1 Introduction
Food security has become an extremely important global issue, and spike in prices of important crops such as rice and wheat has occurred in recent years. Rice (Oryza sativa L.) is the second widely cultivated crop in the world. It belongs to the genus Oryza, family Gramineae (Poaceae) and tribe Oryzeae. It also comprised two subspecies, Indica and Japonica [1]. The production of rice should be increased up to 40% by 2030 due to the growing global population. Hence, the production of rice varieties with higher potency and stable yield is indispensable to overcome the grain yield reduction and arable land limitation [2]. Plant tissue culture technique covers a wide scope of development, maintenance and usage of genetic variability to improve field, vegetable, crops and aromatic/medicinal plants. In several crops, in order to develop transgenic varieties, tissue culture-based genetic transformation has been commercially utilized [3]. However, the lack of an efficient tissue culture method to regenerate the plantlets remains a main barrier for genetic modification of a wide range of plant species. Tissue culture has been verifying some potential, such as producing virus-free plants, improving propagation and developing genetic diversity in the short term [4,5]. One of the most promising goals for plant genetic modification is cultivar improvement, which could be achieved through plant regeneration using somatic embryogenesis [6,7]. The availability of an efficient in vitro regeneration protocol is an essential requisite for doing well transformation and regeneration processes [8]. Furthermore, genetic modification of rice to improve main characteristics needs to have a reliable and well-organized in vitro culture method [9]. The in vitro culture protocol offers material sources that could serve as recipients of introduced exogenous DNA [10]. It also establishes plant propagation and would recognize the genotype that could be used as a recipient of foreign DNA in the following transformation step. Somatic embryogenesis is the common regeneration process in rice. Somatic embryos could be obtained from diverse plant parts, including mature and immature embryos, root, microspores, leaf bases of young seedling, protoplast and young inflorescences and cell suspensions [11–13]. Moreover, the auxin/cytokinin ratio of media resulted in the development of somatic embryos, shoot and root production [14]. We provide here a different regeneration protocol based on initiation of embryogenic calli from mature embryos of Indica rice varieties. The objective of this study was to develop a simple yet effective as well as less genotype-dependent plant regeneration method using embryogenesis that would be of the greatest utility in genetic improvement of Indica rice varieties.
2 Material and methods
2.1 Plant materials
Mature dry seeds of six elite Malaysian Indica rice varieties (MRQ50, MR269, MR276, MR219, MRQ74 and MR84) were used. All seeds were supplied by the Malaysia Agriculture Research Development Institute (MARDI). In addition, the experiment was designed based on Randomized complete block design (RCBD).
2.2 Sterilization procedure
Hull from all mature seeds containing scutellar region of embryo was removed. Dehulled seeds were sterilized with 70% alcohol for 1 min, followed by shaking in a 40% sodium hypochlorite solution containing three drops of polyoxyethylene sorbitan monooleate in an orbital shaker, at 120 rpm for 20 min. Finally, the explants (seeds) were rinsed with sterile distilled water five times.
2.3 Establishment of embryogenic calli from mature embryos
Five sterile seeds were placed in each Petri dish (100 mm × 15 mm) containing 20 mL of MS media supplemented with 2,4-D (MS salts and vitamins, 300 mg/L casamino acid, 2 mg/L 2,4-D, and 2.8 g/L gelrite, pH 5.8). Each four Petri dishes were measured as a replicate and totally 12 Petri dishes, three replicates, were analyzed for every one variety. Then, the cultured seed were incubated in the dark at 25 °C. After three weeks, yellowish white embryogenic calli had developed on the scutellar surface.
2.4 Proliferation of calli
The produced calli were transmitted to the MS media supplemented with 2, 4-D containing MS salts and vitamins, 300 mg/L casamino acid, 2 mg/L 2,4-D, and 2.8 g/L gelrite, pH 5.8 for proliferation of the calli. The calli were kept in the dark at 25 °C for 10 days.
2.5 Shoot production from embryogenic calli
The healthy surviving MR219 embryogenic calli were transferred to the MSKN regeneration medium (MS salts, MS vitamins, 2 mg/L kinetin, 1 mg/L NAA, 300 mg/L casamino acid, 30 g/L sucrose, 0.1 g/L myo-inositol, 2.8 g/L gelrite, pH 5.8). The cultures were kept in the dark for 20 days. The emerging shoots were harvested and then transferred to the fresh regeneration media (MSKN). The cultures were placed in the light at 27 °C with a 16-h photo period (110 μmol/m2/s) for 20 days.
