1 Introduction
Pea (Pisum sativum L.) is a popular leguminous vegetable in Bangladesh. It is grown mainly for green pods and immature seeds, which are consumed as vegetables. Pea has also a great agronomic value. In crop rotation, it helps in improving soil fertility and the yield of succeeding crops. Diseases are one of the main constraints for successful pea cultivation. Eleven diseases of pea are reported in Bangladesh, causing about 30–40% yield loss annually [1]. Rhizoctonia solani causing seedling mortality and root rot is one of the major diseases. The fungus most often infects the hypocotyl, epicotyl and seed of pea [2]. The management of R. solani is difficult due to its soil-borne nature. The fungus is present in most of the soil. Once it is established in the field, it remains indefinitely there [3]. Dry sclerotia of the pathogen are reported to survive up to 6 years when stored at room temperature. Several fungicides, effective against R. solani, can be used for soil treatment as well as for treating material to be planted [4]. However, persistent and injudicious use of chemicals is usually associated with some familiar problems such as toxic effects on non-target organisms, development of fungicide-resistant pathogenic strains and undesirable changes on environment. Trichoderma harzianum is one efficient biological control agent and plant growth promoter that is successfully used to suppress R. solani and improve yield in economic crops [5–8]. Many reports also indicate that amending soil with organic amendments such as oil seed cake and plant residues significantly reduces soil-borne pathogens including R. solani and increases crop productivity [9,10]. However, the most economical way of controlling the disease is the use of a resistant variety, but none among the existing ones was reported to be resistant to the pathogen. Also the strong pathogen inocula in the soil limit the performance of any resistant variety.
Because of the above considerations and unattainability of real varietal resistance against rhizoctoniasis in pea, research directed towards reducing the soil-borne inoculum of R. solani should be taken into serious consideration. However, it is very expensive and not sufficient to escape the pathogen in infested field soil with a single control measure/tactic [11]. Thus, in the case of Rhizoctonia diseases, since chemical control was not successful in many situations and host resistance is rare, integrated control has often been pursued as the only means of achieving reliable and economic disease reduction. Integrated control of Rhizoctonia diseases employs combinations of biological, chemical and cultural approaches. Combined strategies are often additive and sometimes synergistic, leading to more effective and reliable disease control. Until now, many integrated systems have been researched and some are being implemented at the farm level. In each occasion, the most critical step to developing an effective integrated disease management system was the identification of the best and compatible mix of biological, chemical and cultural tactics for a given pathogen. Unfortunately, the interest in such comprehensive study and its progress are completely lacking in Bangladesh, hampering the development of integrated management systems in this discipline. In the present work, we conducted experiments to identify the best individual treatment of antagonists, organic amendments and fungicides for their efficacy in R. solani inhibition and investigated the individual and combined effect of them in the experimental field on seedling mortality disease and yield in pea.
2 Materials and methods
2.1 Microorganisms and plant materials
All the isolates (T-1, T-2, T-3, T-4, T-5, T-6, T-7, T-8, T-9, T-10, T-11, T-12, T-13, T-14, T-15, T-16, T-17, T-18, T-19 and T-20) of T. harzianum were collected from the stock cultures of the Plant Pathology Laboratory of the Bangabandhu Sheikh Mujibur Rahman Agricultural University (BSMRAU), Gazipur, Bangladesh. Four isolates of the test pathogen R. solani were isolated from pea, cotton and bush bean. “IPSA motorshuti-3” variety of pea was used as the test plant.
