1. Rice improvement and apomixis
With 500 million tons of milled rice produced over more than 160 million hectares, rice is the leading cereal for human consumption and the staple food for more than half of humanity. It is mainly a self-consumption food crop, with only 10% of production entering international trade, compared with 20% for bread wheat. The varieties of rice (Oryza sativa L.) grown throughout the world are the result of domestication events in Asia, the number of which is still debated, and which led to two partially interfertile subspecies—japonica and indica. The varieties show a wide range of adaptations to a diversity of climates, cropping systems, practices and consumer preferences. Independent domestication took place in Africa to produce the cultivated species Oryza glaberrima Steud, grown mainly in West Africa. The challenges of feeding the world’s population (expected to reach 10 billion in 2050) in a changing climate, while reducing the environmental impact of rice cultivation (saving water and fertilizer resources and reducing greenhouse gas emissions, such as methane in paddy fields), require the co-design of new diversified varieties to meet both specific local constraints and demands and these global adaptation efforts [1].
The creation of new varieties mobilizes accessions present in cultivated species and their wild relatives (over 100,000 in the case of rice) by crossing them to obtain genetically variable progenies, particularly for agronomic traits, from which the breeder will choose the most interesting plants and then lines through selection cycles carried out over subsequent generations. The return to varietal homogeneity is facilitated in rice, as in wheat, by its mode of sexual reproduction by self-fertilization (the plant is said to be autogamous): the gametes present in the male gametophyte (pollen) and the female gametophyte (embryo sac) carried respectively by the stamen and the ovary, most frequently belonging to the same flower, will unite to form the embryo and the endosperm (reserves) of the seed in a process called double fertilization (Figure 1A). The gametes are the end product of a first specialized cell division—meiosis—of an initial diploid cell (two parental chromosome sets), during which the parental chromosomes recombine and are halved in number to become haploid. The cells resulting from meiosis—the functional megaspore and the microspores on the female and male sides—undergo divisions (mitoses) to form gametophytes.
(A) Double fertilization in rice: as in over 70% of angiosperm species, the female gametophyte (embryo sac) of rice is of the Polygonum type, composed of 7 cells resulting from cellularization of an 8-nucleus coenocyte produced by 3 divisions of a single functional megaspore (left). The functional megaspore is one of the four spores produced by female meiosis, the other three spores degenerating. During meiosis, the homologous parental chromosomes of the diploid mother cell, after having replicated their DNA into two sister chromatids, pair up for reciprocal or non-reciprocal exchanges of DNA constituting genetic recombination, then migrate towards opposite poles during the first division of meiosis. During the second meiosis division, the recombined or unrecombined sister chromatids separate and are distributed