1 Introduction
The coastal dunes ecosystems are globally threatened by human activities [1]. Furthermore, they are vulnerable ecosystems subjected to intense ecological stress caused by wind, drought, salt, and erosion [2]. Along the Mediterranean coastal dune ecosystems, microforests of Juniperus macrocarpa Sm. represent one of the main woody vegetation types. This vegetation type is listed in the DIR. 92/43/EEC as priority habitat 2250 “Coastal dunes with Juniperus spp.”.
In the Mediterranean area, the populations of many species of Juniperus L. are formed principally by adults and senescent individuals, and their persistence is therefore linked more to the longevity of individuals than to the recruitment and establishment of young individuals [3]. The causes of this low recruitment are attributed to several factors, such as reproductive problems [4,5], low germination [6], long reproductive cycle [7], summer drought that may limit the survival of seedlings [3], and seed predation [5].
A key stage in the life cycle of plants is seed germination. It is responsive to many environmental factors, including temperature, light, time after dissemination, and soil moisture content [8]. Among them, temperature is the major factor in regulating dormancy, the maximum germination percentage and rate of germination [9], as well as the success or failure of plant establishment [10]. The optimal germination temperatures for coastal Mediterranean species are mainly within the range 5–15 °C [11], and species of this ecosystem are characterized by a low germination rate [12,13]. This “delay mechanism”, with low germination rate and a narrow range of cool temperatures, is considered an advantageous ecological adaptation of Mediterranean species to the unpredictable rainfall pattern [12]. This optimises autumn/winter germination and therefore the duration of the growing season before the beginning of summer drought [14,15].
Previous studies, carried out on some species of the genus Juniperus, showed a wide range of values in germination percentages (i.e. between 7% of J. communis L. and 87% of J. virginiana L.) [16]. Furthermore, seeds of several Juniperus species have physiological dormancy (PD) [17], in which the embryo is unable to develop a radicle due to a physiological inhibition mechanism [18], while studies on physical dormancy (PY) have provided contradictory data [17,19]. In particular, the seeds of J. oxycedrus L. treated with sulphuric acid showed low percentage of germination (< 20%) [7,20] and those of J. excelsa M. Bieb reached ca. 7–8% of final germination [21]. Conversely, Laurent and Chamshama [22] highlighted a significant increase in germination of J. procera Hochst. ex Endl. seeds treated with this method, reaching germination percentages of ca. 78%. Therefore, there is wide variation among Juniperus species in the degree of dormancy [4], which can be also affected by ripeness of the seed, environmental factors during seed development and variations in genotype [20]. A substantial variation among seed sources, seed age, and individuals is also present, as reported for J. virginiana [23], to allow probably less competition and better distribution in time and space and increase the likelihood that some of the seeds may germinate and grow [7].
Specifically, we focused on Juniperus macrocarpa (Cupressaceae), which is a dioecious species, 1–5 m high, very branching, with large canopy [24]. Cone development starts in summer with the fertilization of female cones and ends in the next summer through embryo maturation [7]. Female cones can be found at different stages of maturity on the same plant simultaneously [25]; fruit ripening and dispersal are distributed from October till January [7].
Very few studies have been carried out on seed germination of J. macrocarpa. Pacini and Piotto [7] reached a maximum germination of ca. 25% after warm followed by cold stratification, while cold stratification alone appeared to be totally ineffective for this species. Chemical scarification with sulphuric acid did not improve germination, with final percentages of ca. 20% [7]. Cantos et al. [26] found that intact seeds did not germinate in the greenhouse or in vitro conditions, while seeds without testa did not germinate under greenhouse conditions, and in vitro isolated embryos reached germination levels of about 50%. Juan et al. [25] found that seeds derived from immature cones of J. macrocarpa under greenhouse conditions germinated significantly better (i.e. 49.3%) than those derived from mature ones, suggesting lower levels of dormancy.
