1 Introduction
Most insects have a sexual reproduction, and most Coleoptera with a homogametic female genome and a heterogametic male genome. Parthenogenetic reproduction is however not really exceptional: it was assumed to occur in about one in a thousand [1,2]. Two main types of parthenogenesis are recognized. In the first one, called arrhenotoky, both sexes exist. Males have a haploid genome, but their gametes do not contribute to the descendant genome, while females are diploid, and unique genetic contributor. This reproduction mode, frequent in social Hymenoptera, exists also in Thysanoptera and Homoptera. It is exceptional in Coleoptera, in which it is known in Micromalthus debilis LeConte 1878 (Micromalthidae) and in a few species of Scolytidae [3–5]. In the second type, called telytoky, only females exist. It is observed in most orders of insect. Almost one century ago, telytoky was recognized in no less than 16 families of Coleoptera [6]. Since then, it was described in additional families, Ptiniidae, Ciidae, Hydrophilidae and Scarabaeidae [7–10]. Usually, telytoky is associated with various degrees of polyploidy, which may range from 3n to 10n in Curculionidae [11,12].
In the karyotype compilation of Smith and Virkki [13], there are 77 reports of telytoky amongst 1840 species. Only 3 have a diploid karyotype: Cis fuscipes Mellié, 1848 (Ciidae) [7], Polydrosus mollis Boheman, 1840 and Scepticus insularis Roelofs, 1873 (Curculionidae) [14,15]. Since 1978, a few new cases of telytoky have been recorded in particular in Curculionidae. All have a polyploid karyotype [12]. Finally, diploid karyotypes would account for about 3–4% of parthenogens in Coleoptera. All families of Coleoptera are not equally engaged in telytoky. Most cases are known in Curculionidae and Ptinidae, and amongst Curculionidae, almost all involve the sub-family Entiminae. Genetic studies of some species indicate that the origin of telytoky is not monophyletic [16]. In addition, various levels of polyploidy are occasionally observed beside diploidy in the same species. Thus, parthenogenesis recurrently occurs, through either a genetic predisposition, or environmental constraints or both. To the best of our knowledge, the only example of parthenogenesis in the large super-family Scarabaeoidea was reported in Cyclocephala dominicensis Cartwright & Chalumeau, 1976 (Dynastinae) [10]. Following observations of only female specimens in the Passalidae, Passalini, Spasalus crenatus (Mac Leay, 1819), from the West Indies, we developed breedings from isolated immature stages to demonstrate their parthenogenetic reproduction. Chromosome studies of some specimens show that the karyotype of the parthenogenetic Spasalus is composed of 26 chromosomes, as that of most sexual species of the tribe Passalini [13]. This establishes a rare example of diploidy in a parthenogenetic Coleoptera.
2 Preliminary data
S. crenatus is one of the five known species of Passalids in Lesser Antilles [17], and the only one in Puerto Rico [18,19]. The species is easily recognizable by its small size (18–20 mm), its glabrous and slightly convex body, and its pentaphylous antennal clubs.
Initial observations on the absence of males or a distorted sex ratio in S. crenatus were reported from Puerto Rico [19,20]. In the second report, these authors observed a biased sex ratio of 1:2300, whereas it is usually near 1 in Passalidae. To find parthenogenesis, these authors tried to:
- • obtain the reproduction of isolated females;
- • look for infection by Wolbachia, but both approaches were inconclusive.
No study was redone on the subject. However, the same authors with others published on the ecology of the species from Puerto Rico [21], but without reference to sex ratio.
Another observation on S. crenatus from Lesser Antilles was made by one of us, during a study of morpho-anatomy and phylogenetics [22]: all specimens examined from Puerto Rico (20), Virgin Islands (4), Montserrat (4), Guadeloupe (29), Dominica (10), Martinique (17), Saint-Lucia (7) and Saint-Vincent (3) were females. Nine other specimens were examined from old collections, labelled from Cuba or Hispaniola: all were females. On the continent and Trinidad, S. crenatus is a fairly well known bisexual species, widely distributed, with a sex ratio near 1. Otherwise, one of us [23] studied the species in French Guiana and developed breedings in terrariums: two colonies, from two couples, were maintained in good conditions and reproduced for 1.4 years. Careful examination of the external morphology did not allow us to determine the sex without dissection. Consequently, we suspected that the unusual sex ratio previously found [20] was possibly an artefact, as the method for separating sexes was the use of “described characters of the pygidium” (without other precision). Thus, the observation of a single male, amongst females, remained not demonstrated.