among four spores. The number of chromosomes is thus divided by two in recombined spores, which are haploid. The male gametophyte, the pollen, is trinucleated, and forms after two divisions of a product of meiosis, the microspore (right). The two sperm cells of the pollen grain are the male gametes, which fuse with the nucleus of the egg cell of the embryo sac, the female gamete, and the two nuclei of the central cell to form the embryo and the albumen of the grain, respectively. As shown in the central figure, rice self-pollinates, i.e. gametes from the same plant unite to form offspring. This process of self-fertilization makes or maintains the two parental batches identical throughout the plant’s cycles (homozygous). (B) During diplosporic gametophytic apomixis (right), the diploid mother cell undergoes simple mitosis to form spores and then gametophytes bearing gametes. Parthenogenesis of the egg cell allows a diploid embryo to develop without fertilization (parthenogenetic), while fertilization of the central cell may or may not take place to form the albumen.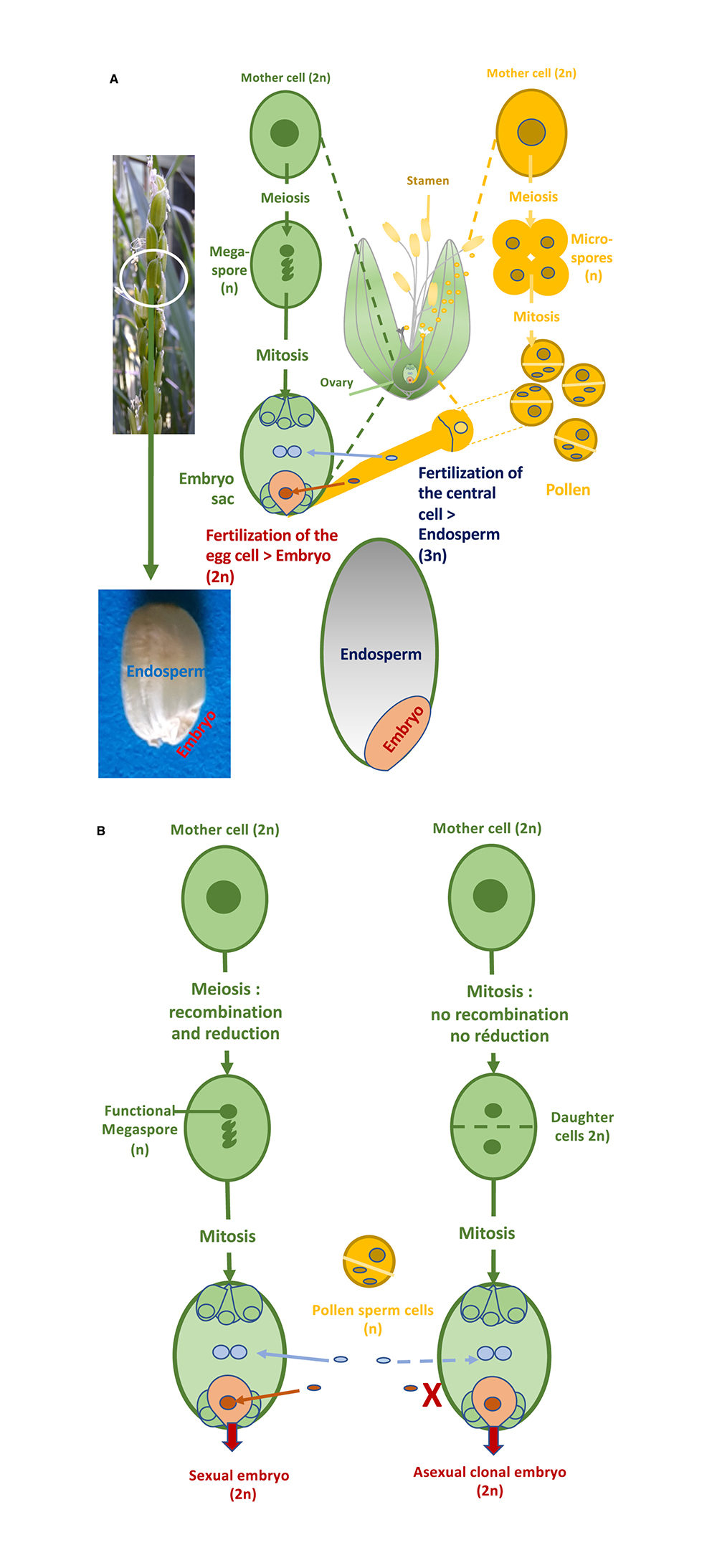
Released rice varieties are generally pure lines, with two largely identical chromosomal sets, ensuring varietal homogeneity. However, there is an advantage in directly using progenies, also homogeneous, formed from hybrid seeds resulting from the crossing of two pure lines, because they exhibit hybrid vigor. Hybrid vigor, also known as “heterosis” already highlighted by Darwin [2] and rediscovered by Shull in maize [3], concerns a whole range of traits, the best known of which is grain yield, which is more stable and increased by 20 to 30%. The genetic and molecular bases of this vigor phenomenon, resulting from the presence and interactions of the two parental genomes in a single hybrid genome, have been extensively studied [4]. Following the example of maize, F1 hybrid rice varieties are used, but as rice plants do not cross spontaneously with high frequency, the use of complex genic or geno-cytoplasmic male sterility systems is necessary to produce hybrid seeds [5]. Fertilization of the female parent by pollen from the male parent often has to be mechanically assisted. The production of F1 seeds is therefore costly: this limits the number of combinations tested and distributed, and above all access to these seeds for small-scale rice growers in the South, all the more so as they have to be renewed with each planting. In fact, F2 seeds harvested from F1 hybrid plants, if sown, will form heterogeneous offspring, particularly in terms of essential agronomic traits (plant height, crop cycle, grain quality, etc.), making them difficult to use by rice growers. This divergence is linked to allele shuffling occurring during meiosis, which recombines homologous chromosomes from the different genomes of the two parents. In order to reproduce an identical hybrid through seeds over successive generations, clonal asexual reproduction would have to be set up in rice. This mode of reproduction could be achieved if the initial cell of the female gamete divided by simple mitosis, without recombination or reduction of the parental chromosomes, forming two identical daughter cells, followed by the formation of an embryo from the egg cell of the embryo sac, without fertilization by the male gamete.