2.2 Isolation and characterization of R. solani
Infected root, leaf, petiole, and stem tissues of pea, cotton and bush bean were collected from the field laboratory of BSMRAU and washed in distilled water to remove sand and soil particles. The specimens were cut into small pieces (5 mm) along with healthy and dead tissue and surface sterilized with 0.1% NaOCl2 for 2 min and rinsed in sterilized water for three times. The plant pieces were blotted dry to remove excess water and plated on water agar. The characteristic colony of R. solani was transferred to fresh acidified PDA (pH 4.5). These isolates were purified by selecting a single hyphal tip (less than 1.5 mm) of each isolate growing on PDA to obtain pure cultures. All isolates were maintained in the short term on PDA slants and on colonized wheat seeds for longer-term storage. Each isolate was arbitrarily named with capital letter codes and a serial number (Table 1). Selected isolates were inoculated onto four replicate PDA plates using 5-mm-diameter mycelial disks taken from the margin of a 3-day-old PDA culture, and the plates were sealed with parafilm strips and incubated in airtight plastic containers at room temperatures (25 ± 2 °C). After 60 h of incubation, observation on several cultural characteristics such as colony colour, mycelial density, zonation number in the culture and sclerotial number per plate was recorded. The mycelial density was rated into I to IV scale as described by Dey et al. [12].
Cultural characteristics and pathogenicity of the isolates of Rhizoctonia solani.
| R. solani isolates | Source Crop |
Colony colour |
Mycelial density | Zonation | No. of sclerotia/plate | % Pre- emergence mortality |
% Post- emergence mortality |
% Total mortality |
| RS1 | Cotton | Brown | IV | 2 | 11.58 | 33.33 | 33.33 | 66.67 |
| RS3 | Pea | Brown | IV | 2 | 14.46 | 90.47 | 4.76 | 95.23 |
| RS7 | Bush bean | Brown | IV | 2 | 10.34 | 85.71 | 9.52 | 95.23 |
| RS10 | Pea | Brown | IV | 3 | 17.34 | 100.00 | 0.00 | 100.00 |
| Control | — | — | — | — | — | 0.00 | 0.00 | 0.00 |
2.3 Preparation of inoculum of R. solani
Wheat grains were soaked in water for 24 h and excess water was drained off. About 100 g of water-soaked grains were taken into a 500-ml Erlenmeyer flask, sealed by cotton plug and were sterilized for 50 min at 121 °C under 15-pound pressure in a autoclave on three consecutive days [13]. Inocula of each isolate were prepared in separate flasks. Sterilized wheat grains were inoculated with 10 disks (5 mm) obtained from the actively growing margin of 4-day-old PDA cultures of R. solani. After 10–12 days of incubation at 25 ± 2 °C in the dark, completely colonized wheat grains were air-dried at laboratory (25 ± 2 °C) temperature and stored at 4 °C until use [14].
2.4 Pathogenicity test
Earthen pots were filled with sterilized soil at the rate of 1 kg/pot. Wheat grain inoculum (WGI) of each isolate of R. solani was thoroughly mixed with the soil at the rate of 20 g/kg soil. The experiment included an uninoculated control where only sterilized soil was used. Seven pea seeds were sown in each pot and grown in the net house. Pre-emergence and post-emergence seedling mortality was estimated at 7 and 30 days after sowing. The causal agent of pre- and post-emergence seedling mortality was confirmed after re-isolation of the pathogen from the ungerminated seeds and the infected roots of pea, respectively.
2.5 In vitro evaluation of T. harzianum isolates for their antagonistic effect on the radial growth of R. solani
A total of 20 isolates of T. harzianum were tested for antagonistic activity against R. solani to select the most effective antagonist as biological control agent of R. solani. Mycelial disks (5 mm in diameter) were cut from the edge of a 4-day-old colony of T. harzianum and R. solani, and were placed simultaneously on the edge of each PDA plate at opposite directions. Four replicate plates were used for each isolate. The plates that received only disks of R. solani served as control. The plates were incubated in the laboratory at room temperature (25 ± 2 °C). Inhibition percentages of the R. solani were calculated based on the growth of the pathogen on PDA plates following the formula suggested by Sundar et al. [15].
