1 Introduction
At the southern edge of their breeding area in North Africa, the Grey Wagtail Motacilla cinerea is known to breed in Morocco and Algeria [1–4]. There is no evidence of nesting in Tunisia [5]. In Algeria, the nesting area is closely associated with the rivers of both the Babor and Djurdjura Ranges [2,6,7].
The diet of the Grey Wagtail has been well studied in Europe [8–12]. The range and composition of the Grey Wagtail's diet during the breeding season generally reflects what is available along the riverbanks and habitat [13]. The diet in Europe is diverse and, although dominated by Diptera [8,9,11], other taxa, such as Trichoptera, Ephemeroptera, Plecoptera, Crustacea, and Gastropoda, can also occur. No study has been undertaken on the diet of the Grey Wagtail at the southern edge of their breeding area in North Africa, particularly in Algeria. Recently, Bougaham et al. [7] described the breeding biology of the species in the region of Bejaia. This study is the first to describe the diet of the Grey Wagtail in the southern part of their living area in North Africa and focuses on the period before and during the breeding season.
2 Materials and methods
2.1 Study site and samples collection
The study area is located inside the Babor Range, south-east of the town of Bejaia, north-eastern Algeria. The study site is a permanent river (Ighezer n’reha) localized close to the area of Souk-El-Tenine and 4 km from Tameridjet (36° 34’ N, 5° 22’ E) in the village of Tinchabine, and lies between 210 and 600 m a.s.l. The width of this river's bed varies between 1 and 17.20 m, with an average of 5.82 m. The study was undertaken along the stream of Ighezer n’reha, where 10 pairs of Grey Wagtail breed [7].
Between February and July 2010, we collected each month 15 fresh faecal samples on the emergent rocks of the stream in the immediate surroundings of the nests. We ensured when collecting samples that the faeces were from Grey Wagtails by only collecting samples in open habitat. The faeces were preserved in ethanol (70%) in labelled Eppendorf tubes.
2.2 Faecal analysis
In the laboratory, Eppendorf contents (faeces macerated in ethanol) were examined in a Petri dish using a zoom binocular microscope (0.7–4.5 × 10 magnification). Preys and prey fragments (heads, elytra, mandibles, thoraxes, abdominal segments, and pronotums) were then sorted and identified using a range of guides [14–20]. Once the prey-taxa were identified and counted, we measured them using graph paper strips to estimate the prey size [19,20]. The prey size classes were determined using the percentage of number (n %) of each length class.
2.3 Diet composition
Diet composition was expressed as a percentage frequency of occurrence (% O, the percentage of faeces in which each prey taxon occurred) and percentage composition by number (n %, the number of individuals of a prey taxon as a proportion of the total number of all prey-taxa in the entire faeces). The diet was expressed using the Shannon–Weaver diversity index [21] and according to the time of the breeding season (pre-laying period: February–March; incubation period: April–May; brood-provisioning period: June–July). The food niche width (B) was calculated [22] to determine the food resource range of the Grey Wagtail.
Diet preferences were described by the application of Costello's graphical method [23]. This graphic visualization uses the frequency of occurrence and a percent measure of abundance and provides a good description of prey occurrence (dominant or rare), predator feeding strategy (specialized or generalized), and the degree of homogeneity of the diet. This method was used to compare the diet of the species across the breeding season: the pre-laying period (February–March), the incubation period (April–May), and the brood-provisioning period (June–July).
Preys were classified according to their habitat use as terrestrial preys (T) and aquatic preys (A). For example, larvae of Ephemeroptera, of Trichoptera or of Plecoptera were deemed to originate from aquatic foraging habitat, as well as larvae of Anisoptera. In contrast, winged adults of Zygoptera, of Carabidae, of Staphylinidae and Chrysomelidae were classified as preys originating from the terrestrial foraging habitat.
To test whether the number of taxa per faeces varied across the breeding season, the values were analysed by means of one-way ANOVA. Chi2 tests of independence were used to test interbreeding period differences in the number frequency of the prey size range. We evaluated the degree of similarity between different periods (pre-laying, incubation, and brood provisioning) of the nesting season of the species using the Sörensen similarity index.