A mode of asexual reproduction by seeds known as “Apomixis” exists in nature in over 400 species of flowering plants, mainly wild but also cultivated, such as forages and Citrus, where it takes place in different ways depending on the origin of the cell giving rise to the clonal embryo (either sporophytic or gametophytic origin) [6]. In its most advanced form (diplosporic gametophytic), apomixis bypasses the allele shuffling and chromosome number reduction involved in sexual reproduction by transforming meiosis into mitosis (apomeiosis), followed by the development of the egg cell by parthenogenesis, without fertilization by the male gamete carried by the pollen (Figure 1B). Fusion of the central polar nuclei with the male gamete may or may not be necessary for the formation of grain reserves (if not, then we speak of autonomous development of the endosperm).
Because of its interest in the production of “immortalized” hybrid formulas, the search for a source of apomixis in cultivated species and their wild relatives has been conducted for many years. Genetic loci involved in the determinism of apomeiosis and parthenogenesis have been identified in wild species [7], but their transfer to cultivated species has so far failed to convert them to apomixis. No source of apomixis has been found in rice [8]. An alternative is therefore to engineer synthetic apomixis in cultivated plants, which has mobilized research over the last 20 years.
2. Development of synthetic apomixis in rice
As described above, three elements are required to mimic diplosporic gametophytic apomixis: (i) conversion of meiosis into mitosis; (ii) development of the egg cell into an embryo without fertilization by the male gamete; and (iii) formation of the endosperm with (or without) fertilization of the polar nuclei by the male gamete.
Regarding the first component, three major mechanisms differentiate meiosis from mitosis: (i) the pairing of homologous parental chromosomes and their recombination; (ii) the monopolar migration of sister chromatids resulting from DNA replication in the first division of meiosis; (iii) the existence of the second meiosis division, which distributes the recombined chromatids into four haploid spores (Figure 2A). The MiMe (Mitosis instead of Meiosis) triple mutation in Arabidopsis abolishes these differences [9]: firstly, recombination and pairing are suppressed by inactivating a member of the recombination initiation complex (e.g. SPO11-1 or PAIR1); next, the joint migration of sister chromatids during meiosis is replaced by their separation through inactivation of the REC8 cohesin; finally, the second meiotic division is suppressed by inactivation of the cell cycle regulator OSD1 (Figure 2A). In collaboration with INRAE Versailles, we have successfully introduced these three mutations into rice [10], and MiMe rice forms unrecombined diploid male and female gametes (containing 2 × 12 chromosomes), i.e. with the genotype of the parent plant. The diploid egg cell is then fertilized by a diploid male gamete and forms a tetraploid clonal sexual embryo, while the endosperm formed after fusion of diploid polar nuclei and a diploid male gamete is assumed to be hexaploid (Figure 2B). Apomeiotic tetraploid rice plants can grow but are sterile.
