1 Introduction
Large-eye seabreams of the genus Gymnocranius Klunzinger 1870 occur in the tropical and warm-temperate coastal waters of the Indo-West Pacific region. They dwell over sandy bottoms at or in the vicinity of coral reefs, at depths ranging from 1 m to about 80 m [1–3]. The genus Gymnocranius, together with the genera Gnathodentex Bleeker 1873, Wattsia Chan and Chilvers 1974, and Monotaxis [Bennett] 1830 form the Monotaxinae, which is one of the two subfamilies of the perciform family Lethrinidae [1]. The genus Gymnocranius currently comprises 10 valid species described between 1830 and as recently as 2013 [4]. These are: G. audleyi Ogilby 1916 [5], G. elongatus Senta 1973 [6], G. euanus (Günther 1879) [7], G. frenatus Bleeker 1873 [8], G. grandoculis (Valenciennes 1830) [9], G. griseus (Temminck and Schlegel 1843) [10], G. microdon (Bleeker 1851) [11], G. oblongus Borsa, Béarez and Chen 2010 [2], G. satoi Borsa, Béarez, Paijo and Chen 2013 [3], and G. superciliosus Borsa, Béarez, Paijo and Chen 2013 [3]. Twelve other species that have been described since 1830 have been recognized as junior synonyms of the foregoing [4]. In addition, G. olivaceus Fourmanoir 1961 [12], is recognized as a junior synonym of Wattsia mossambica (Smith 1957) [1,13]. Sparus ornatus Sevastianoff 1805 [14] has lost its precedence to its junior synonym G. grandoculis because the species name was not used after 1899 (Article 23.9 of the International Code of Zoological Nomenclature [15]).
Large-eye seabreams are sold at local fish markets throughout the tropical and warm-temperate Indo-West Pacific ([1]; authors’ personal observations). Specimens suspected to possibly represent two unknown Gymnocranius species were first noticed at the Kedonganan fish market in Bali in February 2007 (Supplementary Figs. S1A, B and S3A). Provisional species names Gymnocranius sp. D and sp. E were given [3,16]. These two species were subsequently observed by WJC, along with other Gymnocranius species, at several local fish markets in eastern and southern Taiwan and on Penghu Islands in the Taiwan Strait during a biodiversity survey of lethrinid fishes in Taiwanese waters [16]. The two species differed by their patterns of dark bars on the flank and by the colouration of the margin of the caudal fin, which is reddish in Gymnocranius sp. D and yellowish in Gymnocranius sp. E [16]. Other specimens of these two species were simultaneously collected from the Ryukyu Islands by RM, who reached the same conclusions. Two of us (PB, WJC) initially intended to describe Gymnocranius sp. D together with Gymnocranius sp. E. However, as a paper by RM describing Gymnocranius sp. D was already in preparation, all three present co-authors agreed to separately describe the other species. The objective of the present paper is thus to describe Gymnocranius sp. E as a new species based on morphological and molecular diagnoses.
2 Materials and methods
2.1 Material examined
Specimens chosen as type material for the new species, Gymnocranius obesus sp. nov., were deposited at the Division of Fisheries Science, University of Miyazaki (MUFS) and at the National Taiwan University Museums, Taipei (NTUM) collections. Our specimen Let1006 collected from Fugang fishing port (22°47′30″N 121°11′31″E), Taiwan, 03 October 2013 (Fig. 1), catalogued No. NTUM 12079, was selected as the holotype. Seven other specimens were designated as paratypes: MUFS 25522 from Yaku-shima, Ryukyu islands, Japan, 11 August 2008; MUFS 41271 and MUFS 41272 from Okinawa Island, Japan, 27 October 2012; and NTUM 10766 including four individuals with sample Nos. Let998, Let999, Let1004, and Let1005 from Fugang fishing port, Taiwan, 03 October 2013 (Fig. 1). Additional voucher specimens are listed in Supplementary Table S1 and Supplementary Fig. S1.

Type series of Gymnocranius obesus sp. nov. A. Specimen NTUM 12079 (sample Let1006), holotype, standard length (SL) 270 mm, collected 3 October 2013 from off Fugang, Taitung, Taiwan (photographed by WJC). B. Specimen MUFS 25522, paratype, SL 295 mm, from off Anbo, Yaku-shima Island, Kagoshima prefecture, Japan (30°19′N, 130°39′E), 11 August 2008 (photographed by the MUFS fish team). C. Specimen MUFS 41271, paratype, SL 209 mm, purchased at Tomari-Fish Market (26°14′N, 127°41′E), Okinawa Island, Japan, 27 October 2012 (photographed by RM). D. Specimen MUFS 41272, paratype, SL 244 mm, purchased at the same place and same date (photographed by RM). E. Specimen NTUM 10766 (Let998), paratype, SL195 mm, from Fugang fishing port, Taiwan, 03 October 2013 (photographed by WJC). F. Specimen NTUM 10766 (Let999), paratype, SL220 mm, from Fugang fishing port, Taiwan, 03 October 2013 (photographed by WJC). G. Specimen NTUM 10766 (Let1004), paratype, SL192 mm, from Fugang fishing port, Taiwan, 03 October 2013 (photographed by WJC). H. Specimen NTUM 10766 (Let1005), paratype, SL239 mm, from Fugang fishing port, Taiwan, 03 October 2013 (photographed by WJC).