3 New materials and methods
For clarifying the situation on islands, it was necessary to increase the number of observations and make new attempts at breeding. In a first assay, one of us (SB, February-Mar. 2005) collected 224 specimens in Martinique, Dominica, Saint-Lucia and Saint-Vincent (most of them in Dominica, where the species seems to be the most common). All imagines, including mature (black) and immature stages (brown), were taken from their rotten logs and dissected: all were females. From these specimens, twelve mature stages were dissected in search of sperm in spermatheca: none had sperm. In a second assay, two of us (BD & AMD, 2006) collected specimens at various localities in Southern Guadeloupe (Basse Terre). A series of ten mature stages were dissected in INRA laboratories of Guadeloupe, with the aim to establish their karyotype: all were females, and only ovarian material was processed. As expected, brown females had immature ovaries. The cells were subjected to our usual cytogenetic techniques [24]. Nineteen mature stages, five immature stages and two larvae were brought back to MNHN-Paris on February 2006. Five more mature stages were dissected: all were females. Their ovaries were dropped into Carnoy's fixative to perform histology. All the others were maintained together until March 2006, after addition of decayed Birch-wood to the small amount of wood of origin (probably a Mango tree). Then, three independent breedings were developed:
- • breeding S: with a single immature stage;
- • breeding I: with 4 immature stages and the two larvae;
- • breeding A: with 8 mature stages.
Decayed Cherry-wood was added (after freezing to eliminate any living organisms), and soon colonized. All breedings were maintained in almost hermetically closed boxes (volume: 500 to 1000 ml), saturated in humidity and off-luminosity. They were occasionally opened to look for beetles, but could remain closed for months. Observations were as gentle as possible. Thus, beetles could remain hidden in the wood, and their counts are likely to be underestimated. In their process, breedings were comparable to those realized in French Guiana with sexual S. crenatus (see above).
All the material used in the study, from the field or breedings, is deposited in the “Muséum national d’histoire naturelle”, Paris.
4 Results
4.1 Breedings
Breedings were started on March 2006, one of them is still going at spring 2015 (Fig. 1).

(Colour online.) Larva, mature (left) and immature (center) stages of S. puncticollis (Le Peletier & Serville), n. stat., from Guadeloupe, in breeding S. Scale: 9 mm.
In breeding S, the unique immature stage remained apparently alone until December 2006. A third-instar larva was detected in May 2007. It was transformed into an immature stage in July 2007, coexisting with a mature stage, presumably its mother. In January 2008, there was one living mature stage and a dead body. In October 2008, a first instar larva was observed, but we did not try to detect the mother in the wood galleries. In September 2013, one mature and two immature stages were observed. In April 2015, about 15 imagines were observed.
In breeding I, the two larvae were transformed into immature stages and two of the immature stages were found as mature dead body in May 2006 (cannibalism very probably occurred). Two 2nd instar larvae were observed on October 17th. They reached the pre-pupa stage on December 14th and were seen as pupa on December 18th, 2006. On January 22th, 2008, another pre-pupa larva was observed. It was transformed into pupa on January 24th, observed as immature stage on February 5th and died the day after. On September 2013, 4 mature stages and one immature stage were observed.
In breeding A, several larvae were observed during 2006 and 2007. Twelve mature stages and 4 immature stages were counted on July 7th, 2007. After separation of 9 mature stages and 3 immature stages, breeding A was pursued as A1. The 3 immature stages were isolated, and used for starting breedings A2, A3 and A5 in new boxes. Finally, the 9 mature stages were put together, constituting breeding A4.
- • breeding A1: it was maintained as such;
- • breeding A2: a pupa and a third instar larva were observed on July 30th, 2008. Two immature stages and one mature stage were seen on August 6th and finally one immature stage on October 22nd, 2008;
- • breeding A3: one larva and one mature stage were seen on January 22nd, 2008, but they died later;
- • breeding A4: the nine mature stages rapidly colonized the wood and became hardly detectable. Five eggs were found in sawdust in January 28th, 2008, and one immature stage in October 2008;
- • breeding A5: the unique occupant, immature stage, was found dead soon after.
Breedings A1 to A5 were stopped in November 2008.