3 Results
3.1 Diet composition
Overall, 139 prey-taxa were identified (Appendix 1). The number of taxa per faeces varied between 2 and 16 (mean = 6.08 ± 2.79). These prey-taxa were distributed as follows: 126 Insecta, 7 Arachnida, 5 Crustacea, and 1 Gastrapoda. The total number of individual preys was estimated at 727 individuals. Insects accounted for the greatest number of individuals (n = 612, 84.18%), and Arachnida for the second one (14.44%). Gastrapoda and Crustacea occurred rarely (0.96% and 0.41%, respectively). Coleoptera were common in the diet with 331 individuals (45.52%). They are followed by Arachnida (13.75%) and Trichoptera (13.20%). The diet of the Grey Wagtail in the Bejaia area was composed of 38 families. The most frequent prey-families were Staphylinidae, Dytiscidae, Philopotamidae, Potamantidae, and Formicidae.
The diversity of the diet of the Grey Wagtail remains considerable (H’ = 3.56). The food niche width (B) calculated in the region of Bejaia showed a value of 53.16. Aquatic prey-taxa (n = 72, 51.79%) slightly get it on prey-taxa from terrestrial origin (n = 67, 48.20%).
3.2 Diet variation
A total of 91 taxa were found in the diet in the pre-laying period, 60 taxa for the incubation period and 61 taxa for the brood-provisioning period (Table 1).
Ecological features of the diet of the Grey Wagtail during different periods of the nesting season in the region of Bejaia.
| Parameters | Pre-laying | Incubation | Provisioning |
| Total richness (S) | 91 | 60 | 61 |
| Mean richness (s) ± s.d. | 7.20 ± 3.45 | 5.73 ± 2.59 | 5.33 ± 1.80 |
| Prey-taxa mean size (mm) ± s.d. | 8.80 ± 6.50 | 8.75 ± 6.56 | 8.89 ± 6.28 |
| Shannon–Weaver index (H’) | 3.36 | 2.80 | 3.05 |
| Food niche Width (B) | 28.78 | 16.44 | 21.11 |
The mean taxa richness was higher in pre-laying period (7.20 ± 3.45 preys-taxa) (F2, 87 = 3.95; P < 0.05). The mean taxa richness of the adult diet was broadly similar for the other parts of the breeding cycle. For the three periods, the aquatic prey-taxa were more common than the terrestrial ones (Appendix 1). In pre-laying period, the aquatic prey-taxa identified in 30 faeces were 50 of them (54.94%), whereas the number of terrestrial species was 41 (45.05%). It is the same for the incubation period, when the aquatic prey-taxa were more frequent, with 36 prey-taxa (60%), whereas the terrestrial prey-taxa were less abundant (24 prey-taxa). In contrast, during the brood-provisioning period, the Grey Wagtails did not show any preference when choosing the food for their nestlings (31 aquatic versus 30 terrestrial, Appendix 1).
Across all periods, the medium prey-taxa size varied between 8.75 mm for the incubation period and 8.89 mm for the brood-provisioning phase and there are no significant differences between the mean prey-taxa sizes. The diversity (H’) index varies according to the period of the breeding season; the maximum value was 3.36 for pre-laying phase and the minimal (2.80) for incubation period and an intermediate value (3.05) during the brood-provisioning period (Table 1). The food niche width (B) follows the same prey-taxa diversity pattern: the highest value was recorded during the pre-laying period (28.78), whereas the lowest was observed during the incubation period. An intermediate value (21.11) was noted during the brood-provisioning period (Table 1).
3.2.1 Prey size
Overall, the prey size ranges between 0.2 mm (Hydrachna sp1) and 32 mm (Dytiscus sp1). Across all breeding periods, the intermediate prey size (0.2 to 10 mm) was more common (Table 2). They were represented, for example, by Omaliinae sp2 (n = 34) and Gyrinidae sp (n = 30). The large prey-taxa sizes were eaten less often, and varied from 21.4 to 32 mm for example Dytiscus sp2 (n = 3). However, during the pre-laying period, prey-taxa of smaller sizes were recorded (Table 2). The larger prey-taxa sizes were found more frequently during the breeding season (incubation and brood-provisioning periods). Nevertheless, the prey-taxa sizes varying between 16.1 and 21 mm were more dominant in the pre-laying period (Table 2).