2.6 In vitro evaluation of selected fungicides for their fungitoxicity on radial growth of R. solani
Three fungicides, namely Bavistin 50 WP (Carbendazim, methyl-benzimidazol-2-yl carbamate) (BASF Bangladesh Limited, Dhaka, Bangladesh), Ridomil Gold MZ (Metalaxyl 4% + Mancozeb 64%, N-(2,6-dimethylphenyl)-N-(methoxyacetyl)-alanine methyl ester) (Syngenta Bangladesh Limited, Dhaka, Bangladesh), and Provax-200 (Carboxin, 5,6-dihydro-2-methyl-1,4-oxathin-3-carboxanilide) (Hossain Enterprise CC Limited, Dhaka, Bangladesh) were tested against colony growth of R. solani. The fungicides were chosen for the experiments since they are widely used in conventional cultivations, mainly to control soil-borne pathogens, and because they well represent four different fungicide families. Fungicides were added at a concentration of 100, 250 and 500 ppm in an autoclaved PDA medium following poisoned-food techniques [16]. A 5-mm-diameter agar disk of test fungi was cut from a 4-day-old culture and placed in the centre of petri plates containing different concentrations of fungicides. There were four replicates of each treatment. The plates without fungicides served as control. The inoculated plates were incubated at 25 ± 2 °C. The radial growth was recorded after 7–10 days of incubation when the fungus in control plates covered the plates completely. The percent inhibition (PI) of the fungus over the control was calculated using the formula of Sundar et al. [15].
2.7 In vitro evaluation of organic amendments for their fungitoxicity on radial growth of R. solani
An extract from mustard oilcake, sesame oilcake, wheat meal, maize meal, chickpea meal, rice bran and tea waste was prepared by adding 100 g of each in 1000 ml water and fermenting them for 2 weeks in earthenware pot wrapped with a polythene bag. The extracts were filtrated through cheese cloth and added separately to the unautoclaved PDA medium at concentrations of 1.0 and 2.0%. The media were autoclaved at 121 °C for 15 min and then poured (approximately 15 ml) into 9-cm-diameter petri plates. After solidification, each plate was inoculated with a 5-mm mycelial disk of a 4-day-old colony of R. solani. The inhibition of radial growth was computed using the same formula of Sundar et al. [15].
2.8 Individual and integrated effect of T. harzianum T-3, mustard oilcake and Provax-200 on seedling mortality and improvement of yield of pea in the experimental field
A field experiment under artificially inoculated conditions with the most virulent R. solani isolate on pea plant was conducted to determine the individual and integrated effect of the most effective T. harzianum isolate, an organic amendment and a fungicide on seedling mortality in pea. To do this, we selected T. harzianum isolate T-3, mustard oilcake and Provax-200 from our preliminary screening studies and estimated their effect on seedling mortality caused by R. solani RS10 and yield improvement in pea from November 2009 to March 2010 in the experimental field of the BSMRAU. We grew pea plants for this purpose in a non-sterile field soil. This experiment included following eight treatments:
- • T1 = sowing of pea seeds in field soil (control 1);
- • T2 = inoculum of R. solani RS10 + sowing of pea seeds in field soil (control 2);
- • T3 = inoculum of R. solani RS10 + sowing of Provax-200-treated pea seeds in field soil;
- • T4 = inoculum of R. solani RS10 + inoculum of T. harzianum T-3 + sowing of Provax-200-treated pea seeds in field soil;
- • T5 = inoculum of R. solani RS10 + inoculum of T. harzianum T-3 + sowing of pea seeds in field soil;
- • T6 = inoculum of R. solani RS10 + inoculum of T. harzianum T-3 + mustard oilcake + sowing of pea seeds in field soil;
- • T7 = inoculum of R. solani RS10 + mustard oilcake + sowing of pea seeds in field soil;
- • T8 = inoculum of R. solani RS10 + inoculum of T. harzianum T-3 + mustard oilcake + sowing of Provax-200 treated-seeds in field soil.