2.6 Root production
Finally, the healthy shoots were transferred into the rooting medium (MSO) containing MS salts, MS vitamins, 300 mg/L casamino acid, 30 g/L sucrose, 0.1 g/L myo-inositol, 2.8 g/L gelrite, pH 5.8. The cultures were kept exposed to light at 27 °C with a 16-h photo period (110 μmol/m2/s) for 14 days.
2.7 Transfer of regenerated plants to the soil
After 14 days, when a well-developed root system was observed, the culture medium was removed gently from the roots of plantlets using water. After that, the plantlets were transferred individually into the Yoshida culture solution in a greenhouse with a 14-h photo period (160 μmol/m2/s), 95% relative humidity, and 29 °C day/light temperature and kept for 21 days. Subsequently, the plants with vigorous root systems were transferred into the pots containing paddy soil, water (1 L per pot and 500 mL each day until maturity, and sufficient initial fertilizer – a mixture of fertilizers, 2.5 g of (NH4) SO2, 1.25 g of P2O5, 0.75 g of K2O per pot). About 2.5 g of (NH4) SO2 were added into each pot at the initial stage of flowering. Panicles were harvested when 85% of the color of the grains turned straw gold.
2.8 Characteristics evaluated during the experiment
Various important traits were measured during the experiment to explore the responses of the different varieties to the regeneration procedure. The considered traits included callus induction day, number of contaminated seeds, number of dead seeds, percentage of dead seeds, number and percentage of callus induction frequency, number of explants producing embryogenic callus, EF (Embryogenic Frequency), browning rate (%), plant regeneration frequency, fresh weight and dry weight of four- to nine-week-old calli.
2.9 Statistical analysis
The collected data were statistically analyzed by SAS version 9.3. The level of significance was evaluated from analysis of variance (ANOVA). The mean of different traits among varieties were investigated using Duncan's multiple range.
3 Results
Experiments from seed sterilization to plant regeneration in rice (Oryza sativa L.) were conducted in six varieties, MRQ50, MR269, MR276, MR219, MRQ74 and MR84.
3.1 The effects of seed sterilization procedure on different varieties
There were no significant differences among varieties in term of number of contaminated seeds. The lowest number of contaminated seed (3.33) was observed in MR269 and MR276 varieties. While, the highest number of contaminated seed (4.66) was recorded for MR219 and MRQ50 (Tables 1–4).
ANOVA of some traits measured among six Malaysian rice varieties.
| Source of variation | DF | NCS | CID | CIF | NCI | NDS | NEPEC |
| Block | 2 | 1.20ns | 0.50ns | 4.16ns | 0.16ns | 0.16ns | 0.50ns |
| Treat | 5 | 0.667ns | 51.06** | 149.16** | 5.96** | 3.03** | 5.83* |
| Error | 10 | 0.867 | 3.16 | 25.83 | 1.03 | 0.50 | 1.63 |
| Total | 17 | – | – | – | – | – | – |
| CV | – | 23.273 | 12.133 | 8.590 | 8.590 | 16.970 | 21.908 |
ANOVA of some traits measured among six Malaysian rice varieties.
| Source Of Variation | DF | PDS | EF | BR | PRF |
| Block | 2 | 4.16ns | 12.50ns | 2.38ns | 2.38ns |
| Treat | 5 | 75.83** | 145.83* | 681.25** | 681.25** |
| Error | 10 | 12.50 | 408.33 | 5.65 | 5.65 |
| Total | 17 | – | – | – | – |
| CV | – | 16.970 | 21.908 | 3.758 | 6.476 |
Mean comparison of different traits in rice varieties.
| Varieties | NCS | CID | CIF | NCI | NDS | NEPEC |
| MRQ50 | 4.66a | 15.33b | 53.33c | 10.66c | 4.66ab | 6.66ab |
| MR269 | 3.33a | 15b | 70a | 14a | 2.66c | 6.66ab |
| MR276 | 3.33a | 14.66b | 65ab | 13ab | 3.66bc | 4.66bc |
| MR219 | 4.66a | 7c | 58.33bc | 11.66bc | 3.66bc | 7.66a |
| MRQ74 | 4.33a | 16.66a | 51.66c | 10.33c | 5.33a | 4c |
| MR84 | 3.66a | 19.33a | 56.66bc | 11.33bc | 5ab | 5.33abc |
| CV(%) | 23.273 | 12.133 | 8.590 | 8.590 | 16.970 | 21.908 |
Mean comparison of PDS, EF, BR and PRF among different rice varieties.