Number frequency (%) of valued prey size range (mm) of Grey Wagtail for each nesting period in the region of Bejaia.
| Prey size range (mm) | Percentage of number (%) | Inter-period differences | ||
| Pre-laying | Incubation | Provisioning | ||
| [0.2–5.5] | 49.8 | 33.6 | 21.9 | χ2 = 24.58; d.f. = 2; P < 0.0005 |
| [5.5–10.8] | 29.5 | 41 | 46.9 | χ2 = 10.79; d.f. = 2; P < 0.005 |
| [10.8–16.1[ | 9 | 12.9 | 16.5 | χ2 = 5.46; d.f. = 2; P = 0.052 |
| [16.1–21.4] | 7.9 | 2.3 | 6.7 | χ2 = 7.26; d.f. = 2; P = 0.026 |
| [21.4–26.7] | 3.1 | 6.4 | 3 | χ2 = 4.22; d.f. = 2; P = 0.12 |
| [26.7–32] | 0.6 | 3.7 | 4.9 | χ2 = 9.10; d.f. = 2; P = 0.011 |
3.2.2 Abundance in the diet
During the nesting season 2010 (February–July), insects dominate the diet of the Grey Wagtail of the region of Bejaia. Spiders (Araneae sp2 and Araneae sp4) were the predominant prey recorded both in terms of occurrence and number (Fig. 1a).
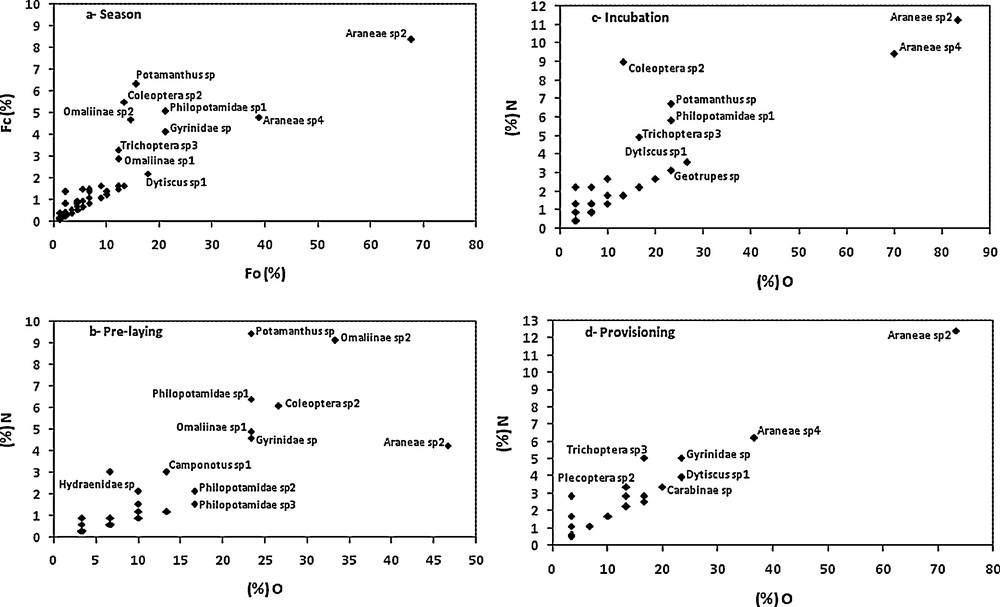
Costello graphical representation of potentials prey-taxa during different periods of the nesting season of the Grey Wagtail. % O: frequency of occurrence. % n: frequency of the number.
In the pre-laying period, Grey Wagtails capture mainly Omaliinae sp2 (33.33%, 9.14%), Potamanthus sp (23.33%, 9.45%), Araneae sp2 (46.66%, 4.26%), Coleoptera sp2 (26.66%, 6.09%), Gyrinidae sp (23.33%, 4.57%), Omaliinae sp1 (2.33%, 4.87%), Philopotamidae sp1 (23.33%, 4.40%), Philopotamidae sp3 (16.66%, 2.13%), Philopotamidae sp2 (16.66%, 1.52%), Camponotus sp1 (13.33%, 3.04%). The other prey-taxa occurred less. Araneae sp4 and Dytiscus sp1 do not occur as prey-taxa.
During the incubation period, the diet was largely dominated by Spiders (Fig. 1c). The Costello graphical method shows that Araneae sp2 (83.33%, 11.26%) and Araneae sp4 (70%, 9.45%) were dominant. These were followed by Dytiscus sp1 (26.66%, 3.70%), Potamanthus sp (23.33%, 6.75%), Philopotamidae sp1 (23.33%, 5.85%), and Coleoptera sp2 (13.33%, 9%). The other insects, for example Trichoptera sp3 (16.66%, 4.95%), were well represented in the analysed faeces. It is to be noted that Geotrupes sp (23.33%, 3.15%) appeared as a new prey taxon in the diet of the species. During the brood-provisioning period, Araneae sp2 (73.73%, 12.42%) constitute singly the potentials prey-taxa of the Grey Wagtail. The other prey-taxa were also important prey items, for example Araneae sp4 (36.66%, 6.21%), Gyrinidae sp (23.33%, 5.08%), Dytiscus sp1 (23.33%, 3.95%), and Trichoptera sp3 (16.66%, 5.08%) (Fig. 1d). This period can also be distinguished from the other ones by the presence of two new potential prey-taxa, Carabinae sp (20%, 3.38%) and Plecoptera sp2 (23.33%, 3.95%).
