1 Introduction
Nowadays, urban areas are in constant expansion (up to 1.2 million km2 between 2000 and 2030 [1]) and are now recognized as a major challenge for biodiversity conservation in the context of global changes [2]. Indeed, the expansion of urban areas may have numerous and significant consequences on the evolution and ecology of population, and more largely on ecosystems. The ecological definition of urban areas, as “large concentrations of people and industrial activity that consume more available energy and material than can be produced, and produce more wastes than can be assimilated within the relatively small areas they occupy” [3], places humans at a keystone position in this environment. Urban ecosystems encompass specific environments regarding parasitism, pollutants or photoperiod, but also disturbances due to the presence of people, their activities, and wishes. For example, urban authorities can decide to manage urban nature to deal with negative perceptions of urban citizens. These social and bio-physical constraints contribute to classify urban ecosystems as “novel ecosystems” and may impact on local biodiversity. However, the functioning of such ecosystem remains poorly known, especially in the context of biodiversity [4].
A recent worldwide study has shown that urbanization decreased avian species richness, but tends to retain native species [5]. Such finding has been confirmed by local studies [6,7] and highlighted that avian species richness and abundances of an urban area can be used as indicators of biodiversity loss when compared to rural environments. It could be interesting to use a similar approach to investigate how the variability of urban environment would affect biodiversity. Indeed, urban areas are heterogeneous environments including built-up areas, open and greenspaces [8]. Recently, it has been advanced that the presence of greenspaces inside the cities could constitute a refuge for avian species and would host a high number of avian species [9]. Therefore, we first hypothesized that the greenspaces of urban areas could constitute a refuge for avian biodiversity [10,11], and we predicted that the avian species richness and abundances would be more important in greenspaces than in built-up areas. However, our indicators of avian biodiversity (species richness and abundances) can be highly variable among seasons. In particular, during the winter season, birds are supposed to be less detected due to lower activity and presences (migratory species) [12]. Therefore, the winter season could hide the positive effect of greenspaces when using such indicators of avian biodiversity. Unfortunately, little is known about the impact of the seasonal variability of avian biodiversity and how it is altering the measurements of avian species richness and abundances [13–15]. We thus hypothesized that seasons may alter our measurements of avian biodiversity and may hide the positive effect of greenspaces in urban areas. If true, we can predict that the positive effect of greenspaces on species richness and abundances should be more pronounced during the breeding season than during the winter season.
In this study, we tested these predictions by sampling avian species in the urban tissue of Annaba (Algeria). We collected the presences (detected or not) and abundances of 28 avian species using the “Indice pontuel d’abondance” for 650 sites distributed in three different types of urban habitats (greenspaces, intermediate and built-up areas) during two different seasons (breeding and winter seasons).
2 Material and methods
2.1 Study area
Our study has been realized in the furthest northeast limit of Algeria, in Annaba (36°.30 N & 37°.03 N &7°.20 E & 8°.40 E, Fig. 1). This city is located at 600 km from the capital Algiers; it extends at about 80 km on the Mediterranean cost, it covers 1412 km2, the climate is typically Mediterranean, with an average annual temperature of 18 °C and an annual rainfall ranging from 650 to 1000 mm with a peak in winter and a deficit in summer. The massive Edough (highest altitude 850 m) borders the city to the north and the west, the Mediterranean Sea to the east, and the alluvial plain of the Seybouse River to the south [16].
Satellite map representing the study zone (From Belabed et al. [28]).
2.2 Data collection
The study was conducted from 13 February 2013 until 31 January 2014 in the urban tissue of Annaba's downtown, including all parks and greenspaces surrounded by urban sites, mainly the Christian cemetery, the course of the revolution, and the plot Alexis-Lambert (Ex-Lambert and place George-Ishaq). We collected the presence and abundances of avian species encountered by visual and audio detection.