Synthetic apomixis in rice. (A) The MiMe (Mitosis instead of Meiosis) triple mutation transforms meiosis into mitosis in Arabidopsis [9] and rice [10]. MiMe abolishes the three essential differences distinguishing meiosis from mitosis namely (i) pairing of homologous chromosomes and recombination, (ii) migration of sister chromatids towards the same pole during the first division of meiosis, and (iii) existence of a second division separating sister chromatids. (B) The combination of MiMe and parthenogenetic initiation of embryogenesis in the egg cell without fertilization, by expression of the BABYBOOM transcription factor in this cell, enables the development of a clonal embryo [13]. Grain formation is completed by fertilization of the central cell nuclei to form the albumen.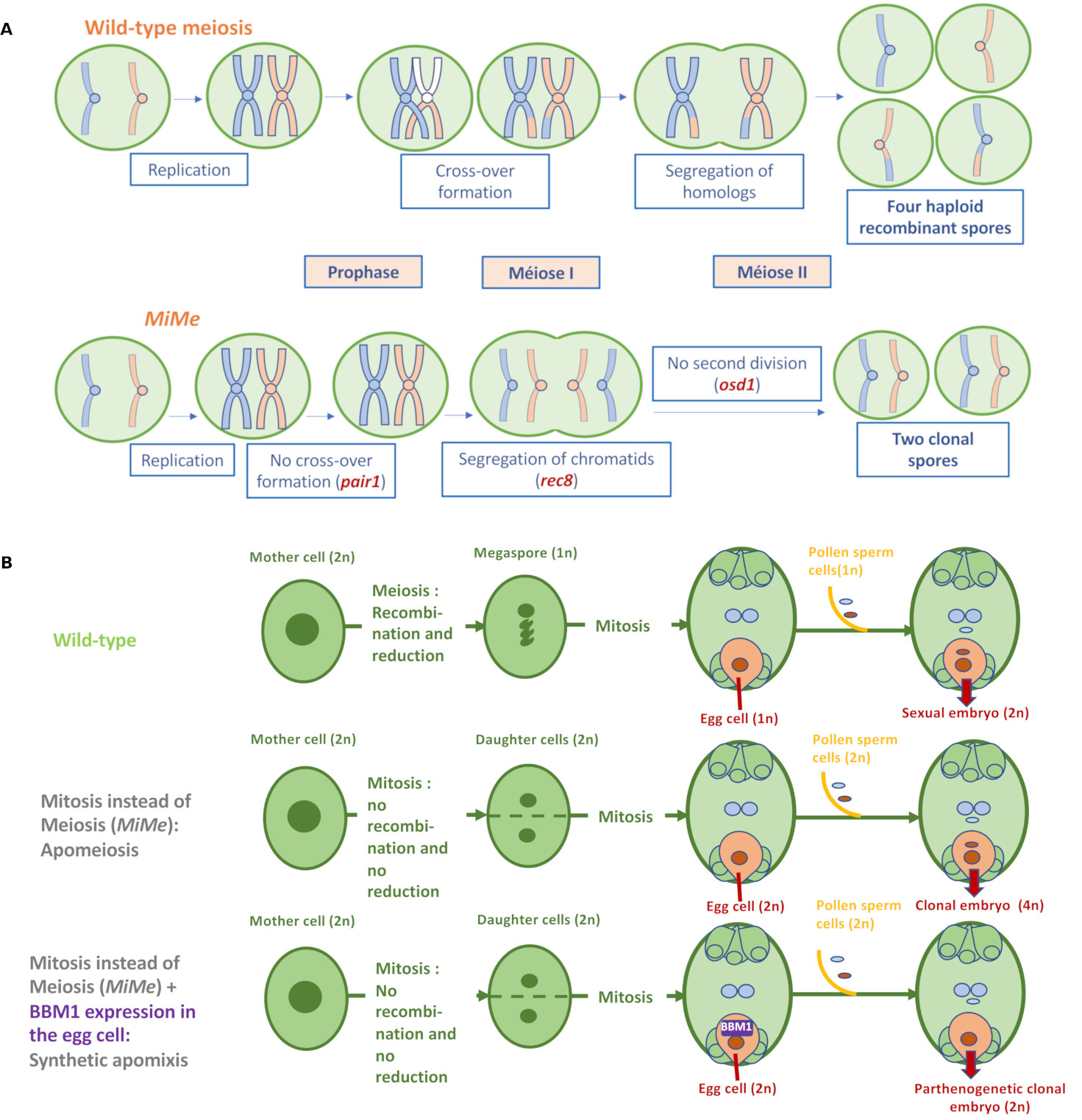
The second component is the parthenogenetic development of the egg cell without fertilization: the study of genes transcribed in isolated rice gametes has identified a candidate transcription factor of the APETALATA 2 family, BABYBOOM (OsBBM1) [11]. The male allele of OsBBM1 is expressed in sperm cells, but the female allele remains repressed in egg cells until fertilization occurs. An ortholog of this gene discovered in rapeseed (Brassica napus) has been previously shown to induce somatic embryogenesis when constitutively expressed in Arabidopsis plant cells [12]. Similarly, constitutive accumulation of OsBBM1 in rice triggers the formation of somatic embryos on various organs [13]. These data, added to the fact that a putative ortholog of OsBBM1 is also present in the locus responsible for facultative apomixis in the wild grass Pennisetum [14], designated OsBBM1 as a good candidate to successfully trigger parthenogenesis by its ectopic expression specifically in the egg cell of the embryo sac, which indeed led to haploid embryos in wild-type rice seeds [13]. Combined with the MiMe triple mutation, egg cell-specific expression of OsBBM1 enabled synthetic apomixis to be obtained, resulting in the production of diploid clonal apomictic plants with a frequency of up to 30%, the rest of the offspring being tetraploid plants resulting from sexual fertilization (Figure 2B) [13].