The comparative material used for morphological examination included: NTUM 10722 (G. griseus; one individual: Let734), Chenggong fishing port, Taiwan; NTUM 10768 (G. griseus; six individuals: Let1000, Let1001, Let1007-Let1010) from Fugang fishing port; NTUM 10808 (G. griseus: one individual: Let1168) from Fugang fishing port; NTUM 10818 (Gymnocranius sp. D: one individual, Let1211) from Penghu Islands; NTUM 10826 (Gymnocranius sp. D: one individual, Let1191) from Hengchun town; NTUM 10831 (Gymnocranius sp. D: one individual, Let1341) from Macclesfield Bank in the South China Sea (114°48′E 16°10′N); NTUM 10835 (Gymnocranius sp. D: two individuals, Let1362, Let1363) from Hengchun town; RMNHN.PISC.D.2248 (G. griseus, lectotype [17]) from the Nagasaki region [17]; RMNHN.PISC.1026 (G. griseus, paralectotype); and additional voucher specimens listed in Supplementary Figs. S2–S4.
Other type materials examined included ANSP 68291 (G. orbis, holotype), MNHN 0000-1317 (Pentapus curtus, holotype), MNHN 0000-8811 (Cantharus grandoculis, holotype), MNHN 2009-0009 (G. oblongus, holotype), MNHN 2009–0010 (G. superciliosus, holotype), MNHN 2011–0103 (G. satoi, holotype), RMNH 5680 (Dentex lethrinoides, holotype, and Lobotes microprion, syntypes), SAM 10037 (D. robinsoni, holotype), and SMF 3042 (D. rivulatus, holotype).
2.2 Measurements on specimens and identification to species
The specimens were photographed shortly after collection to record their fresh colour patterns. Standard length (SL), largest body depth (BD), body depth at origin of first dorsal fin, body depth at the origin of the first anal fin, and pre-dorsal, pre-pelvic and pre-anal lengths were measured to the nearest millimetre. Head length, snout length, eye diameter (ED), inter-orbital width (IOW) and median ray of the caudal fin length (MRC) were measured to the nearest tenth of millimetre using a vernier calliper. All the foregoing measurements followed [1]. Other measurements are listed in Table 1. Identification to species was done according to various complementary sources [1–3,18] or was assessed against the genetic library of Lethrinidae based in WJC's laboratory.
Measurements on the type material of Gymnocranius obesus sp. nov.
| Measurement | Specimen no. | |||||||
| NTUM 12079 (Let1006) |
MUFS 25522 | MUFS 41271 | MUFS 41272 | NTUM 10766 (Let998) | NTUM 10766 (Let999) | NTUM 10766 (Let1004) | NTUM 10766 (Let1005) | |
| Holotype | Paratype | Paratype | Paratype | Paratype | Paratype | Paratype | Paratype | |
| SL (mm) | 270 | 295 | 209 | 244 | 195 | 220 | 182 | 239 |
| Body depth (BD) (mm) | 120 | 132 | 91 | 108 | 87 | 98 | 78 | 106 |
| BD at origin of DF (mm) | 119 | 132 | 89 | 105 | 86 | 97 | 77 | 105 |
| BD at anal-fin origin (mm) | 108 | 120 | 85 | 100 | 81 | 95 | 74 | 95 |
| Head length (HL) (mm) | 87.5 | 104.0 | 76.6 | 87.1 | 61.6 | 72.0 | 61.5 | 78.8 |
| Snout length (mm) | 37.7 | 43.3 | 32.3 | 36.3 | 20.0 | 25.0 | 21.7 | 30.0 |
| Eye diameter (ED) (mm) | 32.1 | 33.4 | 24.9 | 28.1 | 23.7 | 26.4 | 23.8 | 31.5 |
| Inter-orbital width (IOW) (mm) | 32.0 | 36.9 | 24.1 | 28.8 | 23.1 | 27.2 | 20.0 | 28.5 |
| Predorsal length (mm) | 101 | 131 | 93 | 109 | 72 | 83 | 67 | 92 |
| Prepelvic length (mm) | 93 | 122 | 88 | 100 | 66 | 75 | 57 | 86 |
| Pre-anal length (mm) | 168 | 193 | 137 | 159 | 117 | 133 | 107 | 144 |
| Length of median ray of CF (MRC) (mm) | 31.5 | 35.4 | 26.4 | 29.8 | 24.8 | 24.8 | 21.7 | 26.7 |
| DF spines, rays | X, 10 | X, 10 | X, 10 | X, 10 | X, 10 | X, 10 | X, 10 | X, 10 |
| AF spines, rays | III, 10 | III, 10 | III, 10 | III, 10 | III, 10 | III, 10 | III, 10 | III, 10 |
| Pored scales on lateral line | 48 | 47 | 48 | 48 | 49 | 47 | 48 | 48 |
| Ratio of SL to BD | 2.25 | 2.23 | 2.30 | 2.25 | 2.25 | 2.24 | 2.32 | 2.26 |
| Ratio of SL to BD | 2.27 | 2.23 | 2.34 | 2.33 | 2.28 | 2.27 | 2.36 | 2.29 |
| Ratio of SL to HL | 3.09 | 2.83 | 2.73 | 2.80 | 3.17 | 3.06 | 2.96 | 3.03 |
| Ratio of ED to SL | 0.12 | 0.14 | 0.15 | 0.14 | 0.12 | 0.12 | 0.13 | 0.13 |
| Ratio of ED to HL | 0.37 | 0.39 | 0.40 | 0.39 | 0.38 | 0.37 | 0.39 | 0.40 |
| Ratio of ED to IOW | 1.00 | 1.10 | 1.27 | 1.18 | 1.03 | 0.97 | 1.19 | 1.11 |
| Ratio of ED to MRC | 1.02 | 1.15 | 1.16 | 1.14 | 0.96 | 1.06 | 1.10 | 1.18 |
The relative proportions of eye diameter and body depth of the lectotype of G. griseus (RMNHN.PISC.D.2248) were obtained from the sharp photograph reproduced in Supplementary Fig. S2A.