| Varieties | PDS | EF | BR | PRF |
| MRQ50 | 23.33ab | 33.33ab | 46e | 54a |
| MR269 | 13.33c | 33.33ab | 53.33d | 46.66b |
| MR276 | 18.33bc | 23.33bc | 56d | 44b |
| MR219 | 18.33bc | 38.33a | 63c | 37c |
| MRQ74 | 26.66a | 20c | 74.33b | 25.66d |
| MR84 | 25ab | 26.66abc | 87a | 13e |
| CV (%) | 16.970 | 21.908 | 3.758 | 6.476 |
3.2 The responses of different rice varieties to the callus induction media
Calli were variably developed from the scutellar region of seeds and were visible within 6–20 days. The varieties were significantly differentiated in terms of callus-induction day (51.06**), callus-induction frequency (149.16**), number of calli induced (5.96**), number of dead seeds (3.03**) and percentage of dead seeds (75.83**) (Tables 1 and 2). The comparison of the varieties as regards the callus-induction day showed that the minimum number of days needed for callus induction corresponds to MR219 and the maximum one to MR84. The highest and lowest callus induction frequency (70% and 51.66%) and number of induced calli from seeds (14 and 10.33) were observed in MR269 and MRQ74, respectively. After the callus induction step, the number (5.33 and 2.66) and the percentage of seeds dead (26.66 and 13.33) were counted and showed that the highest number of dead seeds corresponded to MRQ74 and the lowest one to MR269, respectively (Tables 3 and 4 and Fig. 1).
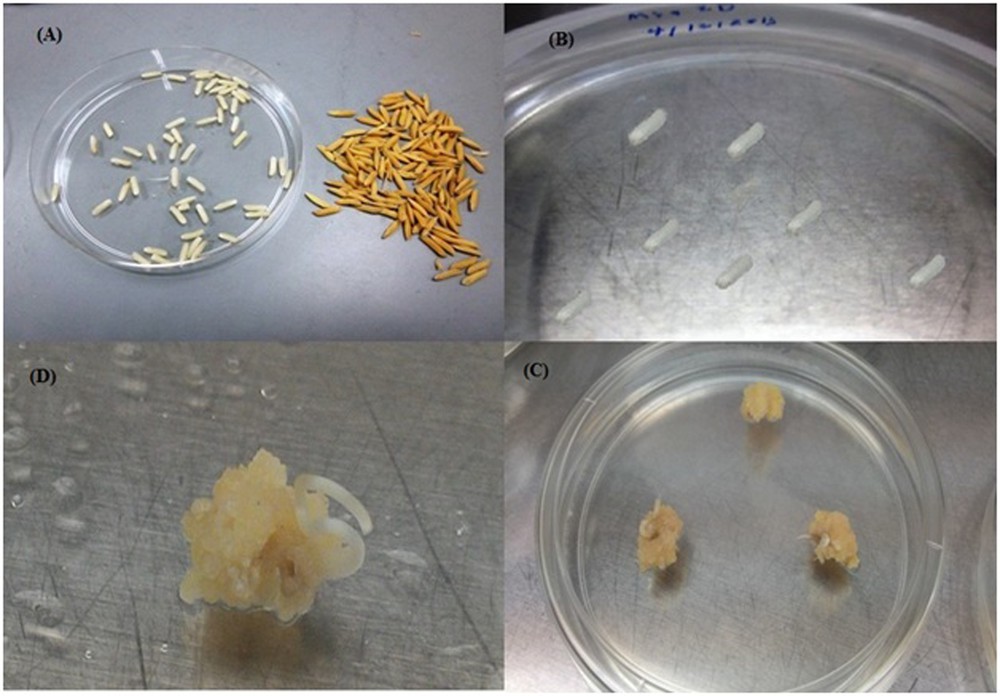
(Color online.) Calli induced from mature embryo. A. Dehusked seeds. B. Cultured seeds on the callus induction media. C and D. Calli induced from mature embryos of MR219.
