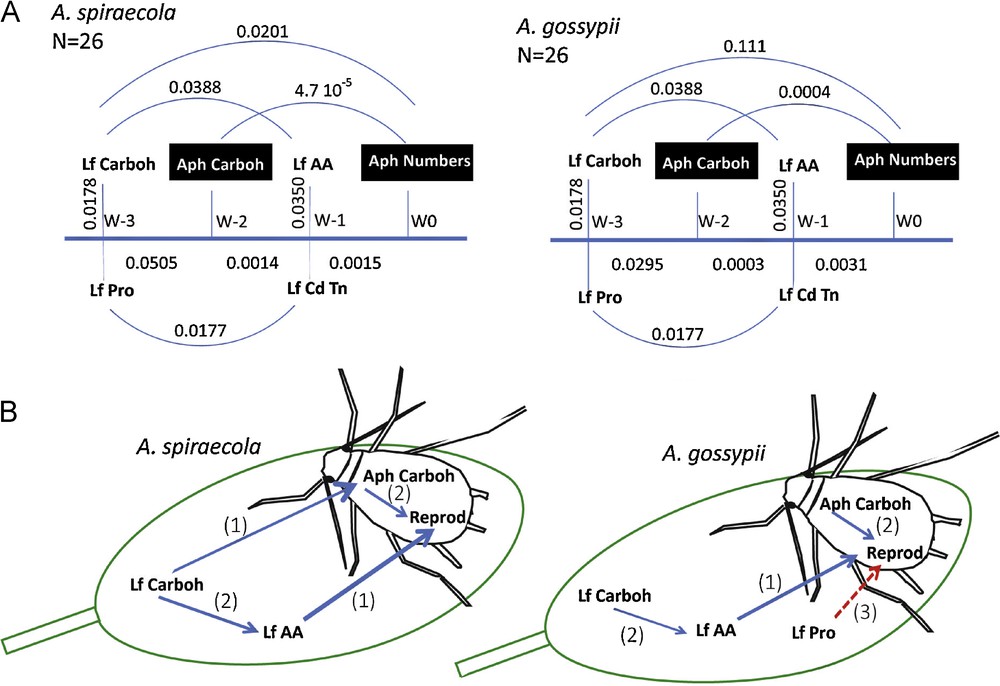
Temporal correlations between foliar metabolite contents, concentrations of aphid energetic compounds and abundance of A. spiraecola and A. gossypii wingless populations. A) Lags and P-values between parameters related to leaves (Lf) and aphids (Aph). Lags were expressed in weeks (W) and the aphid number, considered as the reference, was assigned at week zero. For example, A. spiraecola carbohydrate content precedes aphid abundance by a lag of two weeks, and the probability corresponding to the correlation between both parameter fluctuations (cross-correlation) is 4.7·10−5. Otherwise, there is no lag between leaf amino acid and condensed tannin contents and the probability affected to the correlation between these two parameters is 0.0350. Carboh: total carbohydrates, Pro: proline, AA: amino acids, Cd Tn: condensed tannins. B) Schematic representation of effects of leaf metabolites on the population level of A. spiraecola and A. gossypii. Numbers correspond to lags expressed in weeks. The continuous blue arrows indicate a positive effect on aphid population level and the red dotted arrow a negative one. For interpretation of references to color, the reader is referred to the online version of this article.
3.3 Variations in aphid energetic compounds
Carbohydrate contents in wingless adults were not similar during the whole season (Fig. 2E). Indeed, three peaks were observed for both aphid species: from the middle to end of April, at mid-June and at the end of August. The carbohydrate content was similar for the first two peaks in both aphid populations, whereas the third peak was twice lower for A. spiraecola and has approximately a similar value than the other peaks for A. gossypii. For both aphid populations, cross-correlation tests showed a significant two-week lag of wingless adult population compared to their carbohydrate content with P < 10−3 (Fig. 4A). We also calculated a one-week delay of aphid carbohydrates by comparison to soluble carbohydrates in infested leaves (P ≤ 0.05 for both aphids).
There were two peaks of lipid content in wingless adults in both aphid species: the first at mid-May and a second, approximately twice lower, at the end of July (Fig. 3E). For both aphid populations, cross-correlation tests showed a significant two-week delay of lipid content compared to wingless adult populations with a P-value of 0.012 (Fig. 4A). We observed higher values in A. spiraecola than in A. gossypii from the end of July on (Wilcoxon P = 0.018).
In summary, the main difference between both aphid species was a stronger relationship between wingless A. spiraecola abundance and total foliar carbohydrates (lag = 3, P = 0.0201) than between the same variable and A. gossypii (P = 0.111). Otherwise, it can be noticed a stronger link between aphid carbohydrates and abundance of A. spiraecola (lag = 2, P = 4.7 10−5) than of A. gossypii (P = 0.0004).
