1 Introduction
On coral reefs, most fish species have stage-structured life histories, with a largely sedentary benthic stage (usually juveniles and adults), preceded by a pelagic larval stage with the capacity for long-distance dispersal [1]. After the pelagic phase, fish larvae return to reef habitat in order to continue their development into juvenile and adult stages (i.e., settlement phase) [2]. During the settlement phase, fish larvae are subjected to strong selective pressure to choose a suitable reef habitat that will promote post-settlement survival and growth of individuals [3]. Up to 90% of fish larvae may be removed by predation during the first post-settlement days if they do not select a suitable habitat [4,5]. Thus, many reef fish species show marked selectivity in habitat choice at settlement based on the presence of specific substrates and/or conspecifics, and on the absence of predators or competitors for food and space [1,3]. As it is unlikely that successful habitat selection of fish at settlement is solely a matter of chance; one of the greatest challenges facing the fish species with pelagic larval stages is how to relocate the relatively rare patches of suitable coral reef habitat on which they settle and ultimately reside as adults [6].
Recent studies on coral reef fish larvae have revealed highly developed swimming abilities and the use of sensory modalities to interpret habitat cues when settling to reef habitat [7]. Recognition of suitable settlement habitats has been hypothesized to be based on a combination of some or all of acoustic, chemical, visual, sun compass, rheotactic, magnetic, wave motion and thermal cues. However, only the visual, olfactory and auditory senses are known to be functional in coral reef fishes when they settle into their first reef habitat [1,6,7]. Sound is omnidirectional, and pervasive on coral reefs, as a result of bioactivity, and some recent studies have demonstrated its importance for several species of tropical marine fishes as they settle into their first reef habitat [8–10]. Several species of fish can use chemical cues related to the habitat, to conspecifics or to predators at settlement [11–14]. Vision may be significant over only short ranges, up to 5–10 m [6]. However, vision is especially important in environments where water transparency is high, such as on coral reefs or in non-estuarine back-reef areas [15,16]. Unfortunately, only seven studies have explored the importance of visual cues during habitat selection of coral reef fish larvae [16–22]. For example, Huijbers et al. [22] tested the response of a fish species (Haemulon flavolineatum) toward auditory, olfactory, and visual cues from four different reef patches (seagrass beds, mangroves, rubble, and coral reef). They showed that H. flavolineatum only responds to sound from coral reefs and to chemical cues from mangroves and seagrass beds, whereas conspecific visual cues overruled olfactory cues from mangrove and seagrass water.
In the present study, we aimed to increase scientific knowledge of the visual world of fish during habitat selection, focusing on the larval life history stage to better understand the settlement process. Firstly, we performed laboratory experiments to study the visual recognition of conspecifics (i.e. attraction behavior) and predators (i.e. repulsion behavior) by fish larvae. Secondly, experiments with conspecific cues were performed in outdoor aquaria under natural light in order to identify if fish larvae can visually recognize conspecifics according to lunar light intensity.
2 Materials and methods
2.1 Fish collection and experimental setup
A total of 6 larval fish species were captured with crest nets [23] on the reef crest of Moorea Island (17°31′7.38 S, 149°55′20.89 W), French Polynesia from March to June 2011: Acanthurus triostegus (Linnaeus, 1758 – standard length at larval stage: mean = 25 mm, SD = 4 mm), Chromis viridis (Cuvier, 1830 – mean = 9 mm, SD = 2 mm), Mulloidichtys flavolineatus (Lacepède, 1801 – mean = 78 mm, SD = 6 mm), Ostorhinchus angustatus (Smith and Radcliff, 1911 – mean = 16 mm, SD = 4 mm), Stegastes fasciolatus (Ogilby, 1889 – mean = 15 mm, SD = 3 mm) and Valenciaenna strigata (Broussonet, 1782 – mean = 25 mm, SD = 3 mm). Fish captured during the night were transferred and subsequently maintained in individual aquaria (0.3 × 0.3 × 0.2 m; water temperature: 26–27 °C) supplied with flow-through seawater from the adjacent lagoon, and without any added artificial or natural habitat substrate.