There is much to learn about the stimulation of seed germination in junipers, and more research is called for [16]. In addition, considering the relatively low germination percentages achieved in the few previous studies on J. macrocarpa and the needs for recovery of this taxon, new approaches are needed to better understand its reproductive cycle. Therefore, the aims of this work were to test the effect of the collecting season, of the collection site (plant and soil; site, hereafter), and to check laboratory germination pre-treatments and temperatures on seed viability and germination of J. macrocarpa seeds. The achieved results may be helpful to enable recovery actions of the fragile and threatened J. macrocarpa ecosystems.
2 Materials and methods
2.1 Seed lot details
Ripe cones of J. macrocarpa were collected in 2010 from four dune systems in southern Sardinia at two different times of the dispersal period: autumn (i.e. the beginning) and spring (i.e. the end; Table 1). Cones of the same year were collected from plant and on the soil surface, leading to a total of 16 seed lots. Immediately after collection, seeds were manually drawn out from the fleshy cones and washed by stirring them in water.
Locality and seed lots details.
| Locality | Coordinates (WGS 84) | Elevation (m. a.s.l.) | Distance from the coastline (m) | No of sampled individuals (spring/autumn) |
| Arbus, Medio Campidano, SW Sardinia | 39° 31′ 05′′N 8° 25′ 55′′E |
22 | 150 | 20/24 |
| Buggerru, Carbonia-Iglesias, SW Sardinia | 39° 26′ 18′′N 8° 25′ 51′′E |
32 | 1650 | 20/30 |
| Domus de Maria, Cagliari, SW Sardinia | 38° 53′ 04′′N 8° 51′ 43′′E |
5 | 200 | 20/20 |
| Villasimius, Cagliari, SE Sardinia | 39° 07′ 16′′N 9° 31′ 22′′E |
15 | 62 | 20/20 |
2.2 Viability and germination tests
Besides factors related to seed lots, we included “pre-treatments” and “temperature” as factors to explain seed viability and germination. Specifically, to verify the presence of physiological dormancy (PD), the following pre-treatments were applied: warm (W: three months at 25 °C), cold (C: three months at 5 °C); as well as two combined warm and cold stratifications (W + C and C + W), and control (0), with no pre-treatment. No scarification was applied due to the contradictory data reported in literature for this pre-treatment on juniper seeds [7,20,22]. In addition, scarification could overcome non-deep physiological dormancy [27], therefore, masking the effect of the applied stratifications. After pre-treatments, seeds were incubated under a daily 12-h exposition to light at four constant temperatures: 10 °C, 15 °C, 20 °C, 25 °C, as well as with an alternating temperature regime (25/10 °C). Three replicates of 30 seeds each were sowed in 90-mm diameter plastic Petri dishes with a substrate of 1% water agar. The experimental design was constituted by 3 replicates × 4 localities × 2 seasons × 2 sites × 5 pre-treatments × 5 temperatures. However, due the low seed availability, especially for seeds collected in autumn, only three pre-treatments (W, C and control) were carried out for seeds belonging to Arbus locality (for plant and soil).
Tests lasted for a minimum of four months until a maximum of 10 months; when no additional germination occurred for 15 days, tests were considered ended. The viability of the remaining non-germinated seeds was assessed by a cut test (although the results of this test tend to overestimate seed viability), and the final number of germinated seeds was calculated on the basis of the total number of firm seeds (i.e. that had developed embryos). Therefore, seed viability was assessed as the sum of the germinated seeds plus the viable non-germinated seeds.
2.3 Data analysis
Seed viability and germination percentages were modelled with Generalized Linear Mixed Models (GLMM), using a binomial error distribution and logit link function. To estimate model parameters, the Laplace approximation of likelihood was used. In order to model seed viability, predictors included “locality” as random factor, and “pre-treatment”, “temperature”, “season” and “site” as fixed factors. Seed germination was modelled, including “site” within “locality” as random factors, and “pre-treatment”, “temperature” and “season” as fixed factors. Germination models were performed using the overall data set instead of using the data of each season separately, in order to better understand the effects of pre-treatments on seeds collected in autumn and spring, respectively. Throughout the text, overall means are followed by the standard error (± SE). All statistical analyses were performed using the R 14.6 statistical package.