We used the “Indice ponctuel d’abondance” (IPA) [17] adapted here to the particular case of urban birds that are more visually detectable than forest birds. The IPA consists, for an observer, in staying motionless for several minutes and recording all contacts with birds (audio and visual). The sites of enumeration have been chosen in order to avoid overlapping by maintaining a minimum distance of 100 m between two sites (without binoculars). The count was performed two times by a single observer (H.A.A) on 650 sites localized by GPS. The first one was realized at the beginning of spring and in summer (between 13 February 2013 and 21 August 2013), which includes sedentary species and breeding migratory ones (breeding season). The second was made later in the season (between 15 September 2013 and 31 January 2014) out of the breeding season (winter season). This binomial temporal classification was done in order to maximize the number of sampled sites. Indeed, the data collection on one site was done twice a day for the two seasons: early in the morning (6:00 GMT + 1 to 8:00 GMT + 1) and before the sunset (Approximately 17:00 GMT + 1 to 19:00 GMT + 1). Each observation lasts 15 minutes and consists of recording all birds’ individuals seen or heard. In this context, it was not possible to restrict our sampling during two well-separated temporal classes, such as April–June (breeding season) versus November–January (wintering season). The 650 sites of collection were distributed into three different types of urban areas varying by the intensity of the built-up structure: greenspace, built-up and intermediate. Greenspace habitats are parks, cemeteries, and wooded greenspaces in downtown. Built-up habitats are sites where the building is predominant without greenspaces, and without trees. The intermediate habitats are the sites where the presence of trees and greenery was noted, but with the built nearby [18]. From these data collection, we computed for each site, the presence or absence and the abundances of 28 species during breeding and winter season. We assumed that the non-detection of a species at one given site was due to the absence of this species. This assumption is quite strong for some species for which abundances are low, but allows us to examine how urban environments affect this index of presence of each species. We also computed the species richness for each site for the two seasons, which correspond to the number of species detected.
2.3 Statistical analyses
We investigated the influence of the environment (greenspaces, intermediate and built-up areas) and season (breeding or winter season) on the presence, abundance, and species richness of the avian community on the 650 sample sites by using GLM method. The goal of our analyses was not to obtain an absolute value of abundances and species richness, but rather to draw inference on the factors affecting the relative species abundances and species richness. We therefore used the GLM approach as suggested by [19]. All statistical analyses were performed using SAS (version 9.4). First, the presence was estimated as 0 (no individual of the species was detected) versus 1 (at least one individual of the species was recorded). We used a mixed model with binomial distribution (logit link function, proc glimmix) with the presence as the dependent variable and the species, the environment and the season, plus their interactions as explanatory variables. We included the site nested within the environment as a random factor to take pseudoreplication (the paired nature of our data collection) into account. As our sample sites are close from each other (less than 100 m), we needed to take into account for a potential non-independence of the presences and abundances among the closed sites. To do it, we added latitude and longitude information to the random statement of the model [20] to take into account the spatial autocorrelation among our sampling sites. Non-significant interactions were removed step by step to obtain the final model. Then, a similar mixed model was run on the abundance with the Poisson distribution. The abundance was estimated as the number of individuals recorded when the species was present (i.e. when presence = 1). When the presence was null, the record was not included in this analysis. Finally, we run a mixed model (normal distribution) on the species richness (number of species detected on the sample sites). The species richness was the dependent variable and the season and the environment plus their interactions were the explanatory variables. Again, we included the sites nested within the environment as a random factor to take pseudoreplication into account and added latitude and longitude information to take the potential spatial autocorrelation into account.
3 Results
The presence of birds was found significantly affected by the interactions between species and season, and between species and environments (Table 1). We reran analyses within each one of the 28 species detected to examine how season and environments affected their presences. The results per species are provided in Table 2 and illustrated in Fig. 2. First, stock and common-wood pigeons were never detected in all sampled sites. For all detected species (n = 26) for which we evidenced an effect of the season, the presence was more pronounced during the breeding season than during the winter one (Table 2). The type of urban environment (greenspaces, intermediate or built-up) did not significantly impact the presence of common swift, western cattle egret, black-headed gull, African blue tit, northern house-martin, barn swallow, yellow-legged gull, and European starling (Table 2). In contrast, the presence of birds was positively linked to the degree of greenspaces for the European greenfinch, blue tits, European robin, common kestrel, spotted flycatcher, Eurasian great tit, black redstart, common chiffchaff, European serin, laughing dove, European turtle-dove, black cap and Eurasian blackbird (greenspaces > intermediate > built-up; Fig. 2). Moreover, the presence of birds was higher in intermediate and greenspace environments (intermediate = greenspace > built-up) for the chaffinch, the house sparrow and the common bulbul (Fig. 2). Interestingly, for the feral pigeon, the presence of birds was significantly lower in greenspaces than in intermediate and built-up environments, but was higher in intermediate than in built-up environment (intermediate > built-up > greenspaces). The Eurasian collared dove was the most spread species in our area of research (present in more than 80% of sampled sites). Its presence was higher in greenspaces than in intermediate and built-up environments (green spaces > intermediate = built spaces).