The conspecifics (individuals of the same species than that of the larvae tested in a dual-choice aquarium) and the heterospecifics (individuals of any fish species among the six species captured other than the species tested), used as cue transmitters (Exp. 1 and 3), were juveniles caught with crest nets and maintained in aquaria from 15 to 21 days. The fish grow in aquaria from 2 mm (C. viridis) to 6 mm (M. flavolineatus). Previous studies showed no repulsion effects by the heterospecifics on fish larvae in a dual-choice aquarium [11,20,21].
The predators, used as cue transmitters (Exp. 2), belonged to one of the three fish species captured at adult stage: Saurida gracilis (Quoy and Gaimard, 1824), Neoniphon sammara (Forsskål, 1775), Sargocentron spiniferum (Forsskål, 1775). These species were chosen because they were easy to capture (with hand-nets), easy to keep in a large aquarium (2 × 1 × 0.8 m), and they were key-predators in coral reefs on Moorea [24]. Predators were not fed for 24 h prior to the experiment.
The three experiments described below were conducted the evening (8 p.m. to 11 p.m.) following larval capture (i.e. within 24 h of collection). Two experiments (Exp. 1 and 2) were conducted in the laboratory under artificial lighting conditions (neon lighting of 36 W) provided by evenly distributed florescent light sources [20,21]. The third experiment (Exp. 3) was conducted outdoors under natural lunar light at night (i.e. site isolated from any artificial light: 100 m from the CRIOBE station and on a table placed in the middle of an open field).
In each of the three experiments, a dual-choice aquarium was used (Fig. 1). The aquarium, made with white opaque comassel (60 × 12 × 10 cm), fitted of transparent plexiglass on its sides. During experiments, conspecifics, heterospecifics and predators were set up in adjacent tanks (labelled 1 and 2 – 30 × 20 × 20 cm), put on styrofoam plates and placed at the ends of the dual-choice aquarium (2-cm gap). This experimental system isolate the larva placed in the aquarium from chemical and acoustic cues emitted by conspecifics, heterospecifics and predators [20]. Thus, only visual cues emited from fish were responsible of larva movement in compartment A or C. The larva was introduced into the central compartment B of the aquarium. After 2 minutes of acclimation, separations defining the three compartments of the aquarium were removed. The larva could then move into adjacent compartments (A and C) according to visual cues presented to it (heterospecifics, conspecifics or predators). The time spent in each of the three compartments were recorded for 1 minute. Overall, the fish larvae used in the three experiments were not the same.
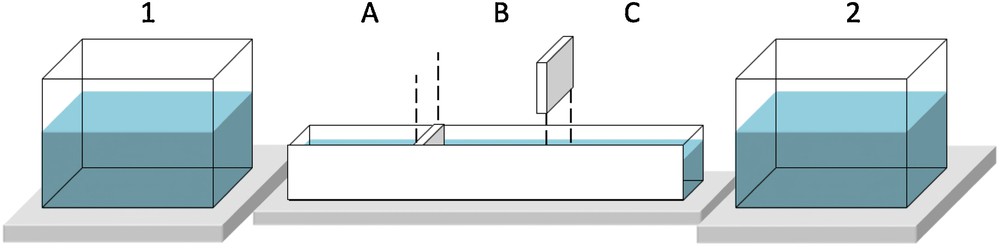
A diagram of the dual-choice aquarium used to highligth the visual attraction of fish larvae to conspecifs cues or the visual repulsion of fish larvae to predators cues.
2.2 Experiment 1: effect of conspecifics on larval fish attraction
Three heterospecifics were placed in one tank and three conspecifics in the other one. To avoid a “size effect” in the choice of fish larvae (i.e., larvae attracted or repulsed by smaller or bigger fish), the heterospecifics used had a relatively similar size to conspecifics. For each test, one larva was placed in the central compartment of the aquarium (Fig. 1). After 2 minutes of acclimation, the time spent in each of the three compartments were recorded for 1 minute. After each test, the dual-choice aquarium was emptied and washed with freshwater. The two tanks having the conspecifics or heterospecifics were inverted after each test. Ten fish larvae of each species were tested in the experiment 1.
2.3 Experiment 2: effect of predators on larval fish repulsion
The sampling protocol was identical to the experiment 1, except that three conspecifics were replaced by three predators (one individual of each species: S. gracilis, N. sammara, S. spiniferum). Ten fish larvae of each species were tested in the experiment 2. For each test, some new fresh predators were used with similar sizes (S. gracilis: 16 cm ± 1.7 cm, N. sammara: 8 cm ± 1.1 cm, and S. spiniferum: 11 cm ± 1.5 cm).
