1 Introduction
Few fish species have survived the increasing aridity of the Sahara since the last humid period at the Holocene, approximately 5000 years BP [1]. Almost all perennial bodies of water—springs, guelta, pounds, sebkhras or lakes—where the occurrence of fishes has been reported since the first Saharan exploration expeditions in the early 20th century, are located in mountainous massifs, rarely elsewhere, and fish diversity is low [2–4]. Currently, 20 species of fish are known in the Sahara, most of them in a very low number of watering places, often in a single spring or guelta [4].
The fish fauna of Chad has been investigated since 1904 following the first expedition Chevalier–Decorse to Lake Chad and Chari River [5]. The first studies on fishes collected in Saharan areas of northern Chad were published by Pellegrin [6,7] who described Tilapia borkuana (= Sarotherodon galilaleus borkuanus) from Ounianga Serir and Labeo tibestii (= L. parvus Boulenger, 1902) from Enneri Debassa in the Tibesti. This author also mentioned the presence of Labeo horie [= L. niloticus (Forsskål, 1775)], Barbus anema (= B. macrops Boulenger, 1911) and Clarias lazera [= C. gariepinus (Burchell, 1822)] in Totous guelta (Tibesti), Hemichromis bimaculatus Gill, 1862, in Ounianga Serir, Barbus deserti (= Barbus macrops) in Archei guelta (Ennedi), and Tilapia zilli (Gervais, 1948) in Archei and Totous gueltas [6]. He also mentioned the presence of Barilius louti [= Raiamas senegalensis (Steindachner, 1870)] in Yezei (Tibesti), where T. zilli and B. macrops were also recorded, the latter species being also collected in Zouarke pool (Tibesti) [6]. Another collection of fishes from northern Chad was studied by Estève [8], who reported the occurrence of Aplocheilus marni [= Epiplatys spilargyreius (Duméril, 1861)] in Tigui (Borkou), L. parvus, B. deserti (= B. macrops) and T. zilli in Archei, and H. bimaculatus and S. g. borkuanus in Ounianga Kebir. S. g. borkuanus was also collected in Fada (Ennedi) by Fowler [9]. Further studies of new collections of fishes from Tibesti, Borkou and Ennedi were made by Daget [10,11], who also re-examined the previous collections deposited in Paris, and by Monod [12]. These authors reported Barbus batesi (= Barbus bynni occidentalis Boulenger, 1911) from Totous, S. g. borkuanus from Enneri Maro (Tibesti), Barbus apleurogramma Boulenger, 1911, from Aoue guelta (Ennedi), and L. parvus from various additional Tibesti and Ennedi gueltas. The taxonomic status of the whole species of fish that were recorded from Sahara was reviewed by Lévêque [2]. Recently, I reported the occurrence of Polypterus senegalus Cuvier, 1829, and Poropanchax normani (Ahl, 1928) in Lake Boukou at Ounianga Serir [4].
In central and southern Chad, after the first studies by Pellegrin [5,13–15] and those by Chabanaud [16] and Fowler [17], the most important work was the monography by Blache [18] which included 175 species from the Lake Chad basin and provided meristic and biological data for the specimens collected. More recently, the fish fauna from the Chad basin was included in the monography of Paugy et al. [19] on the fresh and brackish water fishes of West Africa.
Haplochromine cichlid fishes represent some of the most species-rich adaptive radiations known and are famous for their astonishingly fast rates of speciation [20–22]. Paradoxically, although thousands of haplochromine species are known from central, eastern, and southern Africa [20–26], they are virtually absent from Chad and West Africa, where only a single species has been currently reported [19]. During two zoological surveys in northern Chad in 2013 and 2014, fishes, reptiles, amphibians, and invertebrates were collected in various areas of Borkou, Ennedi and Tibesti [4,27–30]. Among the fishes collected in Lake Boukou at Ounianga Serir, several specimens belonged to an undescribed haplochromine cichlid species. Here I describe the new species from Lake Boukou, which adds to the unique relict fish fauna of Ounianga lakes, one of the most remarkable hotspot for biodiversity in the Sahara desert.