3 Results
3.1 Viability
Seed viability was generally low, with seeds showing an overall mean value of ca. 40%. This varied significantly according to the applied pre-treatments and the incubation temperatures as well as the collecting season, while the site factor had not a significant effect (Table 2). In particular, the season factor showed the highest estimate, and seeds collected in autumn were less viable than those collected in spring, with mean values of 34.18 ± 0.62% and 42.77 ± 0.52%, respectively (Fig. 1). All pre-treatments had a negative effect on seed viability with respect to the control, whose value was 43.57 ± 0.85%.
Generalized Linear Mixed Model (GLMM) results, for the effect on seed viability of the following fixed factors: temperature, pre-treatment, season and site. The locality was considered as random factor (variance: 0.0767; SD: 0.2769). Akaike Information Criterion (AIC): 7595; Bayesian or Schwarz Information Criterion (BIC): 7656; logLik: –3786; deviance: 7571.
| Fixed effects | Estimate | Standard error | z value | P (> |z|) |
| Intercept | –0.5249 | 0.1399 | –3.752 | 0.0002*** |
| T 15 °C | –0.0054 | 0.0193 | –0.280 | 0.7798 NS |
| T 20 °C | –0.1304 | 0.0194 | –6.725 | 1.76e−11*** |
| T 25 °C | –0.3484 | 0.0197 | –17.706 | < 2e−16*** |
| T 25/10 °C | –0.1892 | 0.0195 | –9.723 | < 2e−16*** |
| CW | –0.1630 | 0.0199 | –8.185 | 2.72e−16*** |
| 0 | 0.1916 | 0.0188 | 10.167 | < 2e−16*** |
| W | –0.0509 | 0.0190 | –2.675 | 0.0075** |
| WC | –0.1522 | 0.0199 | –7.647 | 2.06e−14*** |
| Spring | 0.3878 | 0.0125 | 30.938 | < 2e−16*** |
| Soil | 0.0118 | 0.0124 | 0.956 | 0.3389 NS |
** 0.01 > P > 0.001.
*** P < 0.001
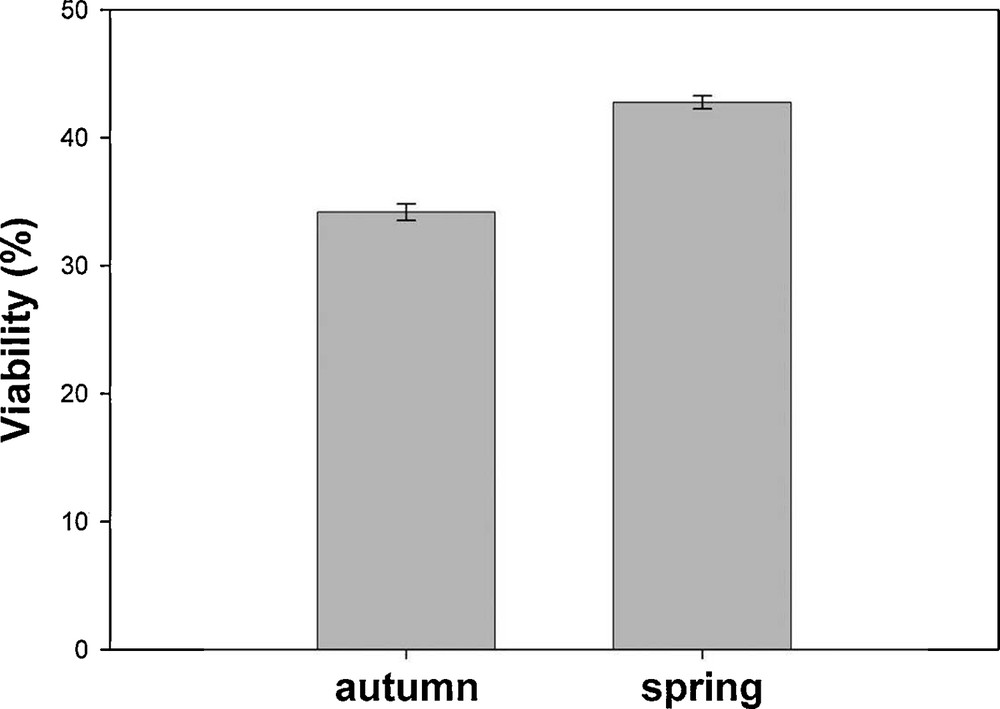
Viability (mean ± SE) for seeds collected in the two seasons.