2.4 Experiment 3: effect of conspecifics according to lunar light intensity
To compare light intensities of different nights, a protocol based on photography was established. Each night, a white sheet of paper was photographed with a Nikon D200 digital SLR camera – 105 mm of 3.2 lens (always positioned at the same distance on a tripod) at the beginning and end of the experiment. All device parameters were set: white balance, aperture length of 3.2, ISO 800, opening time of 2 minutes. These pictures were analyzed using the open source software Image J. An analysis of the gray values of each pixel was performed on all photos. With RGB images, the software used the following formula: gray = log (0.299 red + 0.587 green + 0.114 blue) to obtain a gray value for each pixel of the image. A mean gray value and associated standard error were thus obtained for each picture. These values were averaged per night (photo at the beginning and at the end of the experiment). All camera settings were fixed, allowing value comparison, and subsequently light intensities of each night were compared.
This experiment has only been conducted on A. triostegus during 8 nights due to low capture efficicency of crest nets during this period. A total of 10 A. triostegus larvae were tested each night. The experimental protocol was identical to experiment 1. A Sony Handicam camcorder with an infrared mode (night vision) was used to distinguish the larvae during the experiment without using any artificial light.
2.5 Statistical analyses
The “no-choice” result (i.e., time spent by larvae in the central compartment) was not included in the statistical analysis, which is standard practice for non-responding animals in behavioral studies [14,22,25]. The mean time spent in the central compartment was 24 s in experiment 1, 29 s in experiment 2, and 23 s in experiment 3 (for all larvae tested). Thus, Wilcoxon tests were performed separately for each species to compare the time spent by larvae in the compartment close to the conspecifics’ tank vs. time spent by larvae in the compartment close to the heterospecifics’ tank for experiments 1 and 3. For experiment 2, Wilcoxon tests were performed separately for each species to compare the time spent by larvae in the compartment close vs. opposite to predators. These analyses allowed us to determine if marine larvae were significantly attracted by the visual cues of conspecifics and significantly repulsed by the visual cues of predators.
3 Results
3.1 Experiment 1: effect of conspecifics on larval fish attraction
When larvae experienced visual cues from conspecifics vs. heterospecifics, five of the six species tested were significantly attracted by conspecifics (Wilcoxon test, P < 0.05 for A. triostegus, C. viridis, O. angustatus, S. fasciolatus, and V. strigata – Fig. 2). For example, A. triostegus larvae spent 42 s (mean; standard deviation SD = 7 s) in the compartment close to the conspecifics’ tank and 3 seconds (SD = 2 s) in the compartment close to the heterospecifics’ tank (Wilcoxon test, z-value = 2.5, P-value = 0.01). Only M. flavolineatus larvae were not attracted by conspecifics (z-value = 0.3, P-value = 0.78).
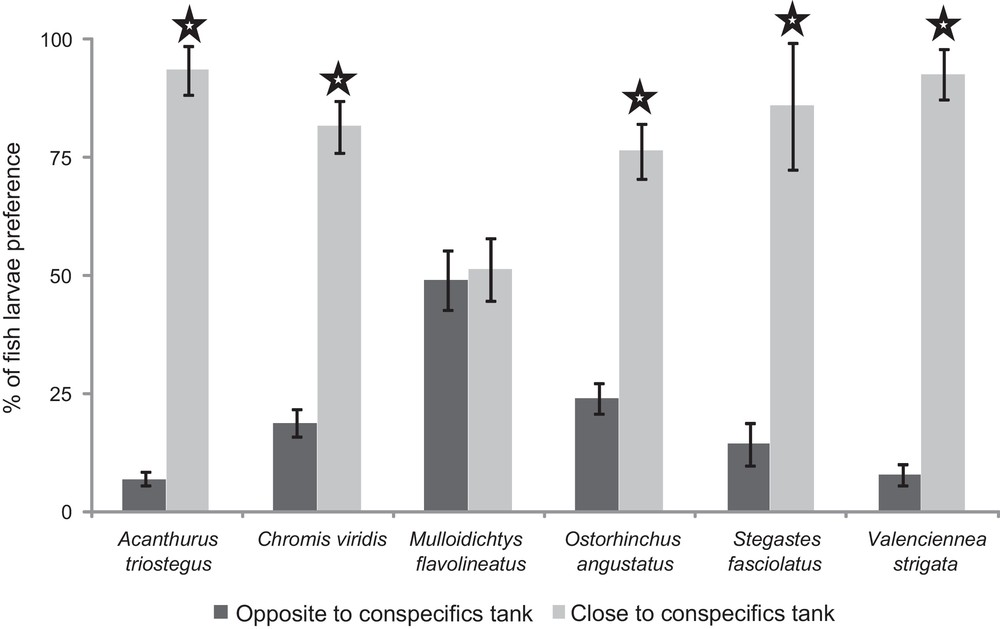
Fish larval preferences (% of time spent in each adjacent compartment: A or C) for visual cues emitted by conspecifics. : the Wilcoxon test comparing the time (absolute time in seconds) spent by larvae in the compartment close vs. the one opposite to the conspecifics’ tank showed a significant difference (P < 0.05). Lines above bars refer to one standard error (computed on 10 fish larvae).