2.3 Genetics
A small piece of muscle was excised from the flank, preserved in 95% ethanol, and stored at −20 °C before genomic DNA extraction. Nucleotide sequences of the mitochondrial cytochrome b (cytb) and/or cytochrome c oxidase I (COI) genes from five G. obesus sp. nov. specimens including MUFS 25522 from Yaku-shima Island, Japan, MUFS 41271 and MUFS 41272 from Okinawa Island, Japan. NTUM 10766 from eastern Taiwan, and NTUM 12079 (holotype) from eastern Taiwan were generated according to the laboratory protocols and procedures of, respectively, [16] and [19]. The chromatograms were edited and assembled using the CodonCode Aligner v. 6.0.2 software (CodonCode Corporation, Dedham, MA, USA). The sequences were deposited in DDBJ (http://http://www.ddbj.nig.ac.jp/; accession Nos. LC213035 to LC213038) and in GenBank (http://www.ncbi.nlm.nih.gov/; accession Nos. KY593332 to KY593334) as a genetic reference for future DNA-identification and other research on large-eye seabreams. The cytochrome b gene sequences were aligned with homologous sequences from G. elongatus, G. grandoculis, G. griseus, G. oblongus, G. satoi, Gymnocranius sp. D, and G. obesus sp. nov. (GenBank accession Nos. AF381260, AF381275, AF381259, KU597061, KX357715, KX357714, and KX357713, respectively) published previously [16,20], and with those of G. superciliosus produced by [3]. The nucleotide sequences were compiled and aligned manually with Se–Al v. 2.0 [21] and MEGA6 [22]. The software PAUP* [23] was used to compute pairwise p distances, to visualize those nucleotides at the cytb locus that are diagnostic of different species, and to determine apomorphic nucleotide sites in G. obesus sp. nov. The COI-gene sequences assigned to the genus Gymnocranius that were available from the GenBank and BOLD (www.boldsystems.org) databases were compared against homologous sequences of reference G. obesus sp. nov. specimens. This screening was aimed at verifying whether any belonged to G. obesus sp. nov., for the purpose of documenting the species’ distribution (see next sub-section).
2.4 Distribution
The geographic distribution of G. obesus sp. nov. was deduced from four potential sources: (1) WJC and PB's database of large-eye seabream specimens, tissue samples, nucleotide sequences and photographs, comprising a total of 329 individuals from 12 locations in the Indian Ocean (in South Africa, Kenya, Îles Éparses, Mayotte, Reunion, Seychelles, Maldives, Aceh, western Sumatra, and northwestern Australia), 12 locations in the Coral Triangle (in Taiwan, the South China Sea, Malaysia, Vietnam, the Philippines, Indonesia, and West Papua), and nine locations in the western Pacific Ocean (in Japan, Papua New Guinea, New Caledonia, Fiji, the Marshall Islands, and French Polynesia); (2) additional information on its occurrence in the Japanese archipelago, which was presented (as “Gymnocranius sp. 1”) at the 2016 annual Asian Society of Ichthyologists meeting by RM and Y. Iwatsuki [24]; (3) GenBank; (4) BOLD.
2.5 Notice
The present article in portable document (.pdf) format is a published work in the sense of the International Code of Zoological Nomenclature [25] or Code and hence the new names contained herein are effectively published under the Code. This published work and the nomenclatural acts it contains have been registered in ZooBank (http://zoobank.org/), the online registration system for the International Commission on Zoological Nomenclature. The ZooBank life science identifier (LSID) for this publication is urn:lsid:zoobank.org:pub:9CEB9BF1-E635-45F0-AC92-FA7A1A10CAA9. The online version of this work is archived and available from the Comptes rendus Biologies (www.sciencedirect.com/science/journal/aip/16310691) and haL-IRD repository (http://www.hal.ird.fr/) websites.
3 Results and Discussion
Species in the genus Gymnocranius are all moderately large and of high commercial value [1–3,10,26]. Despite this, G. obesus sp. nov. has remained unnoticed in the ichthyological literature until recently [3,16,24]. A possible explanation for this oversight is its previous confusion with G. griseus [27,28]. Gymnocranius obesus sp. nov. has also been confused with juvenile or pre-adult G. grandoculis [28].
3.1 Morphological comparison of G. obesus sp. nov. with congeneric species
Measurements on Gymnocranius obesus sp. nov. specimens are provided in Table 1 and in Supplementary Table S1. Homologous measurements on reference specimens of G. griseus and Gymnocranius sp. D are provided in Supplementary Tables S2 and S3, respectively. Morphological features including body shape, caudal fin shape, and pigmentation patterns are here compared across 12 Gymnocranius species, including all 10 species currently recognized as valid, G. obesus sp. nov., and Gymnocranius sp. D (Table 3).
Summary of remarkable morphological features across species in the genus Gymnocranius.