Finally, the third component is the formation, with or without fertilization, of grain endosperm. In the previous study, the endosperm accompanying the parthenogenetic embryo formed in an apparently normal manner, but its ploidy or quality has not been analyzed [13].
3. High-frequency synthetic apomixis in hybrid rice
In a collaboration with UC Davis and the Max Planck Institute in Cologne, we transferred a single construct carrying the elements to engineer in a single step both apomeiosis via the MiMe triple mutation (by CRISPR/Cas9 mutagenesis) and parthenogenesis (Arabidopsis or rice egg cell-specific promoter controlling the OsBBM1 gene) into a commercial rice hybrid (BRS-CIRAD 302) (Figure 3A) [15]. All the constructs and methods have been reported and are detailed in [15]. The indica/indica F1 hybrid BRS-CIRAD 302, released in Brazil in 2010 has been chosen because of its high performance and outstanding grain quality. Fertile regenerated plants exhibited the MiMe triple mutation [15]. The progenies of plants transformed with the construct without the parthenogenetic trigger are all tetraploid as expected and possess the hybrid diagnostic microsatellite markers, and are therefore apomeiotic. When the parthenogenetic trigger is present in the MiMe context, a certain frequency of diploid plants is expected in the progenies: in our case, 80% of the 28 triple mutants observed had a diploid progeny frequency of over 80%. A frequency of 95 to 100% was observed in 4 selected events over the following two generations, indicating that OsBBM1 stably induces parthenogenesis with a high frequency [15] (Figure 3B). Clonal reproduction is supposed to maintain the F1 hybrid genome identical over successive generations: we therefore sequenced at low depth regenerated plants over two generations and observed a sequence rigorously identical to that of the hybrid [15]. This shows that synthetic apomixis, induced by the combination of MiMe mutations and OsBBM1 expression, enables highly efficient clonal propagation of F1 hybrids through seeds. Apomixis should also enable transmission of the F1 hybrid phenotype over generations: Phenotypes were further examined in second-generation progenies of four apomictic hybrids grown with BRS-CIRAD 302 F1 control plants (Figure 3C). No significant differences in plant traits were observed between the apomictic progenies and the hybrid control, which are therefore faithfully and homogeneously recapitulated via synthetic apomixis [15].