3.2.3 Similarity of the diet
The similarity was least (Sörensen index = 35.5%) when pre-laying and brood-provisioning periods were compared and greatest when comparing the incubation and brood-provisioning ones (51%). Comparison between the pre-laying period and the incubation period shows an intermediate value (43.70%).
4 Discussion
The prey-taxa found in the 90 faeces collected in the region of Bejaia enabled us to identify 139 prey-taxa, corresponding to 727 individuals. The insects accounted for the greatest number of individuals (n = 612). This is in similar to what has been found by Santamarina [9] in Spain, Ormerod and Tylor [8] in Wales and Bureš [11] in the Czech Republic, where the Grey Wagtail was mainly insectivorous and captured notably aquatic invertebrates. Among the insects, Potamanthus sp (46) was the most abundant, followed by Coleoptera sp2 (n = 40), Philopotamidae sp2 (n = 37), Omaliinae sp2 (n = 34), and Gyrinidae sp, with 30 individuals. Araneae sp2 and Araneae sp4 (61 and 35 individuals respectively) were well represented in the faeces. Crustacea and Gastropoda also contributed to the diet composition of the Grey Wagtail with 7 and 3 individuals only, respectively. In Europe, Grey Wagtails seem to capture more Diptera than other prey taxonomic categories, for example Trichoptera and Ephemeroptera [8,9]. Indeed, the prey range of the Czech population of the Grey Wagtail was mostly composed of 36% of Diptera, 26% of Plecoptera, 14% of Homoptera, Trichoptera, etc. [11].
It appears that the total specific richness identified in the breeding season (S = 139) was important. The number of prey-taxa per faeces varies between 2 and 16. So the average richness (s) per faeces was 6.08. The variation of prey-taxa number per faeces was important and can be explained by increased fluctuations of the abundance of the prey-taxa captured by the species near the rivers. The insects constitute the dominant (84.18%) constituent of the diet of the Grey Wagtail in the region of Bejaia. The predominance of insects in the diet is related to the fact that insects were the most available preys in the micro-habitats surrounding the river. Arachnida were the second most common (14.44%). Gastropoda and Crustacea were slightly represented (0.41% to 0.96%).
When Spiders are excluded, the Grey Wagtails feed mainly on Potamantidae. This result could be explained by the fact that the Grey Wagtail tends to capture medium-sized prey. Philopotamidae, Staphylinidae and Dytiscidae constitute also a considerable part in the diet of the species. The others items types were slightly represented in the collected faeces. These prey types noted in Bejaia are also recorded across the entire range distribution of Grey Wagtails [8,9,13]. In contrast, our results seem to differ from those of Santamarina [9] in Spain, where Spiders do not appear in the 129 faeces analysed.
The largest number (S) of prey-taxa was found in the faeces collected in the pre-laying period (91) and the lowest mean specific richness (s) was noted in the brood-provisioning period (5.33). The temporal variation in the diet of the Grey Wagtail could correspond to the variation and diversity of preys, to prey-taxa abundance, as well as to the intensity of prey search and catch during the nesting cycle. Moreover, the food niche width of Grey Wagtail varies according to the different breeding periods, with a maximum value recorded during the first months (February–March) of the nesting season. Indeed, the aquatic prey-taxa dominate the diet of the wagtails during the first phase of the nesting season. Then, the diet shifts towards a terrestrial prey-dominated one as the nesting season advances, particularly in the brood-provisioning period. Ecological constraints such as water turbidity and aerial prey availability (terrestrial) stimulate the Wagtails to modify their foraging habitats [12]. Bureš [11] observed less captures of aquatic flying insects during the brood-provisioning period. It is possible that the foraging time or the prey energy value might be no more profitable at the end of the nesting season (brood-provisioning period) [12].