2 Material and methods
The Ounianga lakes consist of two main groups of lakes located between the Tibesti and Ennedi mountains in northeastern Chad (Fig. 1). They are among the very few permanent aquatic ecosystems currently existing in the most arid parts of the Sahara desert [31]. Although in this area, average annual rainfall is less than 5 mm and annual evaporation exceeds 6000 mm, these lakes are maintained by an inflow of fossil groundwater from a large sandstone aquifer that was last recharged during the Early Holocene [32–35]. The four Ounianga Kebir lakes are all hypersaline, with freshwater restricted to a creek and a few springs on the shore of the main lake. At Ounianga Serir, which comprises seven perennial lakes located 50 km east of Ounianga Kebir, only the lowest and largest lake is hypersaline (Lake Teli: 18°55′N/20°52′E, altitude 345 m, surface 6.5 km2, Fig. 2). Lake Boukou, also called Lake Boku (18°54′50″N/20°54′40″E, altitude 363 m, surface 0.2 km2, Figs. 3 and 4), where salt concentration is the lowest of all Ounianga Serir lakes, has a maximum depth of 13 m and present, together with Totous guelta in Tibesti (Chad), the richest fish fauna of the Sahara desert [4].

Satellite view of northern Chad with location of the main lakes and gueltas home of relict fish populations.

The hypersaline Lake Teli at Ounianga Serir.

General view of Lake Boukou, the home Astatotilapia tchadensis sp. nov.

Shores of Lake Boukou where specimens of the new species were collected.
The specimens of the new species described here were collected by night in Lake Boukou using a landing net on 1 March 2013 and 5 March 2014. They were preserved in alcohol and deposited at the Museum national d’histoire naturelle (MNHN) in Paris. Length measurements were taken with a digital caliper. Methods for length measurements and the study of meristic data follow Greenwood [20], Barel et al. [23], and Paugy et al. [19]. Specimens were visually sexed from their gonads and their stomach contents identified using a stereomicroscope.
3 Results
Astatotilapia tchadensis sp. nov.
Holotype: MNHN 2015.0396, formerly IRD TR.4202, a male collected on 1 March 2013 in Lake Boukou (Ounianga Serir, Chad).
Paratypes: MNHN 2015.0397, formerly IRD TR.4201 (Fig. 5), collected on 1 March 2013 and MNHN 2015.0398, formerly TR.4299 (Fig. 6), collected on 5 March 2014, two females from the same locality as the holotype.

Astatotilapia tchadensis sp. nov. Paratype MNHN 2015.0397 just after capture.

Astatotilapia tchadensis sp. nov. Paratype MNHN 2015.0398 in life.
Diagnosis: A large haplochromine cichlid fish from Lake Boukou (Ounianga Serir, Chad) and probably the whole current Lake Chad basin characterized by the following combination of characters: a black bar between the eye and the corner of mouth, rounded orange spots on the anal fin in adult males, scales ctenoid, outer jaw teeth bicuspid, inner teeth tricuspid, 16 scales around the peduncle, no papillose swelling on either side of pharynx, lower limb of first gill arch with 7–8 gill rakers, dorsal fin with 13–14 spines and 9–11 soft rays, anal fin with 3 spines and 8–9 soft rays, 29 or 30 lateral line scales, lower pharyngeal dentition with enlarged molariform teeth in the central rows.
Etymology: refers to the country of origin of the type series and the fossil Lake Chad basin where the species originated.
Description of holotype: standard length 98.3 mm; total length 121.9 mm; body depth 35.3 mm, head length 33.5 mm; head width: 15.5 mm; snout length 10.5 mm; eye diameter 8.6 mm; interorbital width 8.1 mm; predorsal length 33.5 mm; preanal length 64.5 mm; prepectoral length 37.3 mm; prepelvic length 42.4 mm; length of dorsal fin base 50.7 mm; length of anal fin base: 20.1 mm; pectoral fin length 25.2 mm; pelvic fin length 27.5 mm; caudal peduncle length 14.7 mm; depth of the caudal peduncle 13.7 mm; dorsal fin with 14 spines and 9 soft rays; anal fin with 3 spines and 8 soft rays; caudal fin with 23 soft rays; pectoral fin with 13 soft rays; pelvic fin with 1 spine and 5 soft rays; two lateral lines with 18 and 5 scales perforated on the upper and lower lateral lines respectively; 28 + 2 lateral line scales; 5.5 scales between lateral line and origin of first dorsal fin; 11 scales between lateral line and midventral body profile; 5 scales between the pectoral and pelvic fin bases; 2 scales between the upper and lower lateral lines; 16 scales around the peduncle; scales ctenoid; 7 gill rakers on lower limb of first gill arch (total 10 on first gill arch); outer jaw teeth bicuspid; inner teeth tricuspid (3 ranks); no papillose swelling on either side of pharynx; lower pharyngeal bone and dentition: dentigerous surface approximately equilateral, teeth forming the median rows enlarged with molariform and submolariform crowns (Fig. 7).