Output of the generalized mixed models explaining variations in the presence (binary distribution) and abundance (Poisson distribution) of birds according to the 28 considered species, the environment (greenspaces, intermediate or built-up areas), and the season (during the breeding or winter season). All interactions removed from the final model were non-significant.
| Effects | Presence | Abundance | ||||
| DF | F | P | DF | F | P | |
| Species | 27,35641 | 8.01 × 1029 | < 0.0001 | 25,3536 | 68.18 | < 0.0001 |
| Environment | 2,647 | 0.00 | 0.99 | 2,647 | 1.13 | 0.32 |
| Season | 1,35641 | 0.02 | 0.88 | 1,3536 | 0.46 | 0.50 |
| Species × season | 22,35641 | 6.48 | < 0.0001 | 21,3536 | 3.47 | < 0.0001 |
| Species × environment | 50,35641 | 15.69 | < 0.0001 | 45,3536 | 6.69 | < 0.0001 |
Output of the mixed models explaining the variations in the presence (binary distribution) and abundance (Poisson distribution) of birds by species according to the urban environment (green spaces, intermediate or built-up areas) and the season (during the breeding or winter season). NA indicates that statistics were not available due to the total absence of the species or due to its absence only in one season. NC indicates that the models did not converge due to the weak presence of the species. For these rare species for which models did not converge, we reran models with the site as a fixed effect to favor convergence. In these cases, neither the environment nor the season was found significant.
| Species | Latine name | Presence | Abundance |
| Common swift | Apus apus | Env: NC Season: NC |
Env: F2,26 = 0.50; P = 0.61 Season: NA |
| Western cattle egret | Bubulcus ibis | Env: NC Season: NC |
Env: F1,7 = 0.01; P = 0.91 Season: NA |
| European greenfinch | Chloris chloris | Env: F2,647 = 40.79; P < 0.0001 Season: F1,649 = 12.84; P = 0.0004 |
Env: F2,67 = 0.19; P = 0.83 Season: F1,11 = 0.03; P = 0.87 |
| Black-headed gull | Chroicocephalus ridibundus | Env: NC Season: NC |
Env: F1,34 = 2.12; P = 0.15 Season: F1,18 = 2.37; P = 0.14 |
| Feral pigeon | Columba livia | Env: F2,647 = 28.95; P < 0.0001 Season: F1,649 = 0.97; P = 0.32 |
Env: F2,539 = 69.32; P < 0.0001 Season: F1,466 = 150.48; P < 0.0001 |
| Stock pigeon | Columba oenas | Env: NA Season: NA |
Env: NA Season: NA |
| Common wood-pigeon | Columba palumbus | Env: NA Season: NA |
Env: NA Season: NA |
| Blue tit | Cyanistes caeruleus | Env: F2,647 = 32.12; P < 0.0001 Season: F1,649 = 21.96; P < 0.0001 |
Env: F2,52 = 0.40; P = 0.67 Season: F1,2 = 0.78; P = 0.47 |
| African blue tit | Cyanistes teneriffae | Env: NC Season: NC |