3.2 Experiment 2: effect of predators on larval fish repulsion
When larvae experienced visual cues from predators, three of the six species were significantly repulsed by predators (P < 0.05 – M. flavolineatus, O. angustatus, and V. strigata; Fig. 3). For example, M. flavolineatus larvae spent 39 seconds (SD = 9 s) in the compartment opposite to the predators’ tank and 7 s (SD = 5 s) in the compartment close to the predators’ tank (Wilcoxon test, z-value = 2.0, P-value = 0.04). In contrast, A. triostegus, C. viridis and S. fasciolatus larvae were not significantly repulsed by predators (P > 0.05).
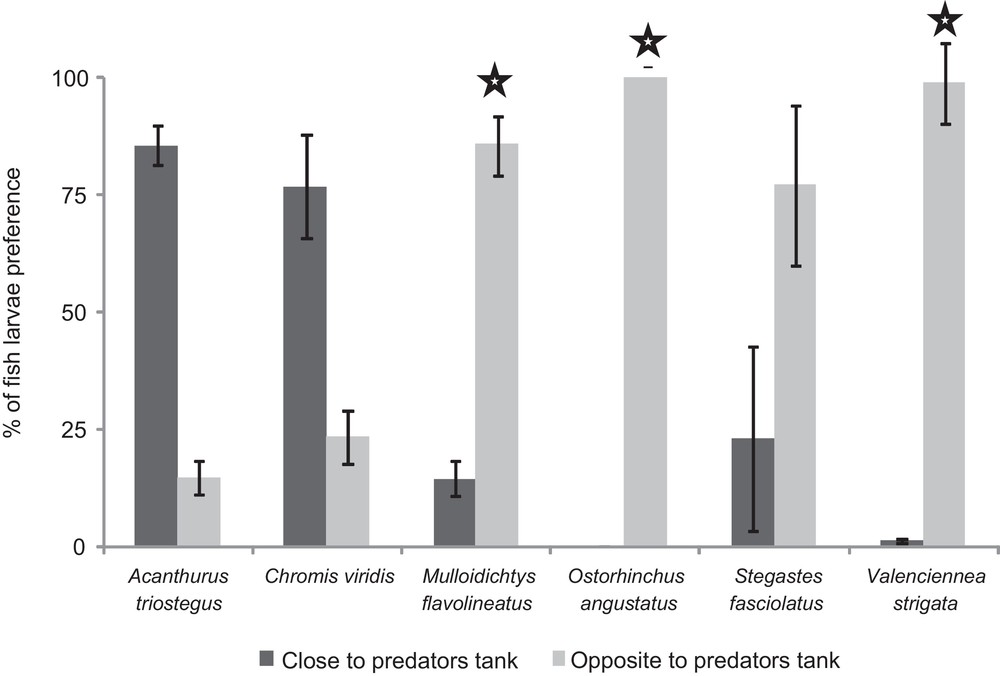
Fish larval preferences for visual cues emitted by predators. : the Wilcoxon test comparing the time spent by larvae in the compartment close vs. the one opposite to the predators’ tank showed a significant difference (P < 0.05). Lines above bars refer to one standard error.
3.3 Experiment 3: effect of conspecifics according to lunar light intensity
The light intensity of sampling nights ranged from 1.16 (new moon) and 2.40 (full moon) (Fig. 4). The attraction of A. triostegus larvae by visual cues of conspecifics varied according to the light intensity (Fig. 4).