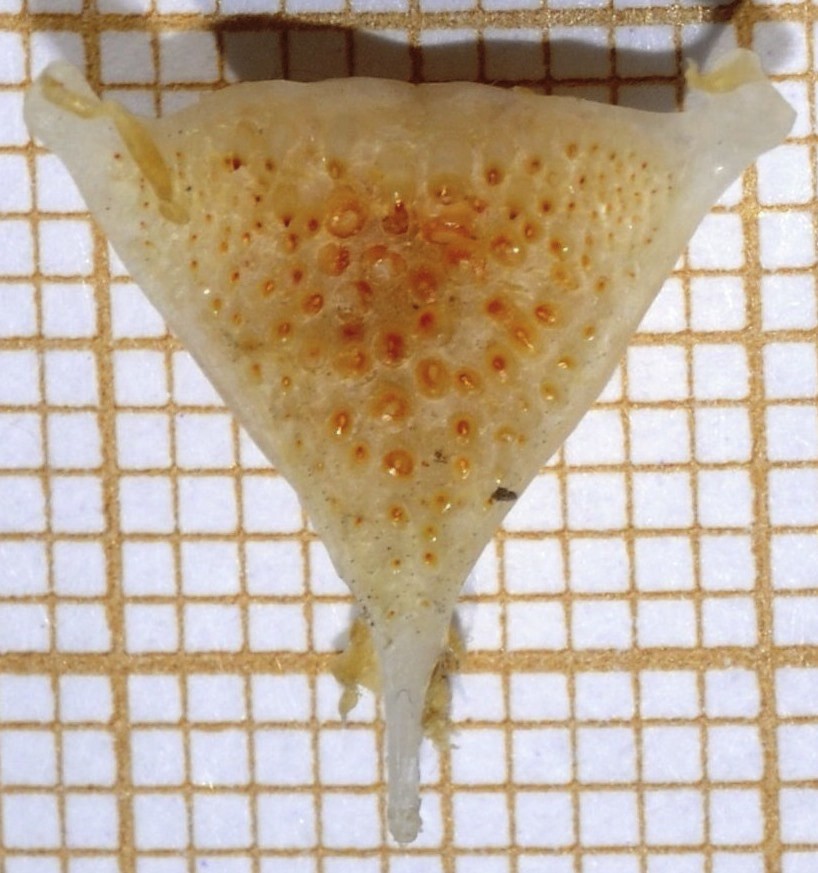
View of the lower pharyngeal bone and dentition of the holotype of Astatotilapia tchadensis sp. nov.
Coloration in preservative: head and body brown; a black bar between the eye and the corner of the mouth; a black spot present on the postero-dorsal angle of the opercula; 6 black cross bars that descend below the midlines of sides; no ocelli on the anal fin but two unpigmented spots; no tilapian mark at the beginning of the soft part of the dorsal fin.