3.2 Germination
Seed germination was low for all the tested conditions, with an overall mean value of ca. 10%, and never higher than 45%, in accordance with seed viability results. Since statistically significant effects were not identified for the site factor on seed viability, this factor was considered as random in the models performed to explain germination (Table 3). All the fixed factors had a significant effect on seed germination, although the highest estimates were recorded for the applied pre-treatments. Warm stratification (W) and control (0) were the most effective on stimulating germination, with mean percentages of 13.61 ± 0.60% and 13.47 ± 0.74%, respectively. On the contrary, cold stratification (C) negatively affected germination (4.96 ± 0.46%). According to the model results, temperatures with a positive effect on germination were the constant ones of 15 and 20 °C, with mean values of 11.35 ± 0.72% and 10.72 ± 0.67%, respectively, and the alternating temperature regime 25/10 °C (9.95 ± 0.62%). Lower values were reached for the extreme constant temperatures of 25 °C (4.74 ± 0.38%) and 10 (6.91 ± 0.50%), both temperatures adding negative sign terms to the model. Regarding the season factor, spring showed a positive significant effect on germination (Table 3), with mean values of 10.68 ± 0.41% with respect to 6.57 ± 0.34%, observed in autumn.
Generalized Linear Mixed Model (GLMM) results, for the effect on seed germination of the following fixed factors: temperature, pre-treatment and season. The locality (variance: 0.0134; SD: 0.1157) and the site nested within the locality (variance: 0.2151; 0.4638) were considered as random factors. AIC: 7119; BIC: 7179; logLik: –3547; deviance: 7095.
| Fixed effects | Estimate | Standard error | z value | P (> |z|) |
| Intercept | –3.6404 | 0.1788 | 20.36 | < 2e−16*** |
| T 15 °C | 0.5725 | 0.0342 | 16.72 | < 2e−16*** |
| T 20 °C | 0.5067 | 0.0346 | 14.66 | < 2e−16*** |
| T 25 °C | –0.4183 | 0.0414 | –10.10 | < 2e−16*** |
| T 25/10 °C | 0.4172 | 0.0350 | 11.91 | < 2e−16*** |
| CW | –0.2358 | 0.0468 | –5.04 | 4.68e−07*** |
| 0 | 1.1290 | 0.0357 | 31.59 | < 2e−16*** |
| W | 1.1424 | 0.0357 | 32.01 | < 2e−16*** |
| WC | 0.3480 | 0.0411 | 8.48 | < 2e−16*** |
| Spring | 0.5668 | 0.0225 | 25.18 | < 2e−16*** |
*** P < 0.001
When analysing the results separately for the season, the same trend was detected, with all the fixed effects being statistically significant and the highest estimates recorded for 0 and W pre-treatments and at incubation temperatures of 15, 20 and 25/10 °C, in both autumn and spring (Table 4).
Generalized Linear Mixed Model (GLMM) results on seeds collected in autumn and spring, respectively, for the effect on seed germination of the following fixed factors: temperature and pre-treatment. Random factors: locality (variance: 5.1640−09 and SD: 7.1861−05, variance: 3.7511−11 and SD: 6.1247−06 for autumn and spring, respectively) and site (nested within locality; variance: 1.5084−01 and SD: 3.8838−01, variance: 3.9112−01 and SD: 6.2540−01 for autumn and spring, respectively). Autumn = AIC: 2747; BIC: 2794; logLik: –1362; deviance: 2725; spring = AIC: 3467; BIC: 3516; logLik: –1723; deviance: 3445.