| Species | Body shape | Shape of CF | Pigmentation patterns | ||||
| General | BD/SL | Head | Head | Flank | Fins | ||
| G. audleyi | High-bodied to oblong | 2.2–2.4 | Snout profile relatively steep | Moderately forked | Parietal scale patch blackish surrounded by brilliant white margin | With scattered brown flecks | Uniformly clear to slightly yellowish |
| G. elongatus | High-bodied to oblong | 2.2–2.4 | Snout somewhat pointed | Strongly forked | No blue ornamentation on snout or cheek | Fourth transversal dark bar running from 6th spine of DF to anterior portion of AF | Clear to yellow–orange; CF margin and tips often deep red |
| G. euanus | High-bodied to oblong | 2.4–2.5 | Sloping steeply | Moderately forked, inner edge of fork slightly convex, with blunt tips | Scattered blue dots on snout and cheek in some individuals | Scattered blackish scales mainly on anterior half of body | AF, CF, DF, PF clear to reddish |
| G. frenatus | High-bodied to oblong; dorsal side convex, ventral side horizontal | 2.3–2.4 | Dorsal profile sloping steeply | Moderately forked, inner edge of fork straight, with pointed tips | Obliquely ascending blue bands on snout and cheek | 5–7 narrow, irregular dark bars | Uniformly clear |
| G. grandoculis | High-bodied to oblong | 2.4–2.5 | Forehead profile moderately steep | Moderately forked with subtle middle notch | Series of narrow undulating, longitudinal lines on cheek and side of snout | Overall silvery with thin brown scale margins. In juveniles, fourth transversal dark bar running from 6th spine of DF to origin of AF | AF, DF, PF clear to yellow–orange; CF frequently dusky grey-brown |
| G. griseus | High-bodied to oblong | 1.9–2.3 | Dorsal and ventral profile of head evenly convex | Moderately forked | No blue ornamentation on snout or cheek | Third transversal dark bar from 1st dorsal spine to lateral line; 4th transversal dark bar from 6th dorsal spine to abdomen | Fins mainly clear to yellowish |
| G. microdon | Oblong | 2.5–3.0 | Forehead prominent | Forked with pointed tips | Blue dots or dashes on cheek | Silvery in adults | AF, DF, PF clear to yellow or reddish. CF sometimes dusky brown |
| G. obesus sp. nov. | High-bodied to oblong | 2.2–2.4 | Protruding, large eye | Moderately forked | No blue ornamentation on snout or cheek | Fourth transversal dark bar running from 6th spine of DF to origin of AF | AF, CF, DF drab with yellowish to yellow margins |
| G. oblongus | Fusiform | 2.6–2.8 | Forehead prominent | Elongate, tips rounded in adults | Sub-horizontal wavy blue lines or dashes on snout and cheek | Fifth transversal dark bar forward-descending | DF, PF, AF and CF drab, brownish or yellowish with reddish to vermilion edges |
| G. satoi | High-bodied to oblong | 2.4–2.5 | Forehead bumpy | Shallowly forked, lobes convex inside and their extremities rounded | Blue speckles against bronze background on snout and cheeks | Silvery | AF, CF, DF, PF reddish to bright vermilion red |
| G. superciliosus | Oblong to elongate | 2.7–3.1 | Forehead prominent | Moderately forked with a subtle middle notch, its lobes slightly convex inside | Snout and cheek with blue speckles | Silvery | AF, CF, DF, PF reddish to red |
| Gymnocranius sp. D | High-bodied to oblong, symmetric dorso-ventrally | 2.2–2.5 | Eye large | Moderately forked with a subtle middle notch | No blue ornamentation on snout or cheek | Fourth transversal dark bar descending from 6th spine of DF to anterior to anus | AF, CF, DF drab with orange to vermilion red edge |
Several species in the genus Gymnocranius, including G. frenatus, G. grandoculis, G. microdon, G. oblongus, G. satoi and G. superciliosus possess blue lines or dots on the snout and cheek. Scattered blue dots on snout and cheek are also present in some individuals of G. euanus, which also has a distinctive head shape (Table 3). The remaining five species, i.e. G. audleyi, G. elongatus, G. griseus, Gymnocranius sp. D and G. obesus sp. nov. do not possess blue ornamentation on snout and cheek. Gymnocranius audleyi has a distinctive parietal scale patch and G. elongatus has a distinctive swallow-like tail. The confusion of G. obesus sp. nov. with the juvenile or pre-adult G. grandoculis [28] was possibly due to the fact that the patterns of dark bars on the flank are similar between the two species, with the fourth dark bar running from the base of the sixth or seventh spine of dorsal fin to the origin of anal fin (Table 3). However, the body is more slender in G. grandoculis and the colour of the anal fin in the adult G. grandoculis is different (Table 3). Additional characters are needed for the morphological distinction of G. obesus sp. nov. from the remaining two species, namely G. griseus and Gymnocranius sp. D.
The lectotype of Dentex (= Gymnocranius) griseus (Supplementary Fig. S2A) had a ratio of SL to BD of 2.6; the eye diameter (ED) was 7.9% of SL. As pointed out previously by specialists of the family Lethrinidae [1,26], the exaggeratedly elongate shape of the lectotype may reflect distortion caused by the preparation of the dried specimen. Measurements on the drawing representing G. griseus in C.J. Temminck and H. Schlegel's original description (Plate 36 of [10]) were the following: ratio of SL to BD = 2.25; ratio of SL to HL = 3.4; ratio of ED to length of caudal fin's median ray (MRC) = 0.89. Neither the body shape of the presumably distorted lectotype of G. griseus nor that of the lithography accompanying the description can be considered as representative of the species. A particular morphological feature enabled us to distinguish G. obesus sp. nov. from G. griseus: the number of front scales on the top of head. We counted four to six front scales (Fig. 2) in G. obesus sp. nov., while nine to eleven were present in G. griseus, including the lectotype, which had nine (RM, pers. obs.; Supplementary Fig. S4). Last, G. obesus sp. nov. differed from G. griseus by the dark-bar patterns on flanks. While the fourth transversal dark bar in G. obesus sp. nov. ran from the basis of the sixth spine of the dorsal fin down to the origin of the anal fin, the one in G. griseus, when visible, ran down from the base of the sixth dorsal spine to the abdomen (Table 3).