Description of paratypes: standard length 77.2 (MNHN 2015.0397) and 73.8 mm (MNHN 2015.0398); total length 94.5 and 81+ mm (caudal fin mutilated); body depth 28.4 and 27.8 mm, head length 28.8 and 26.2 mm; head width: 12.2 and 12.1 mm; snout length 9.1 and 8.4 mm; eye diameter 7.0 and 6.6 mm; interorbital width 6.8 and 6.2 mm; predorsal length 25.9 and 23.3 mm; preanal length 53.1 and 50.5 mm; prepectoral length 28.1 and 27.2 mm; prepelvic length 31.7 and 31.0 mm; length of dorsal fin base 39.5 and 39.2 mm; length of anal fin base: 14.0 and 14.6 mm; pectoral fin length 19.3 and 10.7+ mm; pelvic fin length 19.0 and 12.1+ mm; caudal peduncle length 11.0 and 8.2 mm; depth of the caudal peduncle 10.8 and 10.6 mm; dorsal fin with 13 spines and 10 soft rays (MNHN 2015.0397) or 14 spines and 11 soft rays (MNHN 2015.0398); anal fin with 3 spines and 8 or 9 soft rays; caudal fin with 23 soft rays; pectoral fin with 13 soft rays; pelvic fin with 1 spine and 5 soft rays; two lateral lines with 20 and 11 or 20 and 8 scales perforated on the upper and lower lateral lines, respectively; 28 + 2 or 27 + 2 lateral line scales; 5.5 scales between lateral line and origin of first dorsal fin; 10 scales between the lateral line and the midventral body profile; 4 or 5 scales between the pectoral and pelvic fin bases; 2 scales between the upper and lower lateral lines; 16 scales around peduncle; scales ctenoid; 7 or 8 gill rakers on the lower limb of the first gill arch (total 12 or 13 on first gill arch); outer jaw teeth bicuspid; inner teeth tricuspid (2 ranks); no papillose swelling on either side of the pharynx.
Coloration in life: head and body light brown or silver; a vertical black bar between the front lower part of the eye and the part of the head immediately posterior to the corner of the mouth; two median incomplete horizontal black bars between the eyes; a vertical black bar in the middle of opercula; a black spot present on posterodorsal angle of opercula; 7 black cross bars that descend below the midlines of the sides; three or two rounded orange spots clearly apparent on the anal fin, these spots without contrasting border and without translucent surround (Fig. 8); no tilapian mark at the beginning of the soft part of the dorsal fin; fine orange spots at the basis of the soft rays of the dorsal fin.

The anal fin of paratype MNHN 2015.0398 in life.
Coloration in preservative: head and body brown with black bars poorly contrasting; spots on anal fin unpigmented.
Other specimen: one additional specimen with a standard length of 66 mm and a total length of 82 mm was collected on 1 March 2013 in Lake Boukou. It was photographed (Fig. 9), but not preserved.

The unpreserved specimen of Astatotilapia tchadensis sp. nov. just after capture.
Ecology: emergent aquatic vegetation, mainly Cyperus laevigatus, Typha australis and Phragmites australis, covers approximately the two-thirds of the lake's surface. Submerged macrophyte beds include Potamogeton [35]. Main water chemistry parameters, measured in October 2004 [35], were as follows: conductivity 329 μS·cm−1, O2 7.15 mg·L−1, Ca 44.6 mg·L−1, Mg 6.4 mg·L−1, dissolved inorganic carbon 25.5 mg·L−1, pH 7.5. Surface water temperature was 23 °C in October 2004 and 22 °C on 2 March 2013.
Diet: the head and a piece of thorax of the Poecilliidae fish P. normani were present in the mouth of the holotype at the time of its capture. The stomachs of specimens were either empty (two paratypes) or contained remains of fishes (holotype).
Geographic distribution: Lake Boukou (Ounianga Serir, Chad). I also attribute to the new species the populations from Lake Chad and its basin (Chad, Cameroon, Nigeria, Niger and Central African Republic) previously assigned to Astatotilapia desfontainii, Haplochromis wingati, and Astatotilapia aff. bloyeti (see Discussion).
4 Discussion
4.1 Comparison with other haplochromines
Haplochromine fishes comprise about two thousand species, most of them endemic to the East African Great Lakes where they have undergone explosive adaptive radiations [20–22]. About 1000 haplochromine species belonging to some two dozen genera are endemic to Lake Malawi [21,36], and about 600 haplochromine species are known from the Nile drainage basin, almost all endemic to Lake Victoria or Lake Edward [37,38]. In two papers in 1979 and 1980, Greenwood [20,37] divided the whole haplochromine assemblage (excluding Lake Malawi species flock) into 21 lineages to which he assigned generic rank—including Astatotilapia Pellegrin 1904—or more rarely subgeneric rank. This classification was criticised by further workers (see Van Oijen [26]) and only part of the genera proposed in Greenwood's revision were confirmed by further investigations [25,26]. Regarding the genus Astatotilapia as conceived by Greenwood [20,37], it appears polyphyletic [21,25] and a clear redefinition of this genus is needed [26]. However, the new species from Lake Boukou is both morphologically and geographically close to A. desfontainii, the type species of the genus [20,39], and thus there is little doubt about its generic status contrary to most lacustrine East African species.