During the nesting season (February–July), the potential prey-taxa of the species were: Araneae sp2, Araneae sp4, Philopotamidae sp1, Gyrinidae sp, Potamanthus sp, Dytiscus sp1, Omaliinae sp2, Coleoptera sp2, Trichoptera sp3 and Omaliinae sp2. However, the potential prey-taxa choice varies according to the various nesting periods. In pre-laying period, the diet was more diverse and the species selected preferably the medium prey-taxa sizes, for example Omaliinae sp2 (5 mm) and Araneae sp2 (8 mm). A large prey like Dytiscus sp1 (32 mm) does not appear as a potential prey type of the species in this period. This tendency was different from that noted in the incubation period, when the Spiders (in particular Araneae sp2 and Araneae sp4) were the potentials prey-taxa of the Wagtails. However, besides these two last prey species, the Grey Wagtail tends to capture both aquatic prey-taxa and large preys like Dytiscus sp1 (32 mm) and Philopotamidae sp1 (20 mm). At the end of the nesting season, the availability of the preys in the aquatic environment decreases because of the emergence of the aquatic adult insects that gain however other surrounding micro-habitats such as the shingle sides of the river and the riparian vegetation [11]. Bureš [11] states that the potential preys’ availability under the riparian vegetation (terrestrial habitat, [11] sensu) does not vary significantly during the nesting season. It was thus a predictable food resource in space and time.
The diet of the Grey Wagtail of the region of Bejaia is characterized by a prevalence of aquatic prey-taxa over terrestrial ones. This observation agrees with other ones [8,9,11]. This behaviour indicates a narrow dependence of the species on the aquatic invertebrates to feed [9,11]. In aquatic habitat, the preys are more conspicuous and less mobile than in the surrounding micro-habitats. This habitat appears more favourable to the research of the necessary food [11]. Nevertheless, we notice that the proportion of aquatic prey-taxa decreases as the nesting season advances, whereas that of terrestrial ones increases to reach a maximum of 46.66% at the end of the nesting season (brood-provisioning period). These tendencies could be the reflection of the temporary changes in the food resource availability in these habitats. In northwestern Spain, Santamarina [9] showed that the percentage of aquatic preys was more important during the first months of the year (i.e. the winter) than in the spring and in the summer. It seems that the food resources were more available and more abundant in aquatic environments, in particular during the first months of the breeding season. So wagtails forage in the most diversified and most favourable habitats. Despite the higher proportion of aquatic habitat use, wagtails exploit simultaneously two distinct foraging habitats. A complementary use for two foraging habitats during the nesting season was necessary for the good progress of nesting, which can be changed by breeding requirements and the food supply. The feeding strategy of the Grey Wagtail involves exploiting a large trophic niche throughout the nesting season. The prey-taxa number, the sizes and the particular kind of preys can vary with time. The weather conditions influence also the diversity and the availability of the preys in the considered habitat [24]. In the brood-provisioning period, the aquatic flying insects were less captured [11], because the foraging time or the prey energy value was no more profitable [12].
Comparing the similarity degree between the three phases of the nesting season, we notice that the highest value was recorded between the incubation period and the brood-provisioning phase (51.23%). This could be explained by the effect of food resource availability in the full nesting season that was related to a stronger entomofaune activity during these two periods and with close prey species. In contrast, the lowest value was noted between the pre-laying period and the brood-provisioning phase (35.52%). Moreover, the difference of habitat richness between these periods can play a considerable role. In addition, the species had access to different species, considering that the attended foraging habitats were at least different (aquatic preys predominance during the first months of the nesting season, lower predominance at the end of the season over terrestrial preys). In other words, pairs of nearby periods showed the most similar diet. This result showed the influence of the breeding requirements on the prey-taxa choice during each period of the nesting season. It indicates moreover the importance of the local factors to the Grey Wagtail's faeces composition in the region of Bejaia. The relatively variable values of the similarity index were due to the effect of the heterogeneous prey-taxa distribution in the faeces analysed.
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.
Acknowledgements
The authors thank Laldja Benali and Lamia Hachoud, ecology and environment engineers (University of Bejaia), for their assistance in Grey Wagtail's faeces analysis.
Appendix 1 Percentage occurrence (O %), number (n %) of prey-taxa found in the faeces of the Grey Wagtail Motacilla cinerea in the region of Bejaia during different periods of the nesting season 2010.