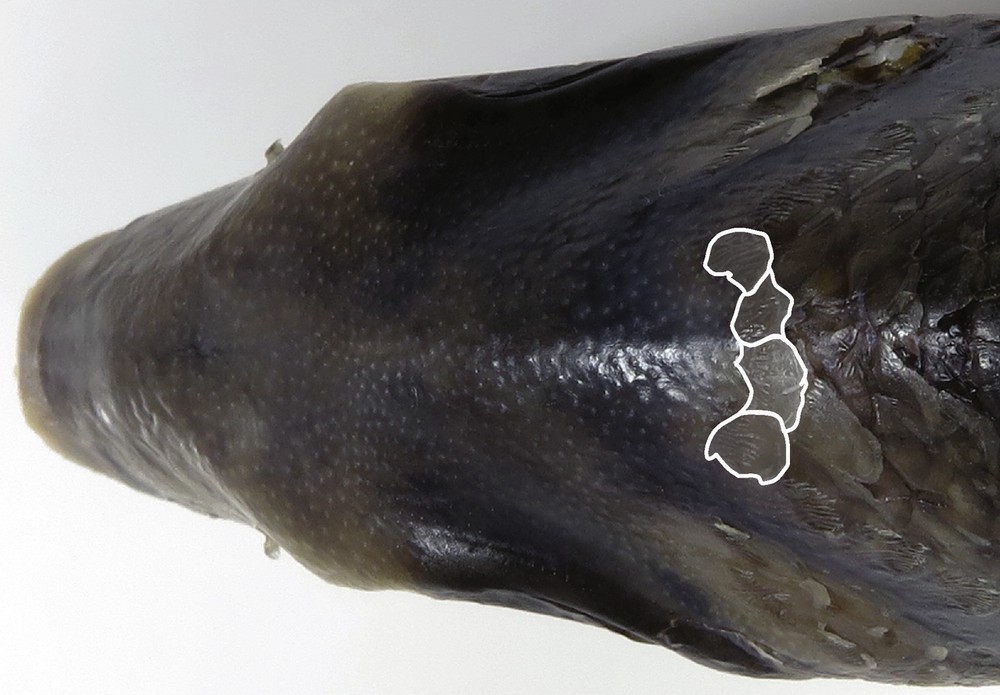
Gymnocranius obesus sp. nov. Dorsal view of head of specimen No. MUFS 41272 (paratype) with front row of scales highlighted in white (photographed by RM).
Gymnocranius obesus sp. nov. was also characterized by its remarkably large eye. The eye diameter (ED) was about equal to or larger than the IOW (ratio of ED to IOW = 1.0–1.3) and generally found to be larger than the MRC, with a ratio of ED to MRC = 1.0–1.2 (Table 1, Supplementary Table S1), while the eye diameters of both Gymnocranius sp. D and G. griseus were generally smaller than both the IOW and MRC (Supplementary Tables S2 and S3). Other body proportions did not clearly differ between G. obesus sp. nov., G. griseus, and Gymnocranius sp. D (Table 1; Supplementary Tables S1–S3): the ratio of SL to BD was 2.2–2.4 in G. obesus sp. nov., vs. 2.1–2.4 and 2.2–2.6 in G. griseus and Gymnocranius sp. D, respectively; the ratio of SL to HL was 3.0–3.2 vs. 3.2–3.5 and 3.1–3.3 in G. griseus and Gymnocranius sp. D, respectively; and the ratio of ED to MRC was 1.0 to 1.2 vs. 0.9–1.1 and 0.8–1.2 in G. griseus and Gymnocranius sp. D, respectively. The upper lip of G. obesus sp. nov. was yellow (Fig. 1; Supplementary Fig. S1); the upper lip in G. griseus was generally drab, with sometimes a yellowish or a reddish hue (Supplementary Fig. S2); the lower edge of the upper lip in Gymnocranius sp. D was vermilion red (Supplementary Fig. S3). Three other morphological features distinguished G. obesus sp. nov. from G. griseus: (1) G. griseus had relatively shorter head, with ratio of SL to HL = 3.2–3.5, compared to 2.7–3.2 in G. obesus sp. Nov; (2) unlike G. obesus sp. nov., the scales on the flank above the lateral line in G. griseus had a darker spot giving the appearance of longitudinal rows. G. obesus sp. nov. and Gymnocranius sp. D roughly had the same deep body shape, but differed by their patterns of dark bars on the flank [16,24] and by the colouration of the caudal fin, whose margin was reddish in Gymnocranius sp. D and yellowish in G. obesus sp. nov. [16].
In summary, G. obesus sp. nov. is distinct from G. griseus, Gymnocranius sp. D, and any other known species in the genus Gymnocranius by its scale counts and by its body, fin and scale colour patterns.
3.2 Genetic comparison of G. obesus sp. nov. with congeneric species
Gymnocranius obesus sp. nov. was differentiated from the seven other congeneric species mentioned in Table 2 by 9.6 to 13.5% nucleotide sequence divergence at the mitochondrial cytochrome b gene. In particular, nucleotide sequences confirmed that G. griseus, Gymnocranius sp. D and G. obesus sp. nov. belong to three distinct genetic lineages. From the sample of eight Gymnocranius species for which cytochrome-b gene sequences were available, the closest relative of G. obesus sp. nov. was Gymnocranius sp. D (Table 2). The genetic distance (p-distance) between the two species was 0.096. One hundred and four diagnostic nucleotide sites were scored between Gymnocranius sp. D and G. obesus sp. nov. Four single-nucleotide polymorphisms were found within G. obesus sp. nov. based on five specimens from Taiwan and Japan. The p-distance between G. obesus sp. nov. and G. griseus was 0.109. The two foregoing values were substantially higher than the genetic distance between G. satoi and G. superciliosus (p-distance = 0.082) or that between G. grandoculis and G. oblongus (p-distance = 0.087) (Table 2). The latter four species being reproductively isolated from one another [29], it is sensible to assume reproductive isolation between G. griseus, Gymnocranius sp. D, and G. obesus sp. nov.