| Season | Fixed effects | Estimate | Standard error | z value | P (> |z|) |
| Autumn | Intercept | –4.1927 | 0.1570 | –26.706 | < 2e−16*** |
| T 15 °C | 0.7855 | 0.0552 | 14.217 | < 2e−16*** | |
| T 20 °C | 0.5008 | 0.0574 | 8.722 | < 2e−16*** | |
| T 25 °C | –0.7629 | 0.0764 | –9.985 | < 2e−16*** | |
| T 25/10 °C | 0.3583 | 0.0587 | 6.100 | 1.06e−09*** | |
| CW | –0.2604 | 0.1067 | –2.440 | 0.0147* | |
| 0 | 1.8355 | 0.0699 | 26.245 | < 2e−16*** | |
| W | 1.8229 | 0.0700 | 26.044 | < 2e−16*** | |
| WC | 0.7854 | 0.0832 | 9.440 | < 2e−16*** | |
| Spring | Intercept | –2.8891 | 0.2260 | –12.782 | < 2e−16*** |
| T 15 °C | 0.4359 | 0.0440 | 9.913 | < 2e−16*** | |
| T 20 °C | 0.5153 | 0.0435 | 11.848 | < 2e−16*** | |
| T 25 °C | –0.2674 | 0.0500 | –5.351 | 8.74e−08*** | |
| T 25/10 °C | 0.4545 | 0.0439 | 10.364 | < 2e−16*** | |
| CW | –0.3365 | 0.0527 | –6.387 | 1.69e−10*** | |
| 0 | 0.7951 | 0.0433 | 18.371 | < 2e−16*** | |
| W | 0.8291 | 0.0431 | 19.231 | < 2e−16*** | |
| WC | 0.1175 | 0.0478 | 2.458 | 0.014* |
* 0.05 > P > 0.01
*** P < 0.001
In particular, while in autumn the highest germination percentages were 11.59 ± 0.97% and 11.44 ± 0.80% for 0 and W pre-treatments, respectively, in spring these values reached 15.36 ± 1.09% and 15.79 ± 0.85%, respectively (Fig. 2). Seeds collected in spring were able to germinate at higher percentages with respect to those collected in autumn also after pre-treatments that negatively affected germination, like CW and C (Table 3), with mean percentages increasing from ca. 2 to 8% (Fig. 2).
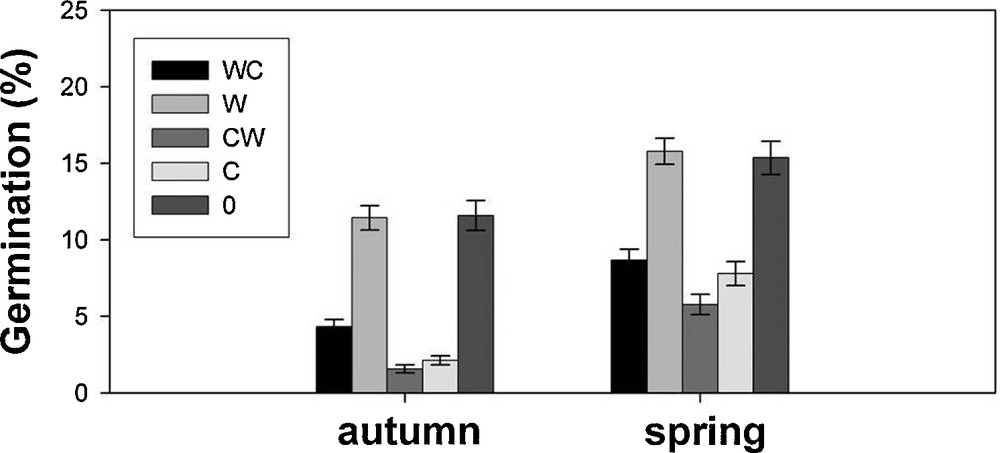
Germination percentages (mean ± SE) after each pre-treatment for seeds collected in the two seasons (autumn and spring).