| Prey-taxa | Pre-laying | Incubation | Provisioning | ||||||
| H | O% | N% | H | O% | N% | H | O% | N% | |
| Gastropoda | |||||||||
| Gastropoda sp. | A | 3.33 | 0.30 | A | 6.66 | 0.90 | |||
| Arachnida | |||||||||
| Araneae sp1. Und. | T | 3.33 | 0.30 | ||||||
| Araneae sp2. Und. | T | 46.66 | 4.26 | T | 83.33 | 11.26 | T | 73.33 | 12.42 |
| Araneae sp3. Und. | T | 6.66 | 0.60 | T | 3.33 | 0.56 | |||
| Araneae sp4. Und. | T | 10.00 | 0.91 | T | 70.00 | 9.45 | T | 36.66 | 6.21 |
| Hydrachna sp1. Und. | A | 3.33 | 0.91 | ||||||
| Hydrachna sp2. Und. | A | 3.33 | 0.30 | ||||||
| Hydrachna sp3. Und. | A | 3.33 | 0.30 | ||||||
| Crustacea | |||||||||
| Amphipoda sp1.Und. | A | 3.33 | 0.30 | ||||||
| Amphipoda sp2. Und. | A | 6.66 | 0.60 | A | 3.33 | 0.56 | |||
| Isopoda sp1. Und. | A | 3.33 | 0.30 | ||||||
| Isopoda sp2. Und. | A | 3.33 | 0.30 | ||||||
| Asellidae sp. Und. | A | 3.33 | 0.30 | ||||||
| Insecta | |||||||||
| Potamanthus sp. Und. | A | 23.33 | 9.45 | A | 23.33 | 6.75 | |||
| Heptageniidae sp. Und. | A | 6.66 | 0.60 | A | 3.33 | 0.45 | A | 6.66 | 1.12 |
| Zygoptera sp. Und. | T | 3.33 | 1.35 | ||||||
| Anisoptera sp. | A | 3.33 | 0.56 | ||||||
| Plecoptera sp1. Und. | A | 3.33 | 0.30 | ||||||
| Plecoptera sp2. Und. | A | 10.00 | 2.70 | A | 13.33 | 3.38 | |||
| Plecoptera sp3. Und. | A | 3.33 | 0.30 | A | 6.66 | 0.90 | A | 23.33 | 3.95 |
| Plecoptera sp4. Und. | A | 3.33 | 0.45 | ||||||
| Perlodidae sp1. Und. | A | 3.33 | 0.45 | ||||||
| Perlodidae sp2. Und. | A | 3.33 | 0.56 | ||||||
| Perloïdea sp. Und. | A | 6.66 | 1.35 | A | 6.66 | 1.12 | |||
| Protonemura sp. Und. | A | 6.66 | 0.60 | A | 13.33 | 1.80 | A | 6.66 | 1.12 |
| Chloroperlidae sp. Und. | A | 6.66 | 0.60 | ||||||
| Chloroperla sp. Und. | A | 3.33 | 0.45 | ||||||
| Orthoptera sp. Und. | T | 3.33 | 0.30 | ||||||
| Blattoptera sp. Und. | T | 3.33 | 0.45 | ||||||
| Hemiptera sp1. Und. | A | 3.33 | 0.56 | ||||||
| Hemiptera sp2. Und. | A | 3.33 | 0.56 | ||||||
| Plea sp. Und. | A | 6.66 | 0.60 | ||||||
| Gerridae sp1. Und. | A | 3.33 | 0.90 | A | 3.33 | 0.56 | |||
| Gerridae sp2. Und. | A | 3.33 | 0.45 | ||||||
| Miridae sp. Und. | T | 3.33 | 0.45 | ||||||
| Corixidae sp. Und. | A | 3.33 | 0.45 | ||||||
| Notonecta sp. | A | 3.33 | 0.56 | ||||||
| Coleoptera sp1. Und. | T | 3.33 | 0.30 | T | 3.33 | 0.45 | |||
| Coleoptera sp2. Und. | T | 26.66 | 6.09 | T | 13.33 | 9.00 | |||
| Gyrinidae sp. Und. | A | 23.33 | 4.57 | A | 20.00 | 2.70 | A | 23.33 | 5.08 |
| Carabidae sp1. Und. | T | 3.33 | 0.30 | ||||||
| Carabidae sp2. Und. | T | 3.33 | 0.30 | ||||||
| Carabidae sp3. Und. | T | 3.33 | 0.56 | ||||||
| Carabidae sp4. Und. | T | 3.33 | 0.30 | T | 3.33 | 0.56 | |||
| Carabidae sp5. Und. | T | 6.66 | 0.90 | T | 3.33 | 0.56 | |||
| Carabidae sp6. Und. | T | 3.33 | 0.56 | ||||||