High frequency synthetic apomixis in one step in hybrid rice [15]. (A) Synthetic apomixis is achieved in hybrid rice by introducing into its genome a single construct, whose variations are schematically shown here. The construct carries the CAS9 gene and specific RNA guides (gRNAs) targeting each of the 3 genes (PAIR1, REC8 and OSD1) to be inactivated, as well as a parthenogenesis-triggering cassette to be expressed specifically in the egg cell (here governed by either an Arabidopsis (T314) or a rice (T315) promoter with egg cell-specific activity). The first construct (T313) is a control construct to produce only apomeiotic MiMe plants, whose progenies are 100% tetraploid. The other two constructs (T314 and T315) designed to convert the hybrid to apomixis lead to the production of MiMe triple mutant plants (n = 28), 80% of which generate diploid clonal seeds with a frequency of over 80%. (B) The ploidy of the progenies of four selected apomictic plants reaches 95–100% of diploid clonal plants over the following two generations. (C) The phenotype of the second-generation progenies of the four apomictic plants (plant growth trays 2–5) is identical to that of the starting hybrid (plant growth tray 1), as is their genomic sequence, verified elsewhere.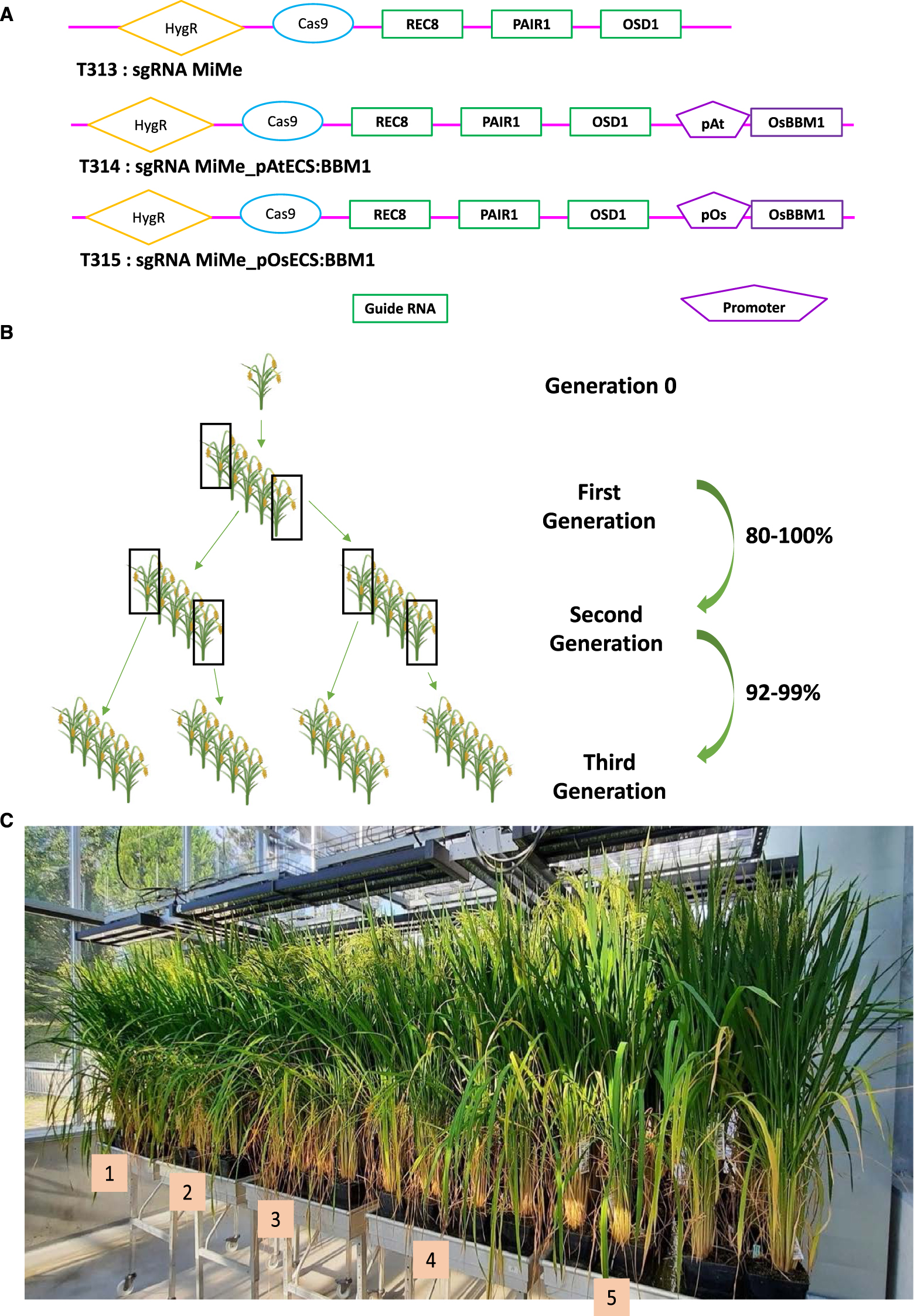
An important trait to examine was grain size and quality. The grain size of apomictic hybrids is comparable to that of grain harvested from F1 hybrids. Analysis of the ploidy of the endosperm during formation showed that, as expected, it was initially hexaploid—and not triploid—and thus a product of the fusion of three diploid nuclei. Finally, grain quality was analyzed, an important factor in a variety’s acceptability to consumers. Apomictic grains showed starch and amylose contents comparable to those of grains harvested from the BRS-CIRAD 302 F1 hybrid, known for its excellent levels of these traits [15].