3.4 Multiple regression analyses
For both aphid species, the lags were corrected (to synchronize aphid abundances and other variables) in order to perform multiple linear regression tests. We did not retain variations in lipid content as their fluctuations present a delay of two weeks relatively to wingless adult abundances. In a first test, Coccinellidae larvae included with leaf metabolites led to a positive and highly significant effect of these predators (P < 0.1%) and a marginal one of amino acid content (around 7.5% for both aphid species). In a second test, the Coccinellidae larvae were removed to focus on leaf metabolites (Table 5). The results indicate a significant and positive correlation between the abundance of both wingless aphids and leaf amino acid contents. Our results show that foliar soluble carbohydrate concentration had a positive effect on A. spiraecola, whereas proline concentration had a negative effect on A. gossypii (Fig. 4B).
Multiple regressions with infested leaves and corrected lags (n = 26).
| Aphis spiraecola | Aphis gossypii | |||
| Coefficient | P-value | Coefficient | P-value | |
| Constant | –452.7 | 0.047 | –161.7 | 0.267 |
| Total soluble carbohydrates | 1.383 | 0.011 | 0.464 | 0.171 |
| Total free amino acids | 1.187 | 0.040 | 0.696 | 0.066 |
| Free proline | –3.380 | 0.186 | –3.437 | 0.048 |
| Condensed tannins | 0.240 | 0.757 | 0.480 | 0.314 |
4 Discussion
4.1 Variations in aphid populations
We showed that the populations of A. spiraecola and A. gossypii recorded on clementine tree leaves had similar variations in the different stages during the growing season. Indeed, each developmental stage (larvae, winged or wingless adults) occurred at the same time for both aphid species. The most important peak of wingless females was observed from the end of April to the beginning of May, i.e. just before the dry season. There is another peak in summer, at a lower level than the autumn one. These seasonal fluctuations of both aphid populations on Citrus species were retrieved in other countries. Indeed, Yokomi and Olfield [56] found a similar variation with an important peak in April–May in California. Two important peaks were also recorded in May and September in Tunisia [17] and in Japan [13]. These authors also found that A. spiraecola population remained significantly more abundant than A. gossypii. However, Marroquín et al. [18] indicated that populations of A. gossypii were more abundant that those of A. spiraecola on various Citrus species in Valencia (Spain).
Our results showed a positive and significant correlation between populations of Coccinellidae and Cecidomyidae larvae and both populations of wingless aphids. Larvae populations were more important during the first peak of aphid development. Moreover, Pearson coefficients were higher for Coccinellidae than Cecidomyidae, suggesting that Coccinellidae would play a greater role in the regulation of aphid populations. However, the positive coefficient given by multiple linear regressions indicated an attraction of predators rather than a regulation of their prey, suggesting that auxiliary fauna was not enough efficient to downsize aphid populations.
4.2 Impact of aphids on the fluctuations of leaf parameters
We evaluated the seasonal fluctuations of leaf soluble carbohydrate concentrations in control and aphid-infested leaves, since these primary metabolites are required for aphid diet [1]. The highest peak of carbohydrates in leaves of clementine trees was detected during the first sap flow, more precisely one week before carbohydrate accumulation in wingless aphids. The content in infested leaves was quite similar to the one in control leaves. Moreover, starch content in leaves was not significantly different between control and aphid-infested leaves (data not shown). Taken together, these results indicate that aphid development did not strongly affected photosynthetic activity. During the second sap flow, the leaf carbohydrate content was lower. Indeed, during this period, photosynthesis decreased as a result of high temperatures and water deficit and leaf carbohydrates are partially translocated to growing fruits [57]. Moreover, the lower carbohydrate content in infested leaves compared to controls may be a consequence of sap harvested by aphids and/or fumagin accumulation on leaves, which decreased photosynthesis activity. During the third sap flow, the progressive increase in leaf carbohydrate content may be explained by:
- • a lower content required by fruits to achieve their maturation, and;
- • a stimulation of photosynthesis by lower temperatures and higher rainfalls to increase carbohydrate reserves for winter.
Amino acids are the main source of nitrogen for aphid diet [33]. We showed that aphid-infested leaves exhibited a higher concentration of total free amino acids compared to control leaves during the whole season, and particularly during the first and third sap flow. Several authors reported that aphids can increase amino acid concentration in sap to enhance the nutritional quality of their feeding [1,58–60]. It is thought that aphid saliva contains molecules that break down leaf proteins, leading to an increase of amino acid translocation [58–61].