The effects of incubation temperatures on seed germination without any pre-treatment for each season are shown in Fig. 3, with seed germinating till ca. 20% at 15 °C, irrespective of the season, while the positive effect of the season was more evident at 20 °C and 25/10 °C reaching also ca. 20%.
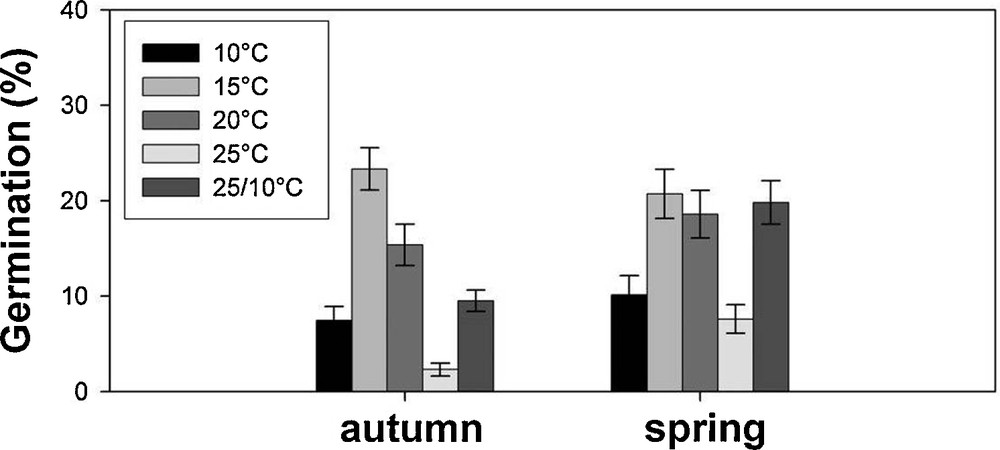
Germination percentages (mean ± SE) at different temperatures for seeds collected in the two seasons (autumn and spring) and incubated without any pre-treatment (i.e. 0).
4 Discussion
The low viability of J. macrocarpa seeds found in this work is in agreement with the previous study carried out on this species by Juan et al. [25]. Low values of seed viability were also obtained for other Mediterranean juniper species [7,28].
The mechanisms behind this low seed viability remain largely unclear [29]. Indeed, low seed viability constitutes a key factor limiting juniper recruitment [30], because this reduces dramatically the potential number of diaspores able to germinate [31]. The results obtained in this work highlighted the fact that the site factor had not a significant effect on the viability of J. macrocarpa seeds, while viability varied significantly according to the collection season, showing lower percentages for seeds collected in autumn than in spring. The differences in viability at the seasonal level might be caused by different temperature conditions and water availability that occur during seed development.
The results of this study, and in particular, the gap between viability and germination percentages, suggest that seeds of J. macrocarpa are dormant. None of the applied pre-treatments improved germination or widened the range of temperatures in which germination can occur. Several authors previously reported seeds of Juniperus as deeply dormant. In particular, Pacini and Piotto [7] argued that the majority of the applied pre-treatments to J. macrocarpa seeds did not remove the dormancy, because these have conditions of very deep dormancy. Furthermore, Pardo and Lazaro [28] suggested that J. oxycedrus seeds have a double dormancy feature, involving both endogenous and exogenous factors. In agreement with these authors, an after ripening period may be needed to break embryo dormancy, influenced by environmental factors, such as temperature [7]. Further studies should be therefore carried out on J. macrocarpa seeds in order to detect the class, type and level of dormancy sensu Baskin and Baskin [27]. Contradictory results are reported in the literature concerning the effects of pre-treatments on seed germination for species belonging to the Juniperus genus [32] and, in particular, few studies were carried out on J. macrocarpa. In this study, the most effective pre-treatment was warm stratification, although the germination percentage was similar to that of seeds germinating without any pre-treatment. The high germination percentages detected after warm stratification are in contrast with the findings of Livingston [33], who found that warm stratification was totally ineffective for J. virginiana, a species growing in temperate grasslands of New England, characterized by stony ground and summer drought. On the contrary, cold stratification negatively affected germination also in combination with warm stratification. The use of cold stratification gave contradictory results in previous studies. Pacini and Piotto [7] found that it was totally ineffective for J. macrocarpa, J. oxycedrus, and J. communis, whereas it increased the germination of J. phoenicea L. [34]. However, this pre-treatment was effective for the seed germination of other juniper species, such as J. excelsa from East Mediterranean and Caucasus areas [21], J. scopulorum Sarg. and J. virginiana [35,36], from Mexico and the United States, respectively. Nonetheless a higher loss in viability after cold stratification than after the other pre-treatments was not detected in this study, suggesting the presence of secondary dormancy.