Preliminary experiments were carried out to determine the range of fungicide concentrations, by exposing R. solani to different concentrations until observing a mycelial growth inhibition lower than 100%. In vitro studies showed an absolute inhibition of mycelial growth of R. solani at doses of 250 and 500 ppm of Provax-200. However, the fungicide concentration for the seed treatment used was 200 ppm. The seed treatment with Provax-200 was done by thoroughly mixing 100 g of seed, 0.02 g of fungicide (200 ppm) and a small amount of distilled water in a conical flask. Inocula of T. harzianum T-3 were prepared by growing on sterilized wheat grain following the same procedure as described for R. solani. The individual plot size was 1.5 m × 1 m and the plot-to-plot distance was 0.5 m. Each plot was prepared by a good tillage. The inoculum of R. solani isolate RS10 was mixed with soil in relevant microplots at the rate of 94 g/m2 soil and moistened to about 50% water-holding capacity. After 21 days, the WGI of T. harzianum T-3 at the rate of 50 g/m2 was mixed thoroughly in the soil of selected microplots. Mustard oilcake was mixed in the soil of treatment microplots at the rate of 200 g per plot 7 days before sowing. Seeds were sown on the same days. Pre-emergence and post-emergence seedling mortality was estimated at 7 and 30 days after sowing. Seed weight and yield were recorded after harvest.
2.9 Experimental design and statistical analysis
In vitro studies were done using a completely randomized design, each treatment with four replications. The field experiment was conducted in Randomized Complete Block Design with eight treatment combinations replicated four times. Data were analysed statistically using the MSTAT-C computer progam. The means were compared using the Fisher LSD (P = 0.05) test [17].
3 Results
3.1 Isolation, characterization and pathogenicity of R. solani
A number of isolates of R. solani were isolated from different crop fields at BSMRAU, of which four were selected for cultural characterization and pathogenicity tests (Table 1). The vegetative hyphae of R. solani isolates were mostly brown in colour and had dense growth with zonation in culture. Brown is the diagnostic colour for vegetative hyphae of R. solani [18]. Zonation, the phenomenon of alternation of periodic zones, has been shown to vary among isolates [19]. The isolate RS10 has three zonations and others have two zonations. The number of sclerotia formation has also been shown to vary among isolates. The highest number of sclerotia/plate was observed in the R. solani isolate RS10, while the lowest was observed in RS1. The pathogenicity test of the four selected isolates of R. solani, namely RS1, RS3, RS7and RS10, was done in pea plants in the pot experiment. All the isolates of R. solani were highly pathogenic to pea, where RS10 showed the highest (100%) and RS1 showed the least (66.67%) seedling mortality (Table 1). Control pots with sterile soil without inoculum of R. solani showed no mortality of seedlings.
3.2 In vitro evaluation of T. harzianum isolates for their antagonistic effect on the radial growth of R. solani
The antagonistic activity of 20 isolates of T. harzianum was tested against R. solani RS10 on PDA by dual culture assay (Fig. 1). All isolates of T. harzianum were highly aggressive in their antagonistic effect, showing more than 60% inhibition of the radial growth of R. solani compared to the control. The percent inhibitory effect among T. harzianum isolates ranged between 77 to 65%, where the highest inhibition was recorded for isolate T-3 and the lowest was for the isolate T-10. Dual petri plate marriage of Trichoderma with pathogen R. solani endorsed the mycoparasitic antagonism of T. harzianum isolates.