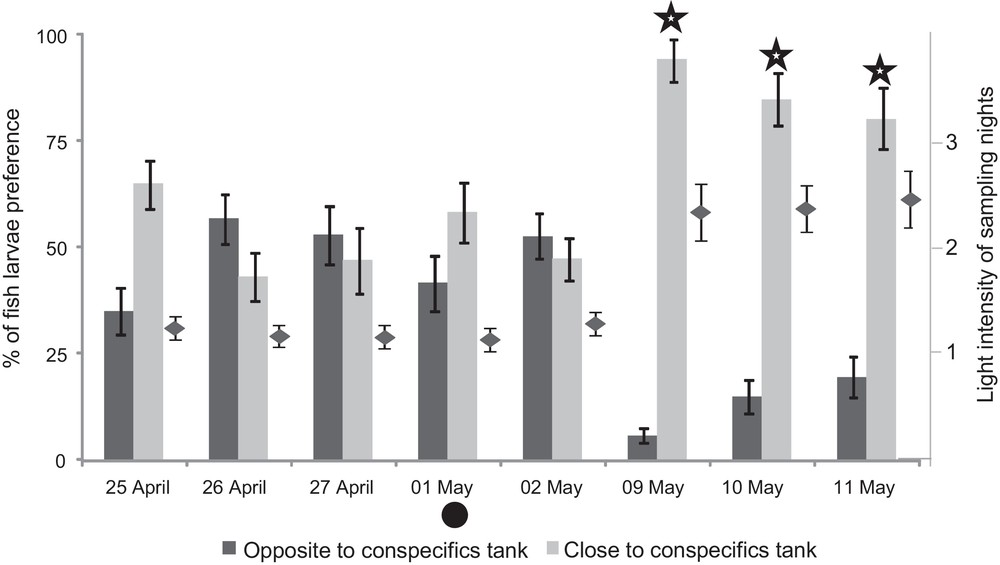
Larval preferences of Acanthurus triostegus for visual cues emitted by conspecifics (y-axis on the left: range from 0 to 100%) according to light intensity of sampling nights (y-axis on the right: range from 0 to 3). The larval preferences were shown by histograms. The light intensities were shown by a black point. : Wilcoxon test comparing the time spent by larvae in the compartment close vs. the one opposite to the conspecifics’ tank showed a significant difference (P < 0.05). Lines above bars or black points refer to one standard error. : new moon.
The five tests conducted during the nights with low light intensity (< 1.26 – nights from 25 April to 2 May) highlighted that fish larvae were not able to distinguish visually between conspecifics and heterospecifics (Wilcoxon test, P > 0.05 for each sampling night). For example, during the night of 27 April (light intensity: 1.18), fish larvae spent 53% (SD = 13%) of their time in the compartment close to the heterospecifics’ tank (Fig. 4). In contrast, the three tests conducted during the nights with high light intensity (> 2.27 – nights from 9 to 11 May) showed that fish larvae were significantly attracted to conspecifics (Wilcoxon test, P < 0.05 for each sampling night). For example, during the night of 9 May (light intensity: 2.27), fish larvae spent only 6% (SD = 3%) of their time in the compartment close to the heterospecifics’ tank, and then 94% (SD = 14%) of their time in the compartment close to the conspecifics’ tank (Fig. 4).
Lastly, no A. triostegus larvae were captured with crest nets during the full moon (i.e. no test conducted); however, the light intensity was measured corresponding to the maximum light intensity (2.40).