Matrix of pairwise genetic distances (p-distance) at the cytochrome b locus, deduced from the nucleotide sequences sampled in 8 species of the genus Gymnocranius.
| Sample | Sample | ||||||||||||
| No. | Species | GenBank | Reference | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| 1 | G. elongatus | AF381260 | [20] | – | |||||||||
| 2 | G. grandoculis | AF381275 | [20] | 0.158 | – | ||||||||
| 3 | G. griseus | AF381259 | [20] | 0.137 | 0.091 | – | |||||||
| 4 | G. obesus sp. nov. | KX357713 | [16] | 0.135 | 0.125 | 0.109 | – | ||||||
| 5 | G. obesus sp. nov. | KY593333 | This study | 0.134 | 0.126 | 0.109 | 0.001 | – | |||||
| 6 | G. obesus sp. nov. | KY593334 | This study | 0.135 | 0.125 | 0.109 | 0.000 | 0.001 | – | ||||
| 7 | G. oblongus | KU597058 | [3] | 0.141 | 0.087 | 0.101 | 0.121 | 0.122 | 0.121 | – | |||
| 8 | G. satoi | KX357715 | [16] | 0.143 | 0.082 | 0.087 | 0.119 | 0.119 | 0.119 | 0.100 | – | ||
| 9 | G. superciliosus | – | [3] | 0.146 | 0.089 | 0.082 | 0.120 | 0.119 | 0.120 | 0.100 | 0.082 | – | |
| 10 | Gymnocranius sp. D | KX357714 | [16] | 0.140 | 0.124 | 0.107 | 0.096 | 0.096 | 0.096 | 0.125 | 0.119 | 0.112 | – |
Thus, we can now formally describe G. obesus sp. nov. A re-description of G. griseus and the formal description (or re-description) of Gymnocranius sp. D are pending taxonomic issues that will be addressed separately by RM.
3.3 Need for a comprehensive phylogeny of the Monotaxinae
It is common taxonomic practice to accompany new species descriptions with an identification key, in particular if former keys are obsolete. Existing identification keys in the Monotaxinae [1,26] are based on morphological characters with no reference to a phylogenetic hypothesis for the sub-family. The key proposed by [1] remains useful to identify most of the currently valid species in the Monotaxinae. The interested reader is able complete it using the information summarized in Table 3. The additional, specific information provided in this paper should further enable the interested reader to easily distinguish G. obesus sp. nov. from all other species, including, in particular, G. griseus and Gymnocranius sp. D.
Our position is that identification keys should be based on phylogenetically informative characters and that any revision of a taxon should be well grounded in a robust phylogenetic framework. An elaborate identification key of the sub-family Monotaxinae should be proposed at some stage, but not before the potential new species in Gymnocranius have been solved out and a comprehensive, robust phylogeny has been produced. A revision of the Monotaxinae currently is in preparation, which will be based on the phylogeny of all valid species including G. griseus once it has been re-described, G. obesus sp. nov., Gymnocranius sp. D, and four other cryptic lineages in the genera Gymnocranius and Monotaxis (WJC and PB, unpublished).
4 Taxonomy
Gymnocranius obesus sp. nov.http://zoobank.org/urn:lsid:zoobank.org:act:83F64FF2-41C9-47E8-B5B9-C284DC8F1AF5 (Table 1; Fig. 1A–H; Fig. 2; Supplementary Fig. S1). Previously referred to as Gymnocranius sp. E [3,16], Gymnocranius sp. 1 [24], G. griseus [27,28], and G. grandoculis [28].
4.1 Types
Holotype: NTUM 12079 (Fig. 1A). Paratypes: MUFS 25522 (Fig. 1B), MUFS 41271 (Fig. 1C), MUFS 41272 (Fig. 1D), and NTUM 10766 (four individuals; Fig. 1 E–H). See details in sub-section 2.1 and in legend to Fig. 1.
4.2 Description
The new species is described under Gymnocranius Klunzinger, 1870, because it has the following general characteristics of the species of this genus ([1]; Table 3): laterally compressed, ovate body; profile of the head in front of the eye convex, slope of the snout relatively steep; adult specimens often develop a bony ridge on the nape and a bony shelf above the anterior part of the eye; mouth small, posterior part of the jaw usually anterior to the level of the anterior edge of the eye; each jaw with two or three slender canines at the front, conical (molariform in G. euanus) teeth on the sides, and a range of numerous villiform teeth behind the front teeth; eye relatively large, a pair of close-set, round nasal openings on each side of the snout in front of the eyes, usually a thin flap of skin on the rear edge of the anterior opening; dorsal fin continuous with 10 spines and 10 (occasionally 11) soft rays; anal fin with 3 spines and 10 (occasionally 9) soft rays; pectoral fin rays 14; caudal fin strongly to moderately forked (moderately forked in G. obesus sp. nov.), usually with pointed or elongate tips (round in G. euanus and G. satoi); pored scales on lateral line 47 to 53 (48 in the holotype of G. obesus sp. nov.; 47 to 49 in paratypes and other voucher specimens); rear part of the cheek with three to five transverse scale rows (four in Gymnocranius obesus sp. nov.); remainder of the cheek, preorbital, snout, and inter-orbital region scaleless; inner surface of the pectoral fin base scaleless. Their body colour is generally silvery; the cheek region below the eye is often marked with either a dark bar (sometimes faint); the fins are clear to yellow or reddish (yellowish in Gymnocranius obesus sp. nov.). Freshly caught specimens, especially juveniles or small adults, often show a pattern of five to eight transversal dark bars [1].
Gymnocranius obesus sp. nov. specimens possess the following diagnostic combination of characters: body deep, 2.2–2.4 times in standard length (2.25 in holotype), apparently invariable across a range of sizes (Table 1), and plumpy (hence the species epithet); lower edge of the eye slightly above a line from tip of snout to middle of caudal fin; eyes protruding and large, eye diameter (ED) reaching 37–40% of head length (37% in holotype), usually close to or larger than inter-orbital width (IOW) with an ED/IOW ratio of 0.97–1.27 (1.00 in holotype) (Table 1); caudal fin moderately forked, the median rays nearly equal to or slightly shorter than ED; a row of four to six front scales on the dorsal side of the head (four in holotype) (Fig. 2); body sides with five to eight transversal dark bars often visible, even in adults (Fig. 1; Supplementary Fig. S1), the fourth one running from the base of the sixth or seventh spine of the dorsal fin to the origin of the anal fin (Fig. 1; Supplementary Fig. S1); anal, caudal and dorsal fins drab with yellowish to yellow margins, sometimes diffuse mottling or spotting on dorsal, pelvic, anal, and caudal fins (Fig. 1; Supplementary Fig. S1); no visible blue lines or spots on snout and cheek below eye in adults (SL > 270 mm) (Fig. 1; Supplementary Fig. S1); upper lip yellow (Fig. 1; Supplementary Fig. S1).