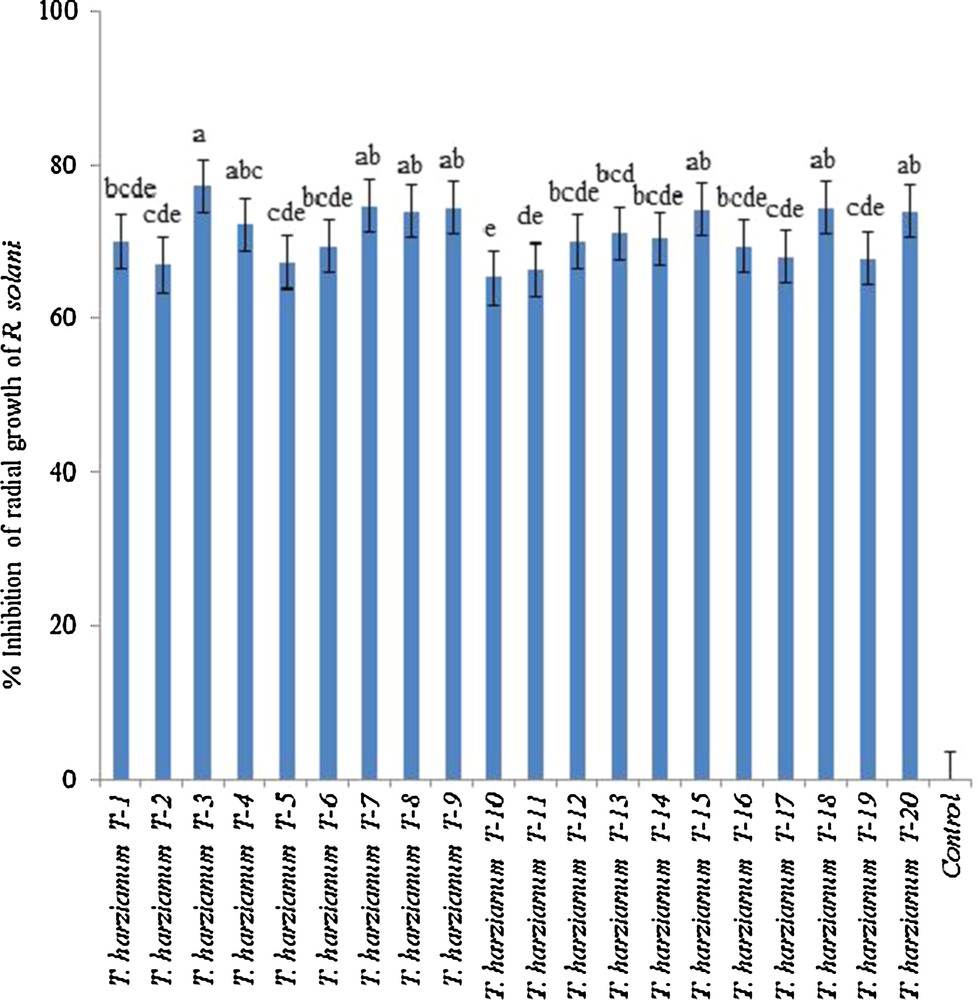
In vitro inhibition of radial growth of Rhizoctonia solani by isolates of Trichoderma harzianum. Inhibition percentages of R. solani were calculated by computing the radial growth of the fungus in the presence and absence of antagonistic isolates of T. harzianum in dual culture on PDA. Error bars are Standard Error (SE) from four replicates. Different letters on the bars indicate significant differences between Trichoderma isolates (Fisher's LSD test, P = 0.05). The data presented are from representative experiments that were repeated at least twice, with similar results. Masquer
In vitro inhibition of radial growth of Rhizoctonia solani by isolates of Trichoderma harzianum. Inhibition percentages of R. solani were calculated by computing the radial growth of the fungus in the presence and absence of antagonistic ... Lire la suite
3.3 In vitro evaluation of selected fungicides for their fungitoxicity on the radial growth of R. solani
Bavistin 50 WP was the most effective fungicide, completely inhibiting the radial growth of R. solani, even at the lowest concentration (100 ppm) (Fig. 1). Provax-200 was identical to Bavistin 50 WP in inhibiting R. solani at higher doses such as 250 ppm and 500 ppm. Media amended with 100 ppm of Provax-200 caused only 83.61% inhibition of the mycelial growth of R. solani. Ridomil was found to be significantly inferior in inhibition of the radial growth of R. solani among the tested fungicides. At 500 ppm concentration, Ridomil caused only 38.74% inhibition of the mycelial growth of R. solani.