4 Discussion
The replenishment and persistence of most coral reef fish species depends on larvae finding suitable adult habitat at the end of their offshore dispersive stage [7]. Despite the importance of perception of information during the habitat selection, the role of visual cues during settlement has been largely overlooked because larval settlement occurs primarily during night [6]. However, several researchers are aware of the importance of visual cues for small scale habitat selection during a bright night [16–22]. For example, Igulu et al. [16] showed that fish larvae of Lutjanus fulviflamma preferred seagrass and coral above mangrove roots. Fish larvae were more attracted towards visual cues of a combination of conspecifics or heterospecifics and seagrass microhabitats than to seagrass microhabitats alone, but showed a significantly stronger preference for visual cues of conspecifics than of heterospecifics when placed in preferred seagrass or non-preferred mangrove microhabitats. Similarly, Booth [18] demonstrated in aquaria that for Dascyllus albisella sight played a role in choice of the settlement habitat. Despite the low number of replicates in experiment 1, our study confirm these previous studies in showing the visual attraction of fish larvae to conspecifics for five of the six species tested (Fig. 2). For example, V. strigata larvae were significantly attracted by visual cues of conspecifics (93% of the time spent in the compartment close to conspecifics’ tank – Exp. 1). Only M. flavolineatus larvae were not attracted by conspecifics, although the juveniles of this species live in school at settlement [26]. Maybe, they could use other sensory cues (acoustic or chemical cues) to recognize their conspecifics and live in school [6,7]. Thus, several works have shown that coral reef fish have a highly developed sensory system at larval stage [6,7,27–29]. Fish larvae therefore have the sensory abilities to perceive visual information emitted by conspecifics, heterospecifics and predators. Arvedlund and Kavanagh [6] suggested that the color patterns or behavior of conspecifics could act as a visual cue aiding the choice of habitat once a reef fish is settled. Although the study was conducted on adult stage, Katzir [30] showed Dascyllus aruanus (fish with three black bands on their white body) recognized the conspecifics only by the central band. Unfortunately, no study highlighted which visual signal is really important for fish larvae (shape, size, color pattern, behavior). Yet, the shape of fish changes during the ontogeny [31]. Therefore, some fish larvae could be more attracted by young juveniles (with a shape close to that of larvae) than by old juveniles or adults (with a different shape). This is a fruitful avenue for future research to use some lures to identify the type of visual signals used by coral reef fish larvae to recognize the conspecifics.
Our results also showed that some larval coral reef fish were capable of recognizing predators (M. flavolineatus, O. angustatus, and V. strigata). For example, V. strigata larvae were significantly repulsed by predators (99% of the time spent in the compartment opposite to the predators’ tank – Exp. 2). Some studies also showed innate predator recognition by coral reef fish larvae. Dixson et al. [32] showed that both newly hatched larvae and settlement-stage larvae of anemonefish, Amphiprion percula, distinguished between chemosensory cues of predatory and non-predatory fish species. Vail and McCormick [33] showed innate predator recognition in settlement-stage damselfishes, using patch reefs manipulated to release a predator scent. Lastly, Dixson et al. [34] showed that A. percula larvae innately distinguish between piscivorous and non-piscivorous fishes based on chemosensory cues in the diet. The innate recognition of potential predators is highly advantageous, particularly when organisms are young or are transitioning to new environments such as fish larvae at settlement. However, the repulsion behavior of fish larvae in the present study should be, nevertheless, moderated as only 50% of species tested were repulsed, and the lack of replication in the experiment 2 implies that more data would be required to generalize our results. Moreover, some fish larvae showed a surprising behavior (A. triostegus and C. viridis). For example, A. triostegus larvae spent 85% of their time in the compartment close to the predators’ tank (Fig. 3).
Overall, our laboratory experiments showed that fish larvae could detect the presence of conspecifics (Fig. 2) and predators (Fig. 3). However, their visual abilities fluctuated highly according to light intensity of the nights (Fig. 4). Our outdoor experiment demonstrated that the conspecific visual attraction was not effective during nights with low light intensity. In contrast, the three tests conducted during high light intensity highlighted that fish larvae were significantly attracted to conspecifics (Fig. 4). Yet, reef colonization occurs at night, and generally around the new moon [35,36]. Fish larvae would mainly settle during the night with low light intensity in order to reduce the reef predation [3,15]. Therefore, our outdoor experiment raises the question of trade-off for fish larvae to settle:
- • during the night with high light intensities to favor the visual recognition of conspecifics;
- • during darker nights to reduce reef predation.
In their review, Arvedlund and Kavanagh [6] suggested 18 future directions to better understand the sensory world of fish larvae during their research of a suitable habitat at settlement. Especially, they suggested that conducting more nocturnal studies of fishes would be beneficial because in nature, settlement happens at night. Thus, future studies should examine the importance of larval settlement during darker nights to avoid the reef predation vs. the importance for fish larvae to visually detect the presence of conspecifics and predator during their research of a suitable settlement habitat.
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.
Acknowledgments
This research was supported by the Institute for Pacific Coral reefs (www.ircp.pf) and the Polynesian firm, Tahiti Perles (grant IRCP – Tahiti Perles to R.G. Lanyon).