| Harpalinae sp. Und. | T | 3.33 | 0.90 | T | 16.66 | 2.55 | |||
| Carabinae sp. Und. | T | 6.66 | 0.60 | T | 20.00 | 3.38 | |||
| Dytiscidae sp1. Und. | A | 3.33 | 0.30 | ||||||
| Dytiscidae sp2. Und. | A | 3.33 | 0.30 | ||||||
| Dytiscidae sp3. Und. | A | 10.00 | 0.91 | A | 3.33 | 0.45 | A | 3.33 | 0.56 |
| Dytiscidae sp4. Und. | A | 3.33 | 0.30 | ||||||
| Dytiscidae sp5. Und. | A | 3.33 | 0.30 | A | 3.33 | 0.45 | |||
| Dytiscidae sp6. Und. | A | 3.33 | 0.30 | ||||||
| Agabus sp. | A | 3.33 | 0.91 | ||||||
| Dytiscinae sp. Und. | A | 10.00 | 0.91 | A | 13.3 | 1.80 | A | 13.33 | 2.82 |
| Dytiscus sp1. Und. | A | 3.33 | 0.30 | A | 26.66 | 3.60 | A | 23.33 | 3.95 |
| Dytiscus sp2. Und. | A | 3.33 | 0.45 | A | 6.66 | 1.12 | |||
| Dytiscus sp3. Und. | A | 3.33 | 0.30 | ||||||
| Colymbetes fuscus | A | 6.66 | 0.91 | A | 3.33 | 0.56 | |||
| Colymbetinae sp1. Und. | A | 3.33 | 0.30 | A | 3.33 | 0.56 | |||
| Colymbetinae sp2. Und. | A | 6.66 | 1.12 | ||||||
| Hydroporinae sp1. Und. | A | 6.66 | 0.91 | ||||||
| Hydroporinae sp2. Und. | A | 6.66 | 0.90 | A | 3.33 | 0.56 | |||
| Hydroporinae sp3. Und. | A | 6.66 | 0.90 | ||||||
| Hygrotus sp. Und. | A | 6.66 | 0.60 | A | 3.33 | 0.45 | A | 3.33 | 0.56 |
| Hydrophilidae sp1. Und. | A | 10.00 | 0.91 | A | 3.33 | 0.45 | A | 6.66 | 1.12 |
| Hydrophilidae sp2. Und. | A | 3.33 | 0.30 | A | 3.33 | 0.56 | |||
| Hydrophilidae sp3. Und. | A | 6.66 | 0.60 | ||||||
| Hydrophilidae sp4. Und. | A | 6.66 | 0.60 | A | 3.33 | 0.45 | |||
| Hydrophilidae sp5. Und. | A | 3.33 | 0.30 | A | 3.33 | 0.45 | |||
| Hydrophilidae sp6. Und. | A | 6.66 | 0.60 | ||||||
| Hydrophilinae sp. Und. | A | 6.66 | 1.12 | ||||||
| Hydrochus sp. Und. | A | 10.00 | 1.21 | ||||||
| Staphylinidae sp1. Und. | T | 3.33 | 0.30 | ||||||
| Staphylinidae sp2. Und. | T | 3.33 | 0.30 | ||||||
| Staphylinidae sp3. Und. | T | 10.00 | 0.91 | ||||||
| Staphylinidae sp4. Und. | T | 3.33 | 0.60 | T | 3.33 | 0.45 | |||
| Gauropterus fulgidus | T | 3.33 | 0.30 | ||||||
| Omaliinae sp1. Und. | T | 23.33 | 4.87 | T | 16.66 | 2.25 | |||
| Omaliinae sp2. Und. | T | 33.33 | 9.14 | T | 10.00 | 1.35 | T | 3.33 | 0.56 |
| Omaliinae sp3. Und. | T | 3.33 | 1.69 | ||||||
| Oxythelinae sp1. Und. | T | 13.33 | 1.21 | ||||||
| Oxythelinae sp2. Und. | T | 13.33 | 1.21 | ||||||
| Oxythelinae sp3. Und. | T | 6.66 | 0.91 | T | 6.66 | 0.90 | T | 3.33 | 2.82 |
| Oxythelinae sp4. Und. | T | 3.33 | 0.30 | ||||||
| Oxythelinae sp5. Und. | T | 3.33 | 0.30 | ||||||
| Elateridae sp1. Und. | T | 6.66 | 0.60 | ||||||
| Elateridae sp2. Und. | T | 3.33 | 0.30 | ||||||
| Elmidae sp. Und. | A | 3.33 | 0.30 | A | 3.33 | 0.45 | |||
| Geotrupes sp. Und. | T | 23.33 | 3.15 | T | 13.33 | 2.25 | |||
| Nitidulidae sp. Und. | T | 6.66 | 0.60 | ||||||
| Aphodius sp1. Und. | T | 3.33 | 0.30 | ||||||