3.4 In vitro evaluation of organic amendments for their fungitoxicity on the radial growth of R. solani
In vitro evaluation of organic amendments revealed that mustard oilcake, sesame oilcake and tea waste significantly inhibited the colony growth of R. solani compared to control plate (Table 2). Mustard oilcake showed more hyphal inhibition (60.28%) of R. solani than sesame oilcake (49.17%) and tea waste (43.89%) at the rate of 2% concentration. Wheat meal, rice bran and maize meal at the rate of 1% concentration showed no effect on radial growth of R. solani, while at 2% concentration showed a very insignificant inhibition of the fungus.
In vitro evaluation of fungicides on inhibition of the radial growth of R. solani.
| Treatment | Concentration (ppm) | % Inhibition of radial growth of R. solani |
| Provax-200 | 100 | 83.61b |
| 250 | 100.00a | |
| 500 | 100.00a | |
| Bavistin | 100 | 100.00a |
| 250 | 100.00a | |
| 500 | 100.00a | |
| Ridomil | 100 | 12.50e |
| 250 | 17.78d | |
| 500 | 39.17c | |
| Control | 0.00 | |
| LSD | 1.078a |
a Mean values within a common letter do not differ significantly (P = 0.05).
3.5 Individual and integrated effect of T. harzianum T-3, mustard oilcake and Provax-200 on seedling mortality and improvement of yield of pea
The individual and integrated effects of T. harzianum T-3, mustard oilcake and Provax-200 were evaluated in the experimental field in controlling seedling mortality and increasing seed yield in pea. The treatments were tested in non-sterilized field soils. When the soil samples were collected from these plots, the density of R. solani surviving in the soil was moderate, i.e. R. solani was present in 55 out of 96 soil samples determined using the buckwheat stem colonization method [20] and approximately 56% of isolates were pathogenic to chick pea. Therefore, the soil-borne inoculum of the natural population of R. solani contributes to some extent (7.57%) to the seedling mortality in pea plants (T1) (Table 3). However, the highest percent seedling mortality was recorded in plots where field soil solely received the WGI of R. solani (T2, pathogen-inoculated control). Treatment with T. harzianum, mustard oilcake and Provax-200 had substantial positive effect on the reduction of percent pre- and post-emergence seedling mortality in pea over controls (Table 4). Consequently, the lowest total percent seedling mortality (4.01%) was recorded in plants receiving a combined treatment with T. harzianum T-3, mustard oilcake and Provax-200 (T8). The next lowest percent disease index was observed in plants where mustard oilcake was applied solely (T7) or in combination with inocula of T. harzianum T-3 (T6) to the field soil. Similarly, a significant variation among the treatments was observed for 1000 seed weight and seed yield. The highest yield was observed in T8, where integrated treatments with T. harzianum T-3, mustard oilcake and Provax-200 were incorporated; the lowest was in control plots (control 2; T2), where untreated pea seeds were sown in field soil inoculated with R. solani isolate RS10. Other treatments (T3, T4, T5, T6 and T7) showed a statistically similar effect on 100 seed weight and seed yield.
In vitro evaluation of organic amendments on inhibition of radial growth of R. solani.
| Treatment | Concentration (%) | % Inhibition of radial growth of R. solani |
| Wheat meal | 1 | 0.00h |
| 2 | 0.87h | |
| Mustard oilcake | 1 | 30.56d |
| 2 | 60.28a | |
| Tea waste | 1 | 6.39f |
| 2 | 43.89c | |
| Til oil cake | 1 | 28.61e |
| 2 | 49.17b | |
| Rice bran | 1 | 0.00h |
| 2 | 4.44g | |
| Maize meal | 1 | 0.00h |
| 2 | 4.72g | |
| Control | 0.00 | |
| LSD | 0.999a |
a Mean values having a common letter do not differ significantly (P = 0.05).