Env: F1,28 = 0.03; P = 0.87 Season: F1,5 = 0.01; P = 0.94 |
| Northern house-martin | Delichon urbicum | Env: NC Season: NC |
Env: F1,28 = 1.05; P = 0.31 Season: F1,3 = 1.63; P = 0.29 |
| European robin | Erithacus rubecula | Env: F2,647 = 25.42; P < 0.0001 Season: F1,649 = 15.02; P = 0.0001 |
Env: F2,38 = 0.24; P = 0.78 Season: NA |
| Common kestrel | Falco tinnunculus | Env: F2,647 = 16.86; P < 0.0001 Season: F1,649 = 11.74; P = 0.0007 |
Env: F2,45 = 0.14; P = 0.87 Season: F1,6 = 0.08; P = 0.78 |
| Chaffinch | Fringilla coelebs | Env: F2,647 = 41.66; P < 0.0001 Season: F1,649 = 19.14; P < 0.0001 |
Env: F2,79 = 0.83; P = 0.44 Season: F1,17 = 1.67; P = 0.21 |
| Barn swallow | Hirundo rustica | Env: NC Season: NC |
Env: F2,39 = 4.99; P = 0.01 Season: NA |
| Yellow-legged gull | Larus michahellis | Env: NC Season: NC |
Env: F1,31 = 0.66; P = 0.42 Season: F1,11 = 1.35; P = 0.27 |
| Spotted flycatcher | Muscicapa striata | Env: F2,647 = 50.92; P < 0.0001 Season: F1,649 = 42,46; P < 0.0001 |
Env: F2,81 = 0.77; P = 0.47 Season: F1,7 = 2.17; P = 0.18 |
| Eurasian great tit | Parus major | Env: F2,647 = 32.63; P < 0.0001 Season: F1,649 = 29.78; P < 0.0001 |
Env: F2,50 = 0.57; P = 0.57 Season: F1,6 = 0.48; P = 0.51 |
| House sparrow | Passer domesticus | Env: F2,647 = 11.06; P < 0.0001 Season: F1,649 = 0.91; P = 0.34 |
Env: F2,334 = 22.86; P < 0.0001 Season: F1,233 = 18.69; P < 0.0001 |
| Black redstart | Phoenicurus ochruros | Env: F2,647 = 31.89; P < 0.0001 Season: F1,649 = 18.93; P < 0.0001 |
Env: F2,79 = 0.39; P = 0.68 Season: F1,2 = 2.95; P = 0.23 |
| Common chiffchaff | Phylloscopus collybita | Env: F2,647 = 34.80; P < 0.0001 Season: F1,649 = 21.98; P < 0.0001 |
Env: F2,65 = 0.92; P = 0.40 Season: F1,13 = 4.48; P = 0.05 |
| Common bulbul | Pycnonotus barbatus | Env: F2,647 = 22.03; P < 0.0001 Season: F1,649 = 19.00; P < 0.0001 |
Env: F2,81 = 0.02; P = 0.98 Season: F1,10 = 0.07; P = 0.80 |
| European serin | Serinus serinus | Env: F2,647 = 47.78; P < 0.0001 Season: F1,649 = 4.51; P = 0.03 |
Env: F2,57 = 1.16; P = 0.32 Season: F1,11 = 1.20; P = 0.30 |
| Laughing dove | Spilopelia senegalensis | Env: F2,647 = 25.50; P < 0.0001 Season: F1,649 = 1.08; P = 0.30 |
Env: F2,39 = 2.10; P = 0.14 Season: F1,14 = 2.82; P = 0.12 |
| Eurasian collared-dove | Streptopelia decaocto | Env: F2,647 = 3.19; P = 0.04 Season: F1,649 = 0.24; P = 0.63 |
Env: F2,618 = 73.17; P < 0.0001 Season: F1,594 = 114.24; P < 0.0001 |
| European turtle-dove | Streptopelia turtur | Env: F2,647 = 14.56; P < 0.0001 Season: F1,649 = 1.44; P = 0.23 |
Env: F2,19 = 2.96; P = 0.08 Season: F1,9 = 5.11; P = 0.05 |