The nucleotide sequence of the COI gene of the holotype of G. obesus sp. nov. (GenBank KY593332) was: 5’- C T C T A T T T A G T A T T C G G T G C A T G A G C T G G G A T A G T A G G A A C C G C C C T A A G C C T T C T C A T C C G A G C G G A A C T T A G T C A A C C A G G C G C T C T C C T G G G G G A C G A C C A G A T T T A C A A T G T A A T C G T T A C A G C A C A C G C C T T C G T A A T G A T T T T C T T T A T A G T A A T A C C A A T T A T G A T C G G A G G C T T T G G A A A T T G A C T T A T C C C C C T A A T G A T C G G G G C C C C T G A C A T G G C A T T C C C T C G A A T G A A C A A C A T G A G C T T T T G A C T T C T C C C C C C T T C C T T C C T A C T G C T C C T A G C C T C C T C A G G C A T T G A A G C C G G A G C A G G T A C C G G A T G A A C A G T C T A C C C C C C A C T A G C T G G T A A C C T T G C T C A C G C T G G A G C A T C T G T T G A C T T A A C C A T T T T C T C C C T C C A C C T A G C T G G C A T C T C C T C G A T C C T G G G G G C T A T T A A T T T T A T T A C A A C C A T C A T C A A T A T A A A A C C C C C C G C C A T C T C T C A A T A C C A G A C C C C T C T T T T C G T T T G A G C A G T C C T A A T C A C T G C T G T C C T T C T C C T C C T T T C G C T G C C A G T C T T A G C C G C A G G C A T C A C A A T A C T C C T T A C G G A C C G A A A C T T A A A C A C A A C T T T C T T T G A C C C A G C A G G C G G G G G G G A C C C G A T T C T T -3’.
4.3 Diagnosis
Gymnocranius obesus sp. nov. differs from its morphologically close congeners G. griseus and Gymnocranius sp. D by the number of front scales on the top of the head, and by the dark-bar patterns on flanks. While the fourth transversal dark bar in G. obesus sp. nov. runs from the basis of the sixth spine of the dorsal fin down to the origin of the anal fin, the one in G. griseus, when visible, runs down from the base of the sixth dorsal spine to the abdomen (Table 3). That in Gymnocranius sp. D descends from the sixth dorsal spine to the extremity of abdomen, before the anus (Table 3).
Along the cytochrome b gene, the following apomorphic sites have nucleotides shared by all five specimens of G. obesus sp. nov. examined so far, that are not present in G. elongatus, G. euanus, G. grandoculis, G. griseus, G. oblongus, G. satoi, G. superciliosus and Gymnocranius sp. D: Nos. 61, 108, 117, 135, 153, 165, 300, 303, 318, 375, 501, 564, 627, 630, 684, 756, 765, 816, 967, 972, and 1032. These nucleotide sites can be used for the diagnosis of G. obesus sp. nov. relative to G. griseus and Gymnocranius sp. D.
4.4 Habitat and distribution
The type locality is Fugang on the eastern coast of Taiwan. We recorded G. obesus sp. nov. from fish landing places in Bali, the Flores Sea, the Ryukyu Islands and Taiwan (Fig. 3). The species was not found in the Coral Sea despite substantial sampling effort targeting Lethrinidae [2,3,29]. All records south of the Ryukyu Islands were within the western half of the Coral Triangle as defined by [30]. The single specimen sampled from the Flores Sea was caught by handline together with other lethrinids including Lethrinus erythropterus, L. rubrioperculatus and L. semicinctus which typically inhabit the first tens of metres on the reef slope and adjacent flat areas [1]. We compared eighty-eight nucleotide sequences at the COI locus referring to the genus Gymnocranius available from the GenBank and BOLD public databases, to the COI-gene sequence of the holotype of G. obesus sp. nov. The most closely related sequence (GenBank No. JF493563, labelled G. grandoculis) diverged by 7.3% nucleotide difference from it. Thus, at the time of writing (January 2017), there was no evidence of the presence of G. obesus sp. nov. in these two COI-gene sequence databases.
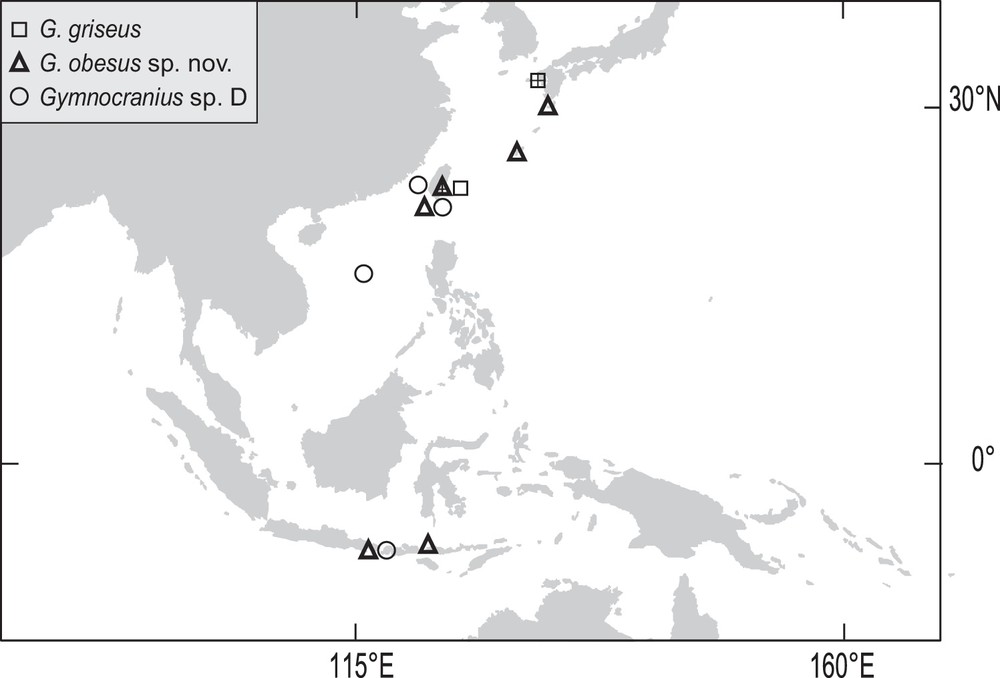
Map of Southeast Asia and northern Melanesia showing the occurrence points of the material examined for the present study (see Table 1, Fig. 1, and Supplementary Material) or reported previously [16], including: Gymnocranius griseus (squares), G. obesus sp. nov. (triangles) and Gymnocranius sp. D (circles). Crosses (+) indicate the type localities of G. griseus (Nagasaki, Japan) and G. obesus sp. nov. (Fugang, Taiwan).