3.3 The effects of proliferation media on development of embryogenic calli among different varieties
The rice varieties used in this study were significantly differentiated based on the number of explants producing embryogenic calli (5.83*), embryogenic frequency (145.83*) in 5% of probability level and browning rate of calli (681.25**) at the 1% of probability level (Tables 1–4). The maximum and minimum numbers (7.66 and 4) and frequencies of embryogenic calli (38.33% and 20%) were recorded in MR219 and MRQ74, respectively. However, the highest browning rate was observed in MR84 (87%) and the lowest rate in MRQ50 (46%) (Tables 3 and 4). The maximal fresh and dry weight of calli during four up to nine weeks of culture was observed in the sixth and seventh weeks in all varieties (Figs. 2 and 3).
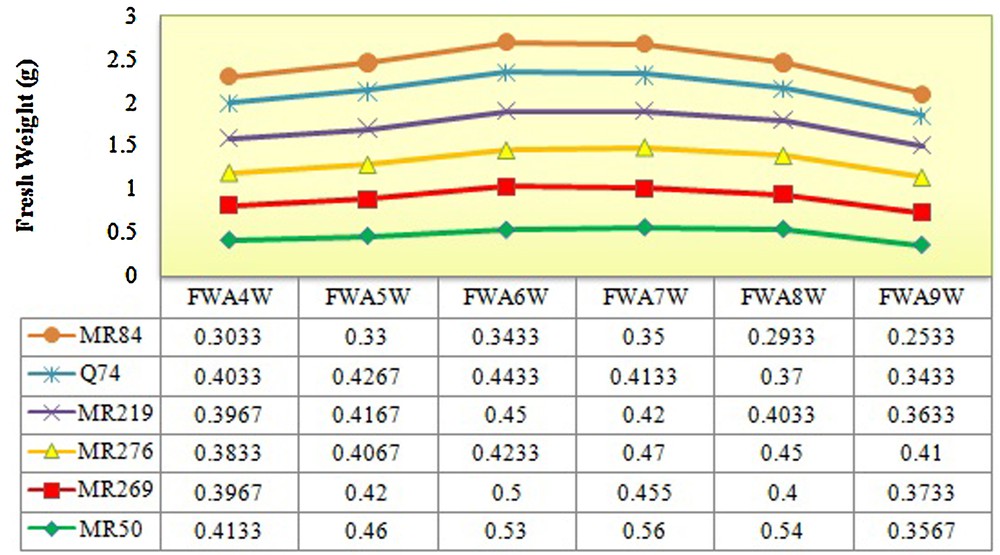
(Color online.) Determination of the fresh weight of calli from the fourth to the ninth week for all varieties. FWA4W: fresh weight after four weeks; FWA5W: fresh weight after five weeks; FWA6W: fresh weight after six weeks; FWA7W: fresh weight after seven weeks; FWA8W: fresh weight after eight weeks; FWA9W: fresh weight after nine weeks.
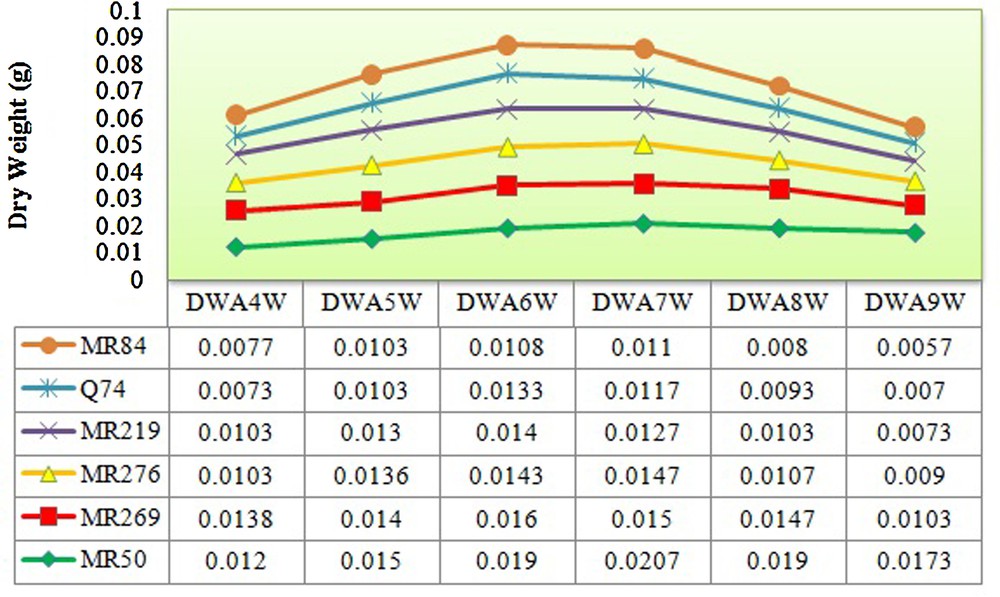
(Color online.) Measured dry weight of calli from forth till ninth weeks for all varieties. DWA4W: dry weight after four weeks; DWA5W: dry weight after five weeks; DWA6W: dry weight after six weeks; DWA7W: dry weight after seven weeks; DWA8W: dry weight after eight weeks; DWA9W: dry weight after nine weeks.