4.2 Chromosome analysis
Ovarian cells from two females provided us with a number of dividing cells, probably not oocytes, sufficient for establishing five karyotypes and counting the chromosomes of 17 metaphases. They had 26 chromosomes, all meta- or sub-metacentric, without evidence of heterochromosomes. By analogy with the chromosomes of other species of Passalini species [25,26], we considered that the Xs formed the largest pair. After C-banding, all the centromeric regions exhibited large heterochromatic blocks (Fig. 2). One autosomal pair carried on its short arms satellite like structures, as in Passalus unicornis Le Peletier & Serville, 1825, an endemic species of the Guadeloupe Archipelago [17], in which it was shown that it was the nucleolus organizer regions (NOR) [26]. This karyotype is thus diploid: 26,XX, and roughly similar to that of females from other species of Passalini with a sexual reproduction.
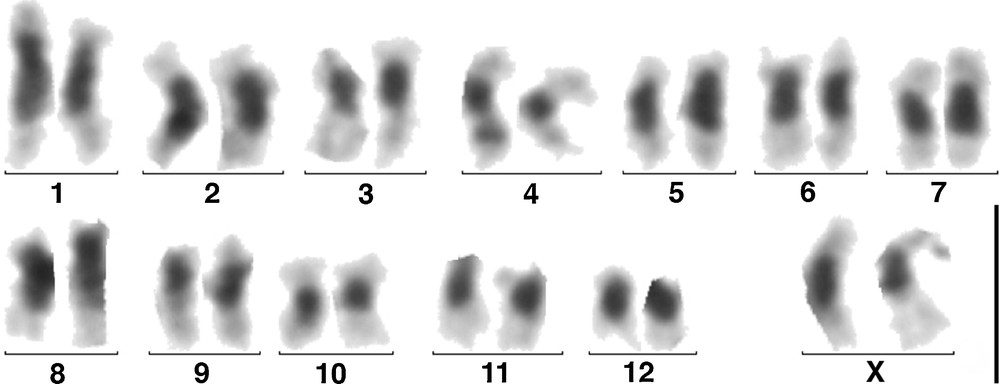
(Colour online.) C-banded karyotype of a parthenogenetic female of S. puncticollis (Le Peletier & Serville), n. stat., from Guadeloupe. Scale: 5 μm.
4.3 Gonad histology
As expected from their external observation by stereomicroscopy, the mature stage gonads were composed of typical ovarian tissue, exhibiting various stages of oogenesis (Fig. 3). Analysis of cross-sections did not allow us to study chromosomes of oocytes, but showed that a single egg per ovariole was developing. This fits with the reduced laying of eggs.
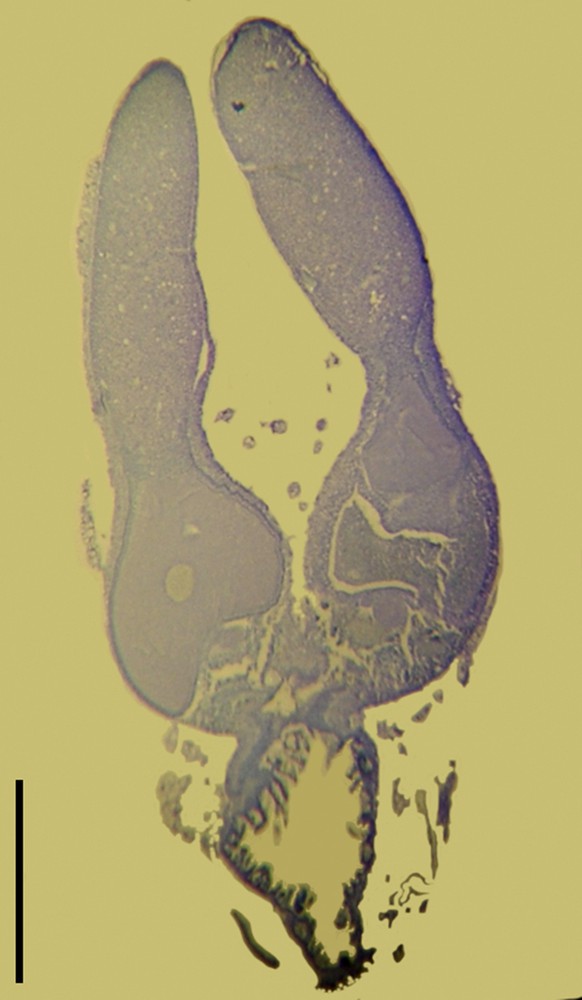
(Colour online.) Ovarian cross-sections of parthenogenetic females of S. puncticollis (Le Peletier & Serville), n. stat., from Guadeloupe. Partial view of immature ovaries exhibiting two eggs at different maturation stages in two adjacent ovarioles. Scale: 0.5 mm.