Haplochromines are virtually absent from West Africa and Chad where only a single species has been reported [19]. This West African species was first mentioned by Pellegrin [14,15] as A. desfontainii (Lacépède, 1802) and by Daget [40] and Blache [18] as H. wingati (Boulenger, 1902). The specimens of Daget [40] were collected in Lake Debo (Mali, central delta of the Niger River) and those of Pellegrin [14,15,41] and Blache [18] in Lake Chad (Bol) and in several rivers of its basin (Chari, Logone and Mayo Kebbi in southern Chad, and Gribingui in Central African Republic). Pellegrin [42] also reported A. desfontainii from Léré, a small town on Lake Tanda in the central delta of the Niger River (Mali). According to Trewavas (see Daget [40]) and Blache [18], all specimens from the Chad and Niger basins—two basins occasionally connected during the rainy season through the Mayo Kebbi and Bénoué flood plains in Cameroon—belong to the same species, which differs from A. desfontainii, the only North African haplochomine (type-locality: Gafsa in Tunisia), by several meristic characters. Further studies by Greenwood [43] have shown that the Chad and Niger basins specimens also differ markedly from H. wingati, a species described from a single adult specimen caught in the Barh-el Jebel at Gondokoro (Sudan), but are morphologically close to Astatotilapia bloyeti (Sauvage, 1883), an East African species [44]. Since no name was available for the species from Chad and Niger basins, it appeared in recent works as Astatotilapia cf. bloyeti or Astatotilapia aff. bloyeti [45,46].
As shown in Table 1, the main meristic characters of Lake Boukou, Lake Chad basin and Lake Debo (Mali) populations are close. Some differences in proportions are observed, e.g. the standard length/body depth and head length/eye diameter ratios (Table 1). However, our four specimens from Lake Boukou have a total length ranging from 82 to 122 mm and a 66–98.3 mm standard length, compared to a maximum total length of 49 mm and a maximum standard length of 39.5 mm for Lake Debo (23 specimens including adults) and a maximum total length of 66.5 mm and a maximum standard length of 54 mm for Lake Chad basin (27 specimens including adults). It is unclear if length differences may explain differences in the size ratios. Colour patterns are close (see Fig. 6 and text in Daget [40], Fig. 138 and text in Blache [18]), but differences with Lake Debo's population includes the colour of anal fin spots (red in Lake Debo, orange in Lake Boukou and the Lake Chad basin), and the number of transverse black bars of the body (8–9 in Lake Debo, 6–7 in Lake Boukou and the Lake Chad basin). I provisionally attribute the populations of the Lake Chad basin to Astatotilapia tchadensis sp. nov., but further studies are needed to confirm this view, and Niger River populations may belong to an undescribed species.
Comparison of the main characteristics of Astatotilapia tchadensis sp. nov from Lake Boukou and the other West African haplochromine cichlid populations.