We analyzed the free proline content, since it can increase in response to various stresses including oxidative stress, drought, high light intensity and biotic stresses [34,35]. Our results showed a high production of proline in April and a second increase from June to September, the latter being probably a response to high temperatures and water deficit [62]. However, proline accumulation in April may be related to a stronger demand for budbreak and/or may be considered as storage of nitrogen compounds [63–65]. Aphid-infested leaves contained less proline than control leaves and the accumulation had a one-week delay compared to control leaves. This difference may be a consequence of the lower leaf carbohydrate content (source of energy for syntheses) and/or the result of a leaf carbohydrate mobilization to other pathways.
We showed that the level of leaf condensed tannins exhibited one peak during each sap flow, which occurred generally two weeks after leaf carbohydrate accumulation. Condensed tannins are secondary metabolites involved in leaf and fruit maturation as well as in response to different stresses [66,67]. Our results showed that infested leaves accumulated slightly less tannins during the first two sap flows, suggesting that leaf energy may be mobilized to other synthesis pathways. Nevertheless, aphid-infested leaves accumulated more condensed tannins than control leaves during the third sap flow, suggesting that they may limit the peak of aphid proliferation in September.
4.3 Effects of variations in plant metabolites on aphids
According to the time series and multiple regression analyses, two contrasting situations can be described between both aphid species, even if the lags between aphid abundance, aphid carbohydrate content and leaf amino acid content are the same for both species (Fig. 4B). It suggests the same trophic requirements for reproduction. However, there are strong differences between both aphid species, as A. spiraecola reproduction is closely linked to the leaf soluble carbohydrate content, whereas A. gossypii reproduction is only indirectly linked with this compound (we found a probability of 0.111). This indicates that A. gossypii would be less adapted than A. spiraecola to the trophic resource variations present in clementine tree, even if sucrose, the major soluble carbohydrate, is considered as a phagostimulant for aphids [68].
We also found that A. gossypii, unlike A. spiraecola, was negatively affected by high values of proline concentration. This is particularly clear when we consider the first sap flow. Indeed, A. gossypii does not benefit from the high availability of carbohydrates in April as A. spiraecola, probably as a result of the synchronized high proline concentration. During the second sap flow, both aphid populations remained at a low level corresponding to a shortage in leaf carbohydrate and proline. During the third sap flow, the difference in aphid abundances observed at mid-September could be linked to rather high levels of proline and soluble carbohydrates three weeks before. The negative effect of high proline levels on A. gossypii seems to be species-specific. In cotton, Liu and Yang [36] found that the development of this aphid population decreased in the varieties with the richest proline content. They also showed that infested plants produced a higher amount of this compound as a result of physiological stress, leading to a better resistance to further attacks. Moreover, Lu et al. [37] conducted experiments on the same aphid species with artificial diets indicating a two-side effect of proline: a minimum level was required for population development but high concentrations had negative effects. It is difficult to compare the concentrations used by these authors with ours since the leaf composition of clementine tree is very complex relatively to an artificial diet. However, this view was contested by Wool and Hales [69] as they invoked the possibility of a reduction of nutritional quality [70] instead of the accumulation of proline. As they did not assayed plant metabolites (including total carbohydrates and free proline), the conclusions of the studies of Liu and Yang [36] and Lu et al. [37] were not invalidated. In the case of clementine tree, we showed that the highest levels of proline were due to intrinsic demand of the plant and to water and temperature stresses, the impact from aphids being probably low.
Our results show that the peaks of lipids in aphids occurred two weeks after the peaks of wingless female abundance. Carbohydrate accumulation by wingless aphids is required to optimize reproduction, whereas lipids are more involved in maintaining the adult population on plant host [71]. During the whole season, lipid content was globally similar for both aphid species, suggesting that this parameter was not decisive to explain why A. spiraecola was more abundant than A. gossypii on clementine trees. At the end of the third sap flow, the lipid content in A. spiraecola was higher than in A. gossypii. We hypothesize that this storage may optimize sexual and oviparous reproduction in A. spiraecola and a subsequent egg production for the following spring, as shown on other aphids [72,73].
In conclusion, we showed that A. spiraecola and A. gossypii had similar temporal variations whatever the stages of development, in parallel with the fluctuations of leaf trophic quality. Moreover, the A. spiraecola populations were more abundant than A. gossypii ones, especially when proline level was high. It seems that this latter species would be limited by an excess of proline in contrast to A. spiraecola. The condensed tannin variations seemed to have no significant impact on aphid populations. In the same manner, auxiliary fauna was probably not enough abundant to limit aphid proliferation. Further studies are required to understand the specific features of A. gossypii genome linked to the sensitivity of this species to proline excess. It could be interesting to analyze plant defences such as other secondary metabolites or cell wall modifications that could act differently on A. spiraecola and A. gossypii [1,59].
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.