4.4 Morpho-anatomy of reproductive apparatus
All dissected mature stages from islands have “normal” endo-ectodermic reproductive apparatus, comparable to that of continental populations. Most internal genitalia are constituted by two meroistic, telotrophic ovaries, each one with two ovarioles. The set is connected to the common oviduct. With respect to the ectodermic genitalia, the spermatecal and accessory glands have normal conformation and cuticular structure. The vagina has one medio-dorsal lobe. Glands are connected to the vagina before the medio-dorsal lobe. The pars vaginalis is large and simple, without clear separation with the medio-dorsal lobe. These observations corroborate with those made on many species of Passalidae, including the two American tribes, Passalini and Proculini (details in [27] for most internal and [22] for external genitalia). In conclusion, the parthenogenetic mode of reproduction of Spasalus on islands is not associated with modifications of the basic endo-ectodermic structures, compared to sexual species of the family.
4.5 Systematics, distribution
S. crenatus is currently known as the only Spasalus species inhabiting the West Indies, including Puerto Rico. The species inhabits also the continent, South America East of the Andes. The type locality is “Brasiliâ” and “Demerarâ” (Guyana). There is no doubt that the continental S. crenatus is the species described by Mac Leay [28]. The comparison made between the populations of the continent and those of the islands does not suggest the existence of clearly different morpho-types. The relatively smaller body size (–.4 mm) of specimens from islands is the only character found for separating the populations (measures of 310 specimens from samples of different sizes on islands versus 223 specimens from the Guyanas, Brazil, Venezuela, Ecuador, Bolivia, Peru, Colombia). The standard deviations of the measures are 1.15 on islands and 1.19 on the continent. They are then little scattered and significant between the populations.
Le Peletier and Serville [29] described Passalus puncticollis, from “America”. Luederwaldt [30] proposed the synonymy of the species with S. crenatus, which was followed by most authors, although Paulian [31] conserved S. puncticollis as a valid species, peculiar to islands. Since then, S. puncticollis remained overlooked. We found various old specimens labelled “puncticollis St F. [from Le Peletier de Saint-Fargeau], Guadeloupe”, in the MNHN. Most of them are contemporary to Le Peletier & Serville and sometimes surely studied by these authors.
The known distribution of S. crenatus/puncticollis includes the islands from Puerto Rico to Saint-Vincent. Citations from Greater Antilles (Cuba, Hispaniola) must be confirmed. To the South, the Grenadines and Grenada are also badly known with respect to the species. One of us collected many Passalids there, but found only Passalus and Pertinax, endemic [17]. In collections, no more Spasalus specimens from these islands were found. At difference, we studied Spasalus specimens from Trinidad: they included males and females with normal sex ratio.
Considering that parthenogenesis replaces sexual reproduction in islands north of Trinidad, we consider that an important event, probably one or more genetic mutations affecting the mode of reproduction, occurred during the northward migration of Spasalus. Further studies are necessary to know if this event is unique or not and if the species occurs in the Grenadines and Grenada Archipelago, but it is clear that the gene flaw is interrupted between southern and northern populations. We propose that all parthenogenetic forms from West Indies belong to the puncticollis species while sexual specimens from Trinidad and the continent belong to the crenatus species. This is in line with other data on Passalids, which affirmed that Trinidad fauna belongs totally to the continent [17].
5 Discussion
Passalidae constitutes one of the rare families of Coleoptera with a subsocial behaviour. They leave in decayed trunks in organized colonies, in which all stages of development coexist necessarily. They are known to have a sexual reproduction, but their behaviour may induce a high level of endogamy. Two species, S. puncticollis and P. unicornis, occur in Guadeloupe. They occupy two similar microhabitats, and may even cohabit in the same dead wood. P. unicornis is bisexual, and its karyotype, 26, XX in the female and 25, X in the male [26] is similar or close to that reported for other species of “Passalus”, s. auct. [25]. It is also close to that of S. puncticollis. Thus, the parthenogenetic character of S. puncticollis is clearly not associated with a gross chromosomal change, such as polyploidy, a character observed in about 95% of parthenogenetic Coleoptera (calculated from Smith and Virkki [13]). In nature, both parthenogenesis and polyploidy are rare and their most frequent association suggests a causal relationship. There may be not a single route to parthenogenesis and various hypotheses were proposed about this relationship [32]. In Coleoptera, particularly in Curculionidae, chromosome formulae of parthenotes vary from 2n to 10n [11,12,15], with frequent odd numbers, such as 3n and 5n. In Curculionidae, populations of the same species may be either parthenogenetic or not. Those with a sexual reproduction are always diploid while the karyotypes of the parthenotes are frequently polyploid. Furthermore, amongst polyploid populations, studies of DNA sequence found several genotypes indicating the occurrence of several polyploidization events [16,33]. Polyploidization appears to be the consequence rather than the cause of parthenogenesis. It is probably originated by the rare, but recurrent fecundation of parthenotes by males of the same or closely related species. This would explain the odd numbers, such as 3n, which cannot be generated by endoreduplication, but by the fecundation of a diploid oocyte by a haploid spermatozoon.