| European starling | Sturnus vulgaris | Env: NC Season: NC |
Env: F2,22 = 1.75; P = 0.20 Season: NA |
| Blackcap | Sylvia atricapilla | Env: F2,647 = 52.31; P < 0.0001 Season: F1,649 = 21,75; P < 0.0001 |
Env: F2,86 = 1.41; P = 0.25 Season: F1,15 = 3.37; P = 0.09 |
| Eurasian blackbird | Turdus merula | Env: F2,647 = 60.25; P < 0.0001 Season: F1,649 = 6.76; P = 0.01 |
Env: F2,117 = 14.05; P < 0.0001 Season: F1,69 = 7.30; P = 0.009 |
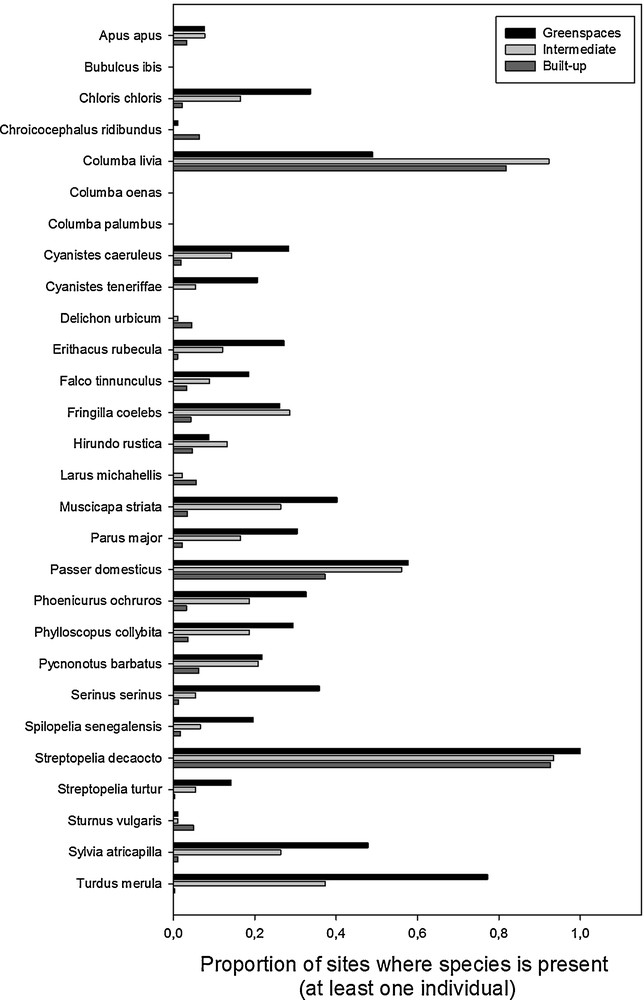
Proportion of presence of the 28 different species during the breeding season from the different environments (greenspaces, intermediate, and built-up areas). While proportions were smaller, the patterns of proportion among environments were similar during the winter season and therefore are not shown.
The abundance of birds was found significantly affected by the interactions between species and environments and between species and season (Table 1). Results per species are provided in Table 2 and show that the feral pigeon, when present, is significantly more abundant in intermediate environment than in greenspaces, but more abundant in greenspaces than in built-up environment (intermediate > greenspace > built-up). Moreover, the abundance of the barn swallow and the Eurasian blackbird was negatively linked to urbanization (greenspaces > intermediate > built-up). For the house sparrow and the Eurasian collared dove, the abundances were lower in built-up environment than in greenspaces and intermediate environments (intermediate = greenspace > built-up). When present, the abundance of the European turtle-dove tended to be positively linked with urbanization (built-up > intermediate > greenspaces; marginally non-significant, Table 2). For the other species, the environment did not affect the abundances (Table 2).
The avian species richness (number of species seen at least once on the sample site) was found significantly affected by the interactions between season and environment (F2,647 = 107.6, P < 0.0001). This interaction was driven by a more pronounced difference in species richness among environments during the breeding seasons than during the winter season (Fig. 3). Overall, species richness was higher during the breeding season than during the winter season (F1,647 = 489.1, P < 0.0001), and was higher in greenspaces than in intermediate and built-up environments (F2,647 = 372.3, P < 0.0001; Fig. 3).
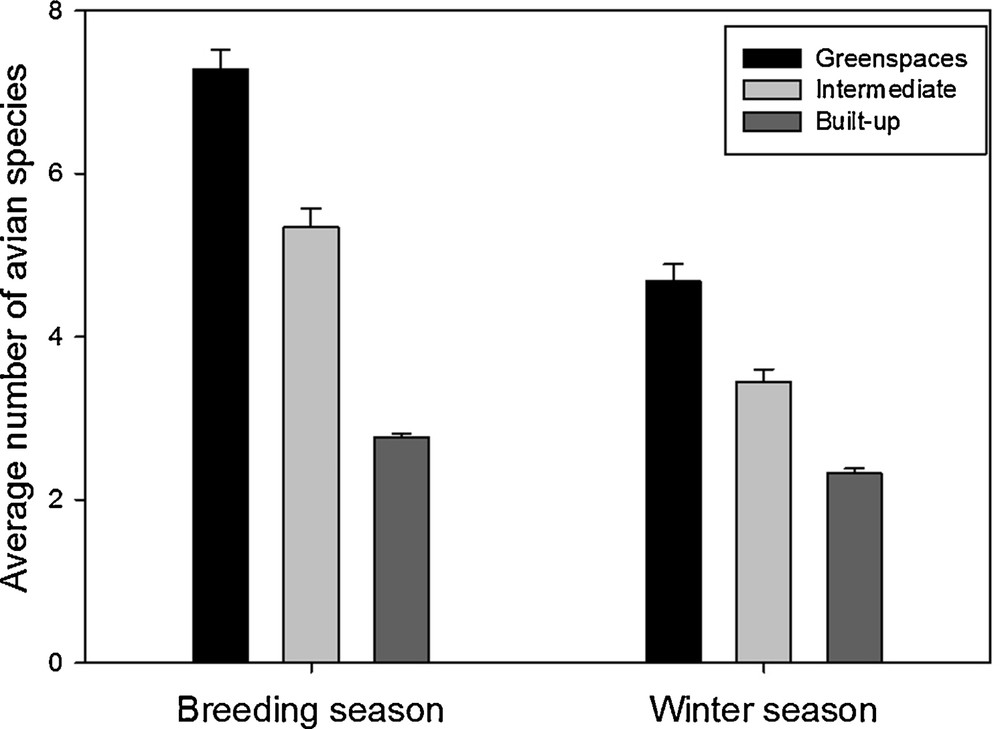
Average number of avian species (±se) from the different environments (greenspaces, intermediate, and built-up areas) during the breeding season and the winter season.