Effect of individual and integrated use of T. harzianum, mustard oilcake and Provax-200 on seedling mortality and seed yield of pea.
| Treatments | Seedling mortality (%) | 1000 seed wt (g) | Total seed wt (g) | Yield (t/ha) | ||
| Pre-emergence | Post-emergence | Total | ||||
| T1 | 5.79d | 1.78de | 7.57e | 131.3d | 396.3c | 2.64c |
| T2 | 15.62a | 11.16a | 26.78a | 131.0d | 372.5c | 2.48d |
| T3 | 6.24d | 4.46b | 13.38b | 142.0bc | 510.0b | 3.40b |
| T4 | 6.24d | 2.22d | 11.60bc | 140.8c | 490.5b | 3.27b |
| T5 | 5.79d | 2.67cd | 10.70cd | 142.0bc | 505.0b | 3.37b |
| T6 | 8.03c | 3.75bc | 8.47de | 141.3bc | 516.3b | 3.44b |
| T7 | 9.81b | 3.56bc | 8.47de | 142.3bc | 523.5b | 3.49b |
| T8 | 3.12e | 0.89e | 4.01f | 147.0a | 597.0a | 3.98a |
| LSD value | 1.647a | 1.147a | 2.311a | 1.679a | 45.07a | 0.301a |
a Means in a column followed by the common letter(s) does not differ significantly (P = 0.05).
4 Discussion
Seedling mortality caused by R. solani is primarily recognized as one of the serious menaces in pea cultivation in Bangladesh. Experiment was conducted in laboratory and field to find out the comparative performance of few microbial antagonists, organic amendments and fungicides and their integration for management of seedling mortality in pea.
As a warmth-dependent pathogen, R. solani is common in Bangladesh soil. In recent years, there has been a considerable expansion in the types of characters available for characterizing R. solani. These include simple scores based on morphological and pathological behaviours. Appreciable diversity among the tested R. solani isolates was particularly found in colony appearance and pattern of sclerotia formation [21]. Frequently, the results of in vitro characterization are reflected functionally by the differences found in pathogenicity. In the current study, sufficient intra-isolate variation in pathogenicity was observed for R. solani. Previously, many workers emphasized the value of combining several morphological and pathological attributes to differentiate between strains of Rhizoctonia [18]. However, Mordue et al. [22] found that these characters were more distinctive among R. solani isolates when they were genetically diverse. Therefore, seedling mortality disease in pea fields in Bangladesh is caused by a mixture of pathomorphologically disparate groups of R. solani. These results are imperative for controlling seedling mortality disease in Bangladesh.
In vitro studies clearly showed that fungal antagonist T. harzianum was able to inhibit the mycelial growth of highly virulent R. solani, although different isolates showed varying levels of antagonism against the pathogen. A similar antagonistic effect of T. harzianum against R. solani infecting many other crops was reported by several workers [7,23]. The species of Trichoderma that are capable of hyperparasitizing pathogenic fungi are known to be highly efficient antagonists [24]. Hyperparasite T. harzianum directly attacks R. solani mycelium, grows on the surface with coiling and penetrates the cell wall in the culture [5,6,25]. A collapse and total disappearance of the parasitized hyphae are common [5,6]. A variety of extracellular lytic enzymes seems to play an important role in parasitism. High chitinase and β-(1,3)-glucanase activities have been reported to be produced by T. harzianum [26,27] and there may be a relationship between the production of these enzymes and the ability to inhibit the pathogen [26–28].
Many reports showed that organic amendments were potential biological control agents against R. solani [6,29]. An organic amendment in the form of mustard oilcake was significantly superior in inhibiting the in vitro growth of R. solani to all other amendments. Similar results were found in the previous studies with mustard oilcake for the inhibition of R. solani and of other pathogens [30–33]. The superior inhibitory effect of mustard oilcake is suggested to be associated with the release of antifungal compounds from it. Brassica tissues contain significant quantities of the thioglucoside compounds, which are hydrolysed to release various forms of isothiocyanates [34]. The biocidal effects of isothiocyanates have long been known [35]. The species of Rhizoctonia were generally the most sensitive to isothiocyanates [30]. This indicates that incorporating mustard oilcake to the soil at the time of sowing could be useful for granting protection to the seedlings from soil-borne diseases by R. solani.