| Aphodius sp2. Und. | T | 3.33 | 0.30 | ||||||
| Onthophagus sp. Und. | T | 3.33 | 0.56 | ||||||
| Chrysomelidae sp1. Und. | T | 6.66 | 0.60 | ||||||
| Chrysomelidae sp2. Und. | T | 3.33 | 0.30 | ||||||
| Chrysomelidae sp3. Und. | T | 3.33 | 0.30 | ||||||
| Chrysomelidae sp4. Und. | T | 3.33 | 0.45 | T | 3.33 | 0.56 | |||
| Chrysomelidae sp5. Und. | T | 3.33 | 0.30 | ||||||
| Chrysomelidae sp6. Und. | T | 3.33 | 0.56 | ||||||
| Otiorryhynchus sp. Und. | T | 3.33 | 0.30 | ||||||
| Curculionidae sp1. Und. | T | 10.00 | 1.52 | T | 3.33 | 0.45 | |||
| Curculionidae sp2. Und. | T | 3.33 | 0.56 | ||||||
| Cetonidae sp. Und. | T | 3.33 | 0.56 | ||||||
| Haliplidae sp1. Und. | A | 3.33 | 0.30 | ||||||
| Haliplidae sp2. Und. | A | 3.33 | 0.30 | A | 10.00 | 1.69 | |||
| Haliplidae sp3. Und. | A | 10.00 | 1.21 | ||||||
| Haliplus sp. Und. | A | 13.33 | 1.21 | A | 16.66 | 2.25 | A | 10.00 | 1.69 |
| Hydraenidae sp. Und. | T | 10.00 | 2.13 | T | 10.00 | 1.80 | |||
| Myrmeleontidae sp. Und. | T | 10.00 | 1.69 | ||||||
| Aphaenogaster testaceo-pilosa | T | 13.33 | 3.38 | ||||||
| Camponotus sp1. Und. | T | 13.33 | 3.04 | T | 3.33 | 0.56 | |||
| Camponotus sp2. Und. | T | 3.33 | 0.45 | T | 3.33 | 1.12 | |||
| Messor barbara | T | 6.66 | 0.60 | T | 3.33 | 0.45 | |||
| Pheidole pallidula | T | 3.33 | 0.45 | T | 3.33 | 0.56 | |||
| Tapinoma nigerimum | T | 6.66 | 2.25 | T | 6.66 | 1.12 | |||
| Monomorium salomonis | T | 6.66 | 3.04 | ||||||
| Plagiolepis barbara | T | 3.33 | 0.56 | ||||||
| Hymenoptera sp. Und. | T | 3.33 | 0.30 | ||||||
| Ichneumonidae sp1. Und. | T | 3.33 | 0.56 | ||||||
| Ichneumonidae sp2. Und. | T | 3.33 | 0.56 | ||||||
| Ichneumonidae sp3. Und. | T | 3.33 | 0.30 | ||||||
| Chalcidae sp. Und. | T | 3.33 | 0.56 | ||||||
| Chrysis sp. Und. | T | 6.66 | 1.12 | ||||||
| Trichoptera sp1. Und. | A | 13.33 | 1.21 | A | 3.33 | 0.45 | A | 13.33 | 2.25 |
| Trichoptera sp2. Und. | A | 6.66 | 0.60 | ||||||
| Trichoptera sp3. Und. | A | 13.33 | 1.21 | A | 16.66 | 4.95 | A | 16.66 | 5.08 |
| Hydroptilidae sp. Und. | A | 6.66 | 1.35 | ||||||
| Philopotamidae sp1. Und. | A | 23.33 | 6.40 | A | 23.33 | 5.85 | A | 10.00 | 1.69 |
| Philopotamidae sp2. Und. | A | 16.66 | 2.13 | A | 3.33 | 0.45 | |||
| Philopotamidae sp3. Und. | A | 16.66 | 1.52 | A | 3.33 | 0.45 | A | 16.66 | 2.82 |
| Rhycophilidae sp. Und. | A | 3.33 | 0.45 | ||||||
| Brachycera sp1. Und. | T | 3.33 | 0.45 | ||||||
| Brachycera sp2. Und. | T | 6.66 | 0.90 | T | 3.33 | 0.56 | |||
| Brachycera sp3. Und. | T | 3.33 | 0.30 | T | 3.33 | 2.25 | |||
| Chironomidae sp. Und. | A | 3.33 | 0.45 | ||||||
| Stratiomyidae sp. Und. | A | 6.66 | 0.60 | ||||||
| Ceratopogonidae sp. Und. | A | 3.33 | 0.45 | ||||||
| Total prey-taxa | 139 | ||||||||
| Aquatic prey | 50 | 36 | 31 | ||||||
| Terrestrial prey | 41 | 24 | 30 |