4 Discussion
As predicted, the presence of greenspaces significantly increases avian species richness (Fig. 3). This trend was observed for most avian species detected in Annaba's agglomeration (Fig. 2). However, the presence of the common swift, western cattle egret, black-headed gull, African blue tit, northern house-martin, barn swallow, yellow-legged gull, and the European starling seems to be unaffected by the presence of greenspaces in urban tissue or we did not have the statistical power to do it because such species are relatively rare. Unsurprisingly, the presence of the feral pigeon was higher in built-up areas, but its abundance was higher in intermediate environment. This species is emblematic of urban areas, as it is present in all cities all over the world [5], and some studies start to understand why this species is well acclimated or adapted to urban environments [21]. The abundances display the same pattern as the presence of avian species. Overall, our study in a Northern African city reinforces the idea that greenspaces significantly increase species richness [11,22], even if a minority of species is more detected in towns. This increase can be due to the characteristics of species that are adapted to their original environment and still failed to adapt or acclimate to built-up environment within the urban environment, which imposes sometimes very contrasted constraints as compared to the original one [10]. Alternatively, this increase in biodiversity may be the result of a better detection of avian species in open areas such as greenspaces. Future studies should try to understand the different patterns observed among towns all over the world and the role and the shape of greenspaces on biodiversity. Indeed, the size of the greenspaces could be a crucial factor for avian diversity and abundances [23] and, therefore need, to be experimentally investigated in future studies.
One species, the collared dove, is particularly interesting, because it is the predominant species in Annaba's agglomeration, whatever the environment. Indeed, even if it is more present in greenspaces, it is also largely present in urban environments (found in more than 85% of urban sites), with important abundances. Therefore, this species seems to be relatively poorly affected by the built-up environment. This result on the collared dove confirmed the results found in a previous study in Algeria [24], which suggests that this species exhibits an extraordinary hardiness and adaptability. This allows it to cope with all environmental constraints, and colonize all habitats in Southern Europe and Northern Africa. In this latter region, it constitutes a serious competitor of feral pigeon in urban areas. The reasons for its rapid expansion in Algeria are poorly known [24,25]. It is possible that the populations of such species are generalist and have undergone some phenotypic adaptations to cope with the heterogeneous environment. In order to better understand the success of this species in cities, it is now needed to investigate the phenotypic diversity and the population dynamic of this species in northern Africa [26–28].
As predicted, the season of data collection is an important factor to consider when investigating the impact of the avian environment on avian diversity. Indeed, our results show that species richness in one site is lower in the winter season as compared to the breeding season (Table 2 and Fig. 3). This is certainly due to the presence of migratory birds that are not present in the site during the winter season, but also to an increase in local movements during the breeding season, which may increase species detection [29,30]. In our case, even if the difference between environments is more important in the breeding season (Fig. 3), we found a similar pattern on species richness among environments (greenspaces, intermediate and built-up) for the winter and breeding seasons. Therefore, even in winter, we were able to observe that built-up areas have less species in lower abundances than intermediate and greenspaces environments.
In conclusion, our work reports for the first time, in a Northern African city, the importance of greenspaces within urban tissues for avian biodiversity. Indeed, our work first confirmed that greenspaces within urban areas significantly increase species richness and abundance of birds. Second, it shows that the season can profoundly affect species detection, and our study underlines the need for collecting data during the breeding season to increase the statistical power to detect environmental effect on such indicators of avian biodiversity.
Acknowledgement
This work was supported by a grant from the University Badji-Mokhtar, Annaba (Algeria).