The negative response to low temperatures detected for J. macrocarpa in the present study is in agreement with the Mediterranean distribution of the species, as seed stratification in a cold-moist medium at 5 °C is especially recommended to overcome dormancy in species from temperate regions [27]. Secondary dormancy was previously detected for other Juniperus species [37,38].
Germination in a narrow range of temperatures (i.e. 15–20 °C) and at a very slow rate is a feature detected for J. macrocarpa seeds that, in agreement with Doussi and Thanos [12], could be associated with autumnal (ca. 18 °C) and wintery (ca. 11 °C) seed germination and seedling establishment. These authors considered that this strategy is ecologically advantageous and tuned to take place in the Mediterranean climate, characterized by a considerable unpredictability of precipitation. Seed germination occurs during the rainy mild winter so that the developing seedlings exploit the subsequent spring prior to the harsh and water stressed conditions of summer [12]. These results are consistent with values obtained for some other species of the genus Juniperus [16,32].
According to the results achieved in laboratory conditions after the different applied pre-treatments and the available climatic data, the phenology of J. macrocarpa seed germination may be graphically summarized as in Fig. 4. The cones of J. macrocarpa do not ripe simultaneously, but their ripening and dispersal are distributed from autumn to spring. This is partially in agreement with the findings of Pacini and Piotto [7], who limited this period to October till January. The seed dormancy detected for this species seems to be a strategy that increases the reproductive success, allowing the occurrence of favourable conditions for germination [39]. In particular, it may allow the formation of a soil seed bank, which could represent a source of new individuals for potential colonization [7]. J. macrocarpa seed germination may occur all along seed dispersal during the rainy season, from autumn (mean temperature of ca. 18 °C and mean precipitations of ca. 176 mm; Fig. 4) to the beginning of spring (mean temperature of ca. 15 °C and mean precipitations of ca. 111 mm; Fig. 4), so that the developing seedlings benefit from the moist conditions of the mild winter (mean temperature of ca. 11 °C and mean precipitation of ca. 189 mm; Fig. 4) and of the following spring. At the same time, the gap, due to seed dormancy, between dispersed and germinated viable seeds may allow the soil seed bank to be established and improved, with germination being the sum of newly dispersed and buried seeds (Fig. 4). Late-spring germination results are limited by the increasing temperatures and by the decreasing rainfall that precede the summer drought when no germination may occur, due to the high temperatures and aridity (mean summer temperatures reach ca. 23 °C, with precipitation of ca. 20 mm; Fig. 4).