3.4 Comparison of plant regeneration responses to MSKN among different varieties
The variance of rice varieties was significantly variable based on the plant regeneration frequency (681.25**) at the 1% level of probability. The majority of plants regenerated from embryogenic calli were obtained from MRQ50 (54%) and the minimum number of plants from MR84 (Fig. 4).
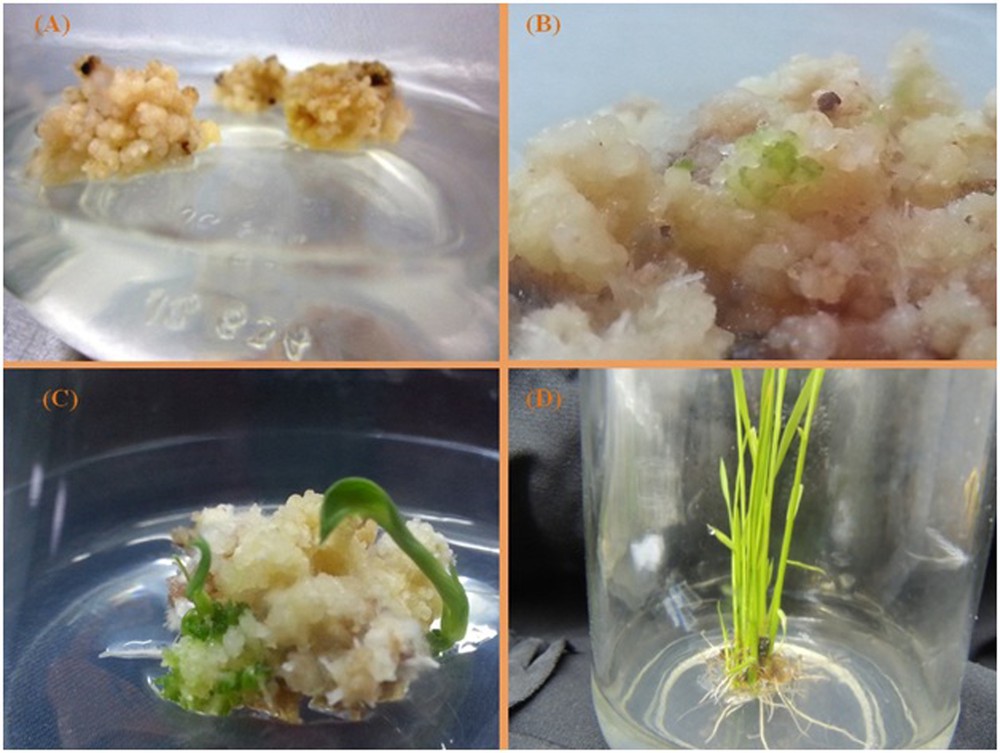
(Color online.) Plant regeneration stages from proliferation calli to regeneration plantlets. A. Proliferation calli. B. Green spots appearing on the callus. C. Shoot production and plant regeneration. D. Shoot, root production and whole plant regenerated.