4.5 Etymology
Epithet obesus is the Latin translation of obese, a reference to the deep and relatively thick body shape of the specimens of this species. We propose the obese large-eye seabream as vernacular name for G. obesus sp. nov.
4.6 Remarks
The type specimens of Lobotes microprion Bleeker 1851 [31] and Gymnocranius orbis Fowler 1938 [32], both with deep body shape, exhibit patterns of dark bars on the flank that have been reported to fit those of G. griseus [1]. Lobotes microprion has also been listed by [26] as a possible synonym of Dentex robinsoni Gilchrist and Thompson 1909 [33], now synonymized with G. grandoculis [1]. Another species with oblong to deep body shape is Pentapus curtus Guichenot 1863 [34] also considered to be a junior synonym of G. grandoculis [1] (but see [2,26]). Dentex lethrinoides Bleeker 1850 [35] from Java and D. rivulatus Rüppell 1838 [36] are also considered to be junior synonyms of G. grandoculis [1].
We found that the dark-bar patterns of the syntypes of L. microprion (RMNH 5680), albeit faint, and those of G. orbis as represented in drawing [32] look more alike those of G. frenatus than G. griseus [24]. Further, the two syntypes of L. microprion have a row of at least eight front scales on the dorsal side of the head and the holotype of G. orbis has a row of 11 (Supplementary Fig. S5). Gymnocranius obesus sp. nov. thus can be easily distinguished from the foregoing two species. From pictures of the holotype of Dentex robinsoni Gilchrist and Thompson 1909 (SAM 10037), we were able to count at least eight scales in the row of front scales on the dorsal side of the head (Supplementary Fig. S5). Similarly, we counted more than seven front scales on the holotype of P. curtus (Supplementary Fig. S5). We also counted eight scales in the row of front scales on the dorsal side of the head of the holotype of D. lethrinoides (Supplementary Fig. S5). Last, we confirm the synonymy of D. rivulatus Rüppell with G. grandoculis. Therefore, we can confidently exclude that G. obesus sp. nov. is synonymous with D. lethrinoides, D. rivulatus, D. robinsoni, G. orbis, L. microprion, or P. curtus.
Disclosure of interest
The authors declare that they have no competing interest.
Acknowledgments
This paper is a contribution of the joint IRD/NTU project on the phylogeography and systematics of emperors and large-eye seabreams, started in 2007. We express our sincere thanks to H. Motomura, T. Yoshida, K. Tashiro, Y. Fukui, and H. Hata (KAUM), Z. Gabsi (MNHN), K. Koeda (NMMB), and R. de Ruiter and M.J.P. van Oijen (RMNH) for facilitating RM's visits to collections. We thank Y. Sakurai (Okinawa, Japan), Mr Jhuo (Fugang fishing port), and H.-C. Ho (NMMB) for collecting and supplying some of the specimens. We thank G.R. Allen, P. Béarez, K.E. Carpenter, A. Collet, N. Hubert, J.-L. Justine, D. Ponton, M.J.P. van Oijen, and R.D. Ward for stimulating discussions in the course of our Lethrinid project. We also thank two anonymous reviewers for insightful comments that greatly helped improve our paper. We acknowledge the special contribution of F. Giancarlo and S. Bari (Proyek BioKor, Indonesia and West Papua). We thank M. Bougaardt and A. Bosman from the South African Museum, Cape Town and M.S. Pérez from the Academy of Natural Sciences of Drexel University, Philadelphia for providing photographs of Dentex robinsoni and Gymnocranius orbis. Issues of the Zoologische Mededeelingen were consulted from the Naturalis repository website (http://www.repository.naturalis.nl/). C.J. Temminck and H. Schlegel's Fauna Japonica was consulted from the Kyoto University Library website (http://kuline.kulib.kyoto-u.ac.jp/). Other 19th-century and early 20th-century books and articles were consulted from the Biodiversity Heritage Library website (http://www.biodiversitylibrary.org). Supported by a Sasakawa scientific research grant from the Japan Science Society (No. 27-506 to RM), by IRD-UMR 250, by the French “Ministère des Affaires étrangères” and by research grants from the Ministry of Science and Technology, Taiwan (MOST 101-2611-M-002-016-MY3 and MOST 104-2611-M-002-002-MY3 to WJC). Designed the study: PB, WJC, RM; contributed samples: PB, WJC, RM; contributed molecular and morphological data: WJC, RM; analysed and interpreted the data: PB, WJC, RM; wrote the paper: PB, WJC. All co-authors read, edited, and approved the final version of the manuscript.