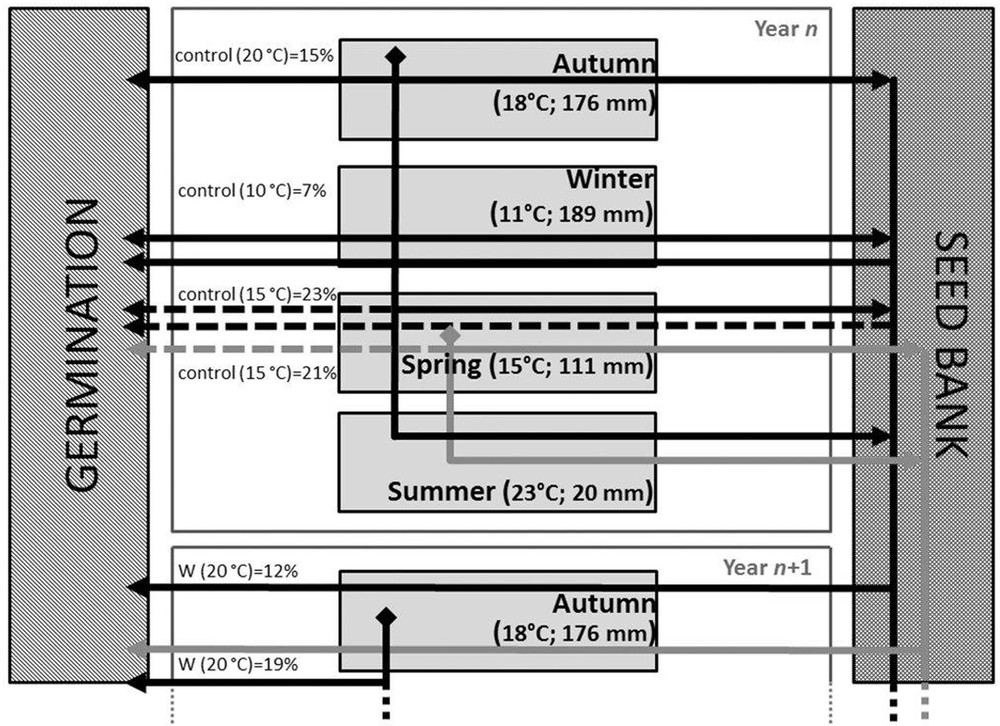
Phenology of J. macrocarpa germination. For each season, mean values of temperatures and rainfall are reported. These mean values were obtained as an average of the climatic data of the four localities acquired from available data at a spatial resolution of one square kilometer, downloaded from the WorldClim website (http://www.worldclim.org) as documented by Hijmans et al. [40]. On the left, the mean germination percentages achieved in laboratory at different temperatures and after different pre-treatments that mimic the correspondent seasons for both autumn and spring dispersed seeds are also reported. Germination event for which favourable conditions of both temperature and rainfall are indicated by continuous lines, whereas long dashed lines represent germination event that are limited by water availability (rainfall).
The results presented in this study could have direct implications on the improvement of in situ conservation actions, such as population reinforcement and regeneration of the habitat of J. macrocarpa. Moreover, they have implications for the planning, the management, and the development of ex situ conservation techniques of the species. Considering that the highest values of viability and germination were obtained from seeds collected in spring, this taxon should be regenerated using cones collected in this season to increase the chances of success in the reproduction under controlled (greenhouse and nursery) or natural conditions. Mature cones may be collected from both plant and soil, as no difference on seed viability and germination were detected as a function of the collection site of the seeds. Sowing should be performed both with fresh seeds and after a warm pre-treatment. Instead, according to the results achieved in this study, autumn should be privileged for sowing in field, considering the higher success rate of seedling survival and establishment [32].
In conclusion, seeds of J. macrocarpa were characterized by low values of viability and germination and high levels of dormancy. The applied pre-treatments were not able to overcome the detected primary and secondary dormancies, highlighting the need for further studies. The germination phenology all along the dispersal season (from autumn to spring) as well as the potentiality of this species to create a soil seed bank were illustrated, although it should be confirmed by specific studies. The narrow range of germination temperatures (15–20 °C) and the slow germination rate detected for seeds of this species are ecologically advantageous to fit the Mediterranean climate, which is characterized by a considerable unpredictability of precipitations [12], a situation that can be amplified by the predicted climate change [41]. Spring was identified as the best season for seed collection, whereas autumn was the best for sowing in the field, providing us with new data for the recovery and conservation planning of this species.
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.
Acknowledgements
The authors wish to thank the BG-SAR staff for the help with seed collection, cleaning and germination tests. Research grants came from the Cagliari Province and from the Providune project (LIFE07 NAT/IT/000519), financed by LIFE+ “Nature and Biodiversity” program.