3.5 Correlation analysis of evaluated traits among rice varieties
Assessment of simple correlation coefficients of traits among six rice varieties illustrated that obtaining embryogenic calli was affected positively by the callus induction day (r = 0.596**). In addition, the frequency of callus induced from the seeds was affected negatively by the number of dead seeds (r = –0.781**). However, the plant regeneration frequency was significantly negatively correlated to the browning rate (r = –1**). The negative but non-significant correlations were founded among different traits, including callus induction day (r = –0.370), number of callus induction (r = –0.262) and embryogenic frequency (r = –0.366) with browning rate and number of dead seeds with embryogenic frequency (r = –0.405) (see Table 5).
Simple correlation coefficients of traits evaluated among six rice varieties.
| Traits | CID | CIF | NCI | NDS | NEPEC | PDS | EF | BR | PRF |
| CID | 1 | ||||||||
| CIF | 0.056 | 1 | |||||||
| NCI | 0.056 | 1** | 1 | ||||||
| NDS | –0.416 | –0.781** | –0.781** | 1 | |||||
| NEPEC | 0.596** | 0.035 | 0.035 | –0.405 | 1 | ||||
| PDS | –0.416 | –0.781** | 1** | 1** | –0.405 | 1 | |||
| EF | 0.596** | 0.035 | 0.035 | –0.405 | 1** | –0.405 | 1 | ||
| BR | –0.370 | –0.262 | –0.262 | 0.458 | –0.366 | 0.458 | –0.366 | 1 | |
| PRF | 0.370 | 0.262 | 0.262 | –0.458 | 0.366 | –0.458 | 0.366 | –1** | 1 |
3.6 Cluster analysis of varieties based on the measured traits
The six Malaysian rice varieties were clustered to two categories based on the measured characters and responses to specific media. Cluster I was composed of the varieties that weakly responded to the media in different stages (MRQ74 and MR84), while cluster II was comprised of four varieties that satisfactorily reacted to the media (MRQ50, MR219, MR269 and MR276) (Fig. 5).
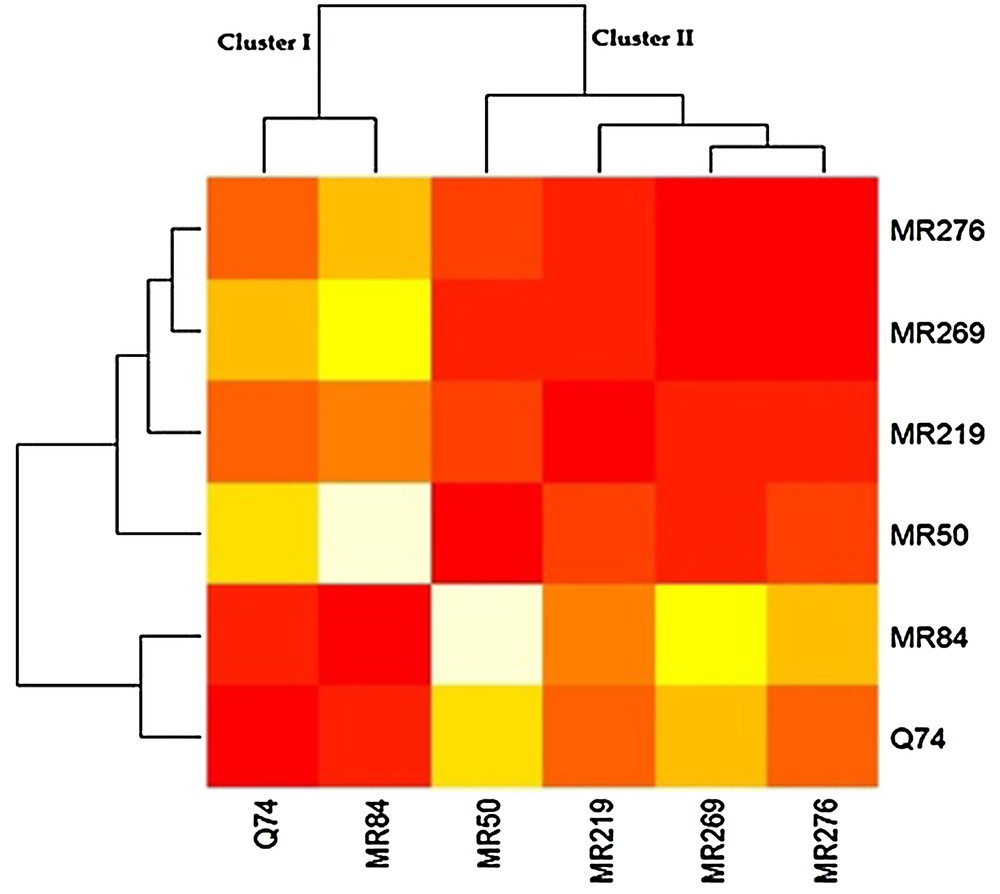
(Color online.) The six rice varieties clustered based on the measured traits.