In Diptera with polytene chromosomes, heterozygosity for structural rearrangements, such as inversions, is particularly frequent amongst parthenotes [1]. Unfortunately, the search for small structural rearrangements remains difficult in beetles, which have no polytene chromosomes and it is almost impossible in the karyotype of S. puncticollis, in which large amounts of variable heterochromatin prevents to pair the chromosomes with confidence. Thus, there is neither indication nor demonstration that parthenogenesis is associated with any chromosome change in S. puncticollis.
According to Maynard-Smith [34], parthenogenetic females would have a much better fitness than sexual females. This could lead to a fairly rapid replacement of the original sexual population by parthenotes. In continental regions, their diploidy might indicate the recent origin of the parthenogenesis, while the observation of various chromosome formulae, as the result of secondary and independent events (fecundation by haploid gametes) might indicate a more ancient origin, giving time for successful fecundations. In this respect, the parthenogenesis of S. puncticollis may be a recent event, but other constraints may favour parthenogenesis occurrence and karyotype stability. The mode of colonization of Caribbean islands is not completely elucidated, but many arguments are in favour of an oversee dispersal from South America [35]. Howden [36] suggested that Passalids could disperse by rafting. South American S. crenatus can fly, but flight muscles were found, sometimes, atrophied in Puerto Rico, possibly reducing their dispersal ability [21]. We have no special data on the fly ability of specimens from other islands, but our numerous observations of hindwings permanently revealed a macropterous type. Like P. unicornis, S. puncticollis is occasionally attracted flying at light. In the likely hypotheses that the origin of S. puncticollis is South America and that its dispersal in the Antilles processed by rafting or by aerosol during hurricane, parthenogenesis is expected to have occurred from southern to northern islands. A relatively similar process could be represented in the Heteroptera, Anthocoridae, Calliodis maculipennis (Reuter, 1884), between Trinidad (parthenogenetic) and the continent (sexual reproduction) [37]. Many authors have pointed out the higher incidence of parthenotes in islands than in continents [38,39], but the relationship between insularity and parthenogenesis remains a matter of controversy. The excess of parthenotes in islands is generally attributed to the better ability of parthenotes to colonize new territories, because a single female is theoretically able to start a new population [38]. It is also possible that insulation, by reducing population sizes, favours homozygosity and thus, expression of recessive mutations. In Drosophila melanogaster Meigen 1830, the model species for genetic studies in insects, recessive mutations can cause parthenogenesis [40,41]. Consequently, the endogamy associated with insulation may favour the expression of such mutations [10]. In any event, the foundation of these processes is that of the geographic parthenogenesis of Vandel [42,43].
6 Conclusion
Passalids of the Greater and Lesser Antilles have been recently revisited in two initial studies [17,44]. From these islands, S. puncticollis constitutes a new and original example of parthenogenesis in Coleoptera. It is the second example amongst Scarabaeoidea, a super-family composed of over 20,000 species. It is also one of the rare instances of parthenogenetic Coleoptera with a diploid karyotype. This may indicate the recent occurrence of parthenogenesis, but it may be also related to insularity: the absence or rarity of males preventing fecundation and thus, addition of a haploid set of chromosomes to the diploid genome of parthenogenetic females, as it occurs in Curculionidae [16,33]. The genetic study of specimens from other islands than Guadeloupe may provide precisions on these points. In particular, the diploid or polyploid status and the presence or absence of genome diversity will inform about either the uniqueness or recurrence of parthenogenesis events and the relationships between parthenogenesis and island colonization.
Disclosure of interest
Highly risked with the North American and Latin American authors working on Scarabaeoidea (including Passalidae).
Acknowledgments
We are indebted to two learned societies which brought their financial support to the project of study of Passalids of the Antilles, started in 2005. As such, we thank especially B. François, the “Société des amis du Muséum”, Paris, and F. Durand, the “Société d’histoire naturelle Alcide-d’Orbigny”, Clermont-Ferrand, France.