Fungicides have continued to be an integral part of preventive control of Rhizoctonia diseases on many crops. In vitro experiments were designed to evaluate the toxicity and the effect of sub-lethal doses of three known fungicides toward R. solani. Bavistin 50 WP and Provax-200 were found the most effective. This is in agreement with results reported by other authors [36–38]. Carbendazim and carboxin are well-known classes of systemic fungicides with strong fungitoxic activity against pathogens. The subsequent application of these fungicides should protect the plant from the attack of pathogens, but also should support the growth of introduced antagonists when they are applied with microbial agents. The idea of combining biological control agents with fungicides is necessary for the development or establishment of the desired microbes in the rhizosphere. But when the chemical control is combined with biological control agents, it frequently inhibits or kills the bio-control agents, which has been a very serious problem for a long time. Therefore, the success of integrated technology depends on the compatibility between the two control agents. Compatibility studies on fungicides with Trichoderma revealed that Bavistin 50 WP checked the growth of T. harzianum completely at all tested concentrations, while Provax-200 allowed the normal growth of fungus even at 500 ppm (data was not shown). Malathi et al. [39] reported that Trichoderma (six strains) could not grow even at 1 ppm of carbendazim. This supports the earlier findings that Trichoderma is incompatible with carbendazim fungicide [38,40,41]. On the contrary, T. harzianum is not only able to tolerate full dose of Provax-200, but its efficacy as an antagonist has also been enhanced in combination with carboxin [42]. Carboxin fungicides have high specificity to only a few numbers of fungi, but limited activity towards others [43]. Therefore, Provax-200 was appeared to be more appropriate than Bavistin 50 WP in the application schedule of integrated disease management strategy with T. harzianum.
Under field conditions, an integrated management strategy that combined the use of T. harzianum, mustard oilcake and fungicide Provax-200 was found to be significantly more superior in reducing seedling mortality incidence and improving grain yield in pea when compared to dual and individual application of them. In the management of soil-borne diseases of several crops, an integrated approach involving microbial antagonist, oilcake and fungicide was found highly effective and resulted in enhanced crop yield when compared with biological (or) chemical treatment alone or in combination [38,44,45]. In this integration, a sub-lethal dose of fungicide weakens the pathogens, an antagonist parasitizes them, and oilcake improves soil nutrients status and properties and enhances the efficacy of both fungicide and antagonist [46,47]. Different mechanisms have been suggested as being responsible for the action of individual bio-agents and fungicides. The bio-control activity of T. harzianum against R. solani includes competition for space and nutrients, secretion of chitinolytic enzymes, mycoparasitism, production of inhibitory compounds, enhancement of plant growth and modulation of induced resistance [27,48–50]. Organic amendment such as mustard oilcake produces volatile and nonvolatile substances during their decomposition and stimulates resident and introduced antagonist-suppressing pathogenic fungi in the soil [51,52]. As regards the mode of action of Carboxin fungicide, it has been known as a strong inhibitor of succinic dehydrogenase enzyme in mitochondria and causing solute leakage (e.g., potassium ions, organic acids, and phosphate-containing materials) from hyphae of R. solani [53,54].
In the current investigation, it is clearly established that the integration of chemical and biological treatments has high potential for success, especially for fungicides with microbial antagonist and oilcake, in controlling seedling mortality (R. solani) in pea. The farmers are therefore advised to use a combination of methods in order to effectively control the disease and improve crop performance. Besides, results on the deleterious effects of fungicides to fungal antagonists indicate that the selection of apposite fungicides that do not affect the native beneficial microflora is required. Otherwise it may need the development of tolerant biotypes of the biological control agents to be utilized in the integrated approach.
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.
Acknowledgments
The authors greatly acknowledge the financial grant from Ministry of Science and Technology, Bangladesh for accomplishing this research project.