4 Discussion
A crucial stage in the beginning of plants tissue culture is the obtaining of cultures free from microbial and fungal contaminations [15,16]. In the present study, the suitability of the sterilization method was tested in six Indica rice varieties (MRQ50, MR219, MR269 and MR276, MRQ74, and MR84). The results showed the low level of seed contaminated in all varieties after culture on the media. The usage of a two-step sterilization treatment has been proven beneficial to particular species (firstly in 70% ethanol and then 40% sodium hypochlorite). One common sterilizing agent that has been used to remove contamination causes in many plant types is sodium hypochlorite [17,18]. In rice, this capability was achieved when 40% sodium hypochlorite were applied for 40 min on the dehusked seeds [19]. Polyoxyethylene sorbitan monooleate, as a wetting agent, used to reduce surface area tension and let better surface contact in the disinfectants. In rice varieties, the lower callus induction and differentiation from mature embryos are the restricting factors of rice improvement through genetic modification [20]. The callus induction as well as embryonic structure formation could be regulated by hormones in plant tissue culture [21–23]. Skoog and Miller found in 1957 that plant morphogenesis and growth could be controlled by auxin and also cytokinin, and made the first hypothesis of hormone balance [24]. The endogenous hormones control the expression of gene in the plant cell and tissue, affect metabolic process, and lastly establish the induction, maintenance and expression of the embryonic potential of plant cells [25]. Various tissue culture abilities in a variety of rice cultivars were mostly due to different genotypes [12,26]. The present study demonstrated that the dissimilar genotype had diverse sensitivity to the callus induction media (MS supplemented with 2,4-D). In addition, maximum and minimum callus induction frequency and number of induced calli from seeds were observed in MR269 and MRQ74, respectively. The study on the rice regeneration procedure independent from the genotype could be an important feature to improve breeding performance in rice genetic modification. In recent times, a number of reports regarding to the regeneration process of various rice subspecies have been made [12,27,28]. Nevertheless, these reports mostly analyzed the effects of diverse concentration of 2,4-D in several rice subspecies. Most of these researches pointed that tissue culture of Indica subspecies was more complicated than that of Japonica rice. Because of the induction of calli merely on the media containing 2,4-D, most of them are non-embryogenic, the rate of plant regeneration remains lower; also, no complete cycle has been documented. The tissue culture and percentage of regenerated plantlets from mature embryos in Indica and Japonica rice subspecies have been studied by Wang et al. [27]. They observed that the percentages of regenerated plants were 17.1–63.3% in Japonica and only 0–24.2% in Indica rice cultivars. Yan et al. [20] reported different rates of regeneration plants from mature embryo for Japonica (9.2–59.5%), Indica (3.6–87.5%) and hybrid rice (17.2–43.2%). In this paper, the regeneration rate for Indica rice varieties was differenced from 13 to 54%. The regeneration procedure of plantlets in order to have different varieties is noted in this paper. As compared to the earlier works, not only the yield of callus induction and proliferation but also the regeneration rate was improved in the present study, which will be useful for improving rice varieties in development programs.
5 Conclusion
Regeneration of plantlets from calli is considered as a main barrier in genetic modification of Indica subspecies of rice. Numerous laboratories around the world are making effort to solve this problem. The higher percentage of regenerated plantlets from different varieties will be achieved, when the best optimized tissue culture protocol is used. In this study, the maximum numbers of plantlets were regenerated from the varieties with the highest rate of embryogenic calli. And also, various varieties, including MRQ50, MR269, MR276 and MR219, were responding satisfactorily, while MRQ74 and MR84 weakly responded to the procedure.
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.
Acknowledgements
The authors wish to acknowledge the Long-Term Research Grant Scheme (LRGS), Food Security project (Grant No. 5525001), Ministry of Higher Education, Malaysia, for the financial support.



Vous devez vous connecter pour continuer.
S'authentifier