| Character | Astatotilapia tchadensis sp. nov. Lake Boukou (n = 4) |
Astatotilapia sp. Lake Chad basina (n = 27) |
Astatotilapia sp. Lake Debob (n = 23) |
| Dorsal fin | XIII–XIV, 9–11 | XIII–XV, 8–12 | XIII–XV, 9–11 |
| Anal fin | III, 8–9 | III, 7–9 | III, 8–9 |
| Lateral line scales | 29–30 | 26–31 | 28–29 |
| Gill rakers (lower limb 1st arch) | 7–8 | 7–9 | 8 |
| Standard length/body depth | 2.6–2.8 | 2.8–3.3 | 2.95–3.25 |
| Standard length/head length | 2.7–2.9 | 2.4–2.8 | 2.5–2.8 |
| Head length/head width | 2.2–2.4 | 1.7–2.3 | 1.85–2.1 |
| Snout length/eye diameter | 1.2–1.3 | 0.7–1.3 | ≈ 0.9–1.1 |
| Head length/eye diameter | 3.9–4.1 | 2.7–3.7 | 3.0–3.5 |
| Caudal peduncle length/depth | 0.8–1.1 | 1.1–1.5 | 1.0–1.2 |
| Interorbital width/eye diameter | 0.94–0.97 | 0.5–1.0 | 0.85–0.95 |
| Standard length | 66–98.3 mm | ?–54 mm | 14–39.5 mm |
| Anal fin spots | Orange | Orange | Red |
Compared to A. desfontainii, the only North African haplochromine, A. tchadensis sp. nov. has a lower number of spines at the dorsal fin (XIII–XIV compared to XIV–XVI) and a lower number of lateral line scales (29–30 compared to 31–34) [40]. The aspect of anal spots is different: A. desfontainii has true ocelli and all spots have a contrasting red or black border [20,47]. It is a rare endangered North African species that has been reported from a few oases in southern Tunisia (Gafsa, Gabès, El Hamma, Tozeur) and northern Algeria (Biskra, Tolga, Oumache) in the basin of the Chotts palaeolake [47,48], and there is also a relict Saharan population near Arak (Mouydir, Algeria) where a 17-cm-long specimen was collected in 1928 [49]. It was the only haplochromine cichlid known from the Sahara. Recent molecular studies have shown that this species represents a distinct lineage that diverged from an East African clade in the Pliocene [46].
A. bloyeti was described from Kandôa, a locality of the Great Ruaha system in Tanzania [44]. It belongs to a poorly understood complex of East African species that needs revision [20,37]. The type of A. bloyeti has a total length of 100 mm and a dorsal fin with 16 spines and 8 soft rays, an anal fin with 3 spines and 7 soft rays, 28 lateral line scales, a total length/body depth ratio of 3.75, and a head length/eye diameter ratio of 3.5 [44]. All these values differ markedly from those of our specimens from Lake Boukou. In addition, this species has true ocelli on the anal fin of males [20], and other head, body and fins colours of males also differ from those of our specimens (see colour pictures of A. bloyeti from Tanzania in Froese and Pauly [50]). Furthermore, its pharyngeal dentition has no molariform crown in the central series [20].
Astatotilapia flaviijosephi (Lortet, 1883), the only haplochromine species distributed out of Africa, is known from the Jordan basin (Syria, Israel, Palestine and Jordan). This species differs from A. tchadensis sp. nov. by several characters, including a dorsal fin with 15 spines and 8 soft rays, an anal fin with 3 spines and 7 soft rays, and 26–28 lateral line scales [51]. Furthermore, its colour pattern is different, with true ocelli on the anal fin [50,51].
Among the few haplochromine species currently known from the Nile River, Lake Albert, Lake Edward or Lake George, and the 600 or more haplochromine species from Lake Victoria and Lake Kyoga [38,52,53], only H. wingati has been previously suspected to be also distributed in the Lake Chad basin [18,40], until a re-examination of the holotype together with additional specimens infirmed this view [43]. Both meristic and morphometric characters allow one to distinguish A. tchadensis sp. nov. from H. wingati and the few taxa of the A. bloyeti species complex known from the basin of the Nile River [43,52]. Furthermore, none of these species nor other Astatotilapia species from tropical Africa have the pharyngeal dentition that characterizes A. tchadensis sp. nov. According to Greenwood [20], only A. flaviijosephi from the Jordan basin develops enlarged teeth with molariform crowns in the central series.
Colour and morphological differences between A. tchadensis sp. nov, A. desfontainii, A. flaviijosephi, and A. bloyeti species complex, together with current knowledge about the phylogeography and explosive speciation of haplochromine cichlid fishes, suggest that these species belong to distinct lineages [21,46,54]. Further molecular studies are needed to establish when West African populations diverged and to investigate relationships between the populations of Lake Boukou, of the Lake Chad basin, and of the Niger River basin.