4. Bringing apomictic hybrids to the field
The very next steps are aimed at improving the fertility of apomictic hybrids, which is still reduced by 20 to 40% compared with F1 hybrids. Main possible causes of this defect are currently being explored: insufficient penetrance of MiMe or defects in the temporality, level and/or territorialization of OsBBM1 expression. The stability of parthenogenetic induction will have to be tested in the field under real and also unfavorable environmental conditions, which are not represented in the greenhouse. These field trials will also confirm the maintenance of hybrid vigor through apomixis over many generations. While the stability of the genome, transcriptome and methylome has been demonstrated in the first greenhouse generations of synthetic apomixis [15, 16], it will be important to further investigate this under field conditions and to assess longer-term consequences of this mode of reproduction on the epigenome. We have recently applied synthetic apomixis to other hybrids, notably distant indica/japonica hybrids, which are expected to show even greater heterosis of the order of 30%.
In addition to grain yield and stability, which are shared with sexually propagated F1 hybrids, hybrids propagated by synthetic apomixis offer additional advantages: the grain endosperm presents a single, predictable F1 genotype, rather than a more randomly segregated F2 genotype, to which the variable grain quality harvested from commercial hybrids has been attributed, at least in part. As the parthenogenetic egg cell of the apomictic hybrid cannot be fertilized, seed purity is maintained during multiplication, which is important for countries in the South without an adequate seed distribution system. The creation and multiplication of hybrids should eventually be facilitated, so that small-scale rice growers can acquire and multiply themselves seeds supplied by national technical institutes. Facilitating the creation of hybrid formulas should make it possible to diversify the locally adapted hybrid combinations made available after co-design with users. It will also avoid the large-scale use of a single male sterile cytoplasm, which can be a source of sensitivity. Finally, the diversification of the tested hybrid combinations should enable effective targeting of tolerance to abiotic constraints and biotic pressures as breeding objectives.
Intellectual property (e.g. of the CRISPR/Cas system) and regulatory bottlenecks will have to be lifted to facilitate the optimization and unleash the implementation of synthetic apomixis in crops by laboratories and National Agricultural Research Systems. Regarding regulatory issues, several strategies, applied before or after generation of the synthetic apomictic events, should allow the elimination of unwanted superfluous T-DNA sequences (i.e. the CRISPR/Cas system and the selectable gene cassette) in the released apomictic hybrid. This will leave only the parthenogenesis cassette integrated, which can be made only with rice elements [15], thereby making apomictic hybrids considered intragenic and not transgenic. If this cassette is transferred to the egg cell of conventional rice via the apomictic diploid pollen sperm cell, it would lead to an unviable triploid embryo.
Management of agrobiodiversity also has to be taken into account: on the one hand, as mentioned above, synthetic apomixis should facilitate the diversification of released hybrid combinations by custom tailoring them to meet the local needs and constraints of farmers and consumers, contrasting in that with the usual release of a few mega F1 hybrids of rice cultivated on dozens of thousands of hectares. In that sense it should favor the maintenance of genotypic diversity in the landscapes. On the other hand, apomictic hybrids cannot be used for crossing anymore by construction, thereby locking their further use for generating new diversity by the farmer and the breeder: if synthetic apomictic hybrids become popular to a point of replacing pure lines it will be crucial to enforce a system to make their parental lines compulsorily accessible to breeders and farmers in public genetic resource repositories.
5. Conclusion
We have shown that a high frequency of clonal seed production is possible by synthetic apomixis, making this mode of reproduction compatible with agricultural use. It enables faithful transmission of the hybrid sequence and phenotype in the progenies, while preserving grain quality. More fundamentally, our work shows that the integration of an additional component inducing autonomous development of the endosperm (without fertilization) is not necessary to achieve a high frequency of apomixis. Moreover, it demonstrates that the epigenetic contribution made by the male gamete does not appear to be necessary for the manifestation of heterosis in an obligate sexual species. Taken together, these results pave the way for international research to improve the system right up to its application in the field [17]. Ultimately, apomixis should enable breeders to exploit more effectively the potential of F1 hybrids that are tolerant to abiotic constraints and biotic pressures, and thus better equipped to meet the global challenges posed by climate change and increasing food demand.
Declaration of interests
The authors do not work for, advise, own shares in, or receive funds from any organization that could benefit from this article, and have declared no affiliations other than their research organizations.
Acknowledgements
The authors would like to thank their collaborators Pr. Raphaël Mercier (INRAE Versailles, France then MPIPZ, Cologne, Germany) and Pr. Venkatesan Sundaresan (UC Davis, Davis, USA), as well as the other co-authors who contributed to the success of the study.




 CC-BY 4.0
CC-BY 4.0