4.2 Ounianga Serir lakes biodiversity
The Ounianga Serir lakes form a complex hydrological system with no equivalent in the Sahara and other deserts on Earth [33,35]. This system, which maintains perennial freshwater lakes despite dramatic levels of evaporation, is the results of a series of factors including a vast groundwater fossil reserve, the occurrence of Aeolian sand dunes alimented by dominant northeastern winds functioning as permeable dams behind which freshwater rising from the surrounding aquifer can accumulate, and extreme evaporation driving the central evaporating pump represented by the lowest hypersaline lake that concentrates salts issued from other lakes. In addition, the presence of freshwater has permitted the development of rich aquatic vegetation—covering approximately two-thirds of the surface of Lake Boukou—which reduce evaporation in freshwater lakes. These lakes constitute an exceptional natural landscape inscribed on the UNESCO world heritage list in 2012. The climatic history of the region during the past 6000 years has been recently investigated through a sediment analysis carried out in Lake Yoa at Ounianga Kebir [1].
The occurrence of fishes in Ounianga Serir lakes was first discovered during the Tilho expedition to Tibesti [6]. Specimens of two species were collected during this expedition: S. g. borkuanus and H. bimaculatus. Recently, three additional species were discovered in Lake Boukou: P. senegalus and P. normani, two species never reported before in the Sahara desert, and T. zilli, a species also known from several Saharan bodies of water [4]. With A. tchadensis sp. nov, six species are currently known from Ounianga Serir lakes. Such diversity in fish species is exceptional for the Sahara desert. In most cases, only one or two species are known in the rare perennial body of waters where fishes have survived since the last humid period during the Holocene [2,3]. In Mauritania, a maximum of four species have survived in Molomhar guelta in the Adrar mountains: B. macrops, Barbus pobeguini, Sarotherodon galilaleus, and Clarias anguillaris [4,55]. In Algeria, three species have been reported from Arak wadi in Mouydir mountains: Barbus deserti (or B. macrops according to map in Lévêque [2]), T. zilli, and A. desfontainii [49]. In Libya, two species are known near Ghat: Barbus deserti and Clarias gariepinus [47]. In western Sahara (Morocco), only Tilapia guineensis has survived in Imlily sebkhra [56], and no relict fish species exists in the Saharan bodies of water of Niger and Mali. In Chad, only Totous guelta in Tibesti mountains present high fish diversity, with six species reported: Labeo niloticus, Labeo parvus, B. macrops, B. bynni occidentalis, T. zilli, and C. gariepinus [6,10]. In other perennial bodies of water in northern Chad, the number of fish species is ranging from one to three [4,6,10]. The relict fish fauna of northern Chad, which comprises 14 species belonging to six families, is clearly Afrotropical and most species are also known from current Lake Chad who extended over 340,000 km2 at the mid-Holocene humid period (≈ 6000 years ago), reaching 18°N in northern Chad [57], with probable connections to Ounianga lakes through rivers issued from the Tibesti mountains and additional lacustrine and fluviatile formations in this area [1,58]. Genetic studies would be useful to better investigate the origin and affinities of current relict species.
The relict fauna of Ounianga lakes also includes the only Saharan populations of the toad Amietophrynus regularis, which is abundant both in Ounianga Kebir and Ounianga Serir lakes, and the frog Phrynobatrachus latifrons, which is known only from Ounianga Serir lakes [29]. In addition, various mammals and numerous birds are permanent or temporary residents of Ounianga lakes and surrounding, a unique biodiversity hotspot in the Sahara. Our data for Chad are in agreement with recent biodiversity studies in Mauritania, which have shown that rock pools and other small pieces of water in the desert are disproportionately important for their tiny size and constitute local hotspots of biodiversity overlooked by global assessments [59]. In the case of Mauritania, despite representing only 0.00004% of the country, they contained with their immediate surroundings 32% of the vertebrate species of Mauritania and 78% of the country's endemics [59]. The perennial bodies of water appear crucial for long-term conservation of biodiversity in the Sahara desert.
Disclosure of interest
The author declares that he has no competing interest.
Acknowledgements
I thank Jean-François Trape, IRD Dakar, who organized the zoological surveys in northern Chad and collected the types of the new species. Two anonymous reviewers provided useful comments on the manuscript.


