1 Introduction
The taxonomy of large-eye breams (Lethrinidae: Monotaxinae), which comprise large, carnivorous species of the Indo-West Pacific coral-reef lagoons and reef slopes, still awaits completion [1,2]. In his revision of the Monotaxinae, Sato [1] noted that specimens labelled “Gymnocranius griseus” (non Temminck and Schlegel 1844 [3]) by Coleman [4] and “G. lethrinoides” (non Bleeker 1850 [5]) by Masuda et al. [6] were actually misidentified and he referred to them as an undetermined species, “Gymnocranius sp.”. Sato's Gymnocranius sp. is a distinctive, bright-coloured, large-sized (up to 50 cm) fish of the reef-associated sandy habitats throughout the Coral Triangle and the western Pacific [1,2]. This fish is occasionally encountered on the stalls of fish markets in southern Japan, western West Papua and the tropical south-western Pacific islands ([2,6]; P. Borsa and S.P., pers. obs.). It is surprising that Sato's Gymnocranius sp. has remained undescribed, nearly three decades after it was first mentioned in the ichthyological literature.
A large-eye bream with reddish fins that resembles Sato's Gymnocranius sp. was first noticed (under “Gymnocranius sp.”) at the Nouméa fish market in August 2002 by P. Béarez [7] (Fig. 1A). This fish was slender than Gymnocranius sp. pictured in Masuda et al. [6] (as “G. lethrinoides”) and Carpenter and Allen [2], although its body proportions fitted Coleman's [4] “G. griseus” from the Great Barrier Reef. The same species was subsequently sampled from the northern and southern lagoons of New Caledonia's Grande Terre, the Chesterfield islands and Fiji, and proved genetically distinct from Sato's Gymnocranius sp. [8,9]. Inferred genotypes at four size-polymorphic intron loci indicated reproductive isolation between the two species in New Caledonia [9,10]. The two species were provisionally named Gymnocranius sp. B and Gymnocranius sp. C, respectively [9–12]. Gymnocranius sp. B and Gymnocranius sp. C were found to be genetically distinct from other Gymnocranius species from the Coral Sea and adjacent regions, including Gymnocranius elongatus Senta 1973 [13], Gymnocranius euanus (Günther 1879 [14]), Gymnocranius grandoculis (Valenciennes 1830 [15]) and the recently described Gymnocranius oblongus Borsa, Béarez and Chen 2010 [8–10], and reproductively isolated from the three latter [9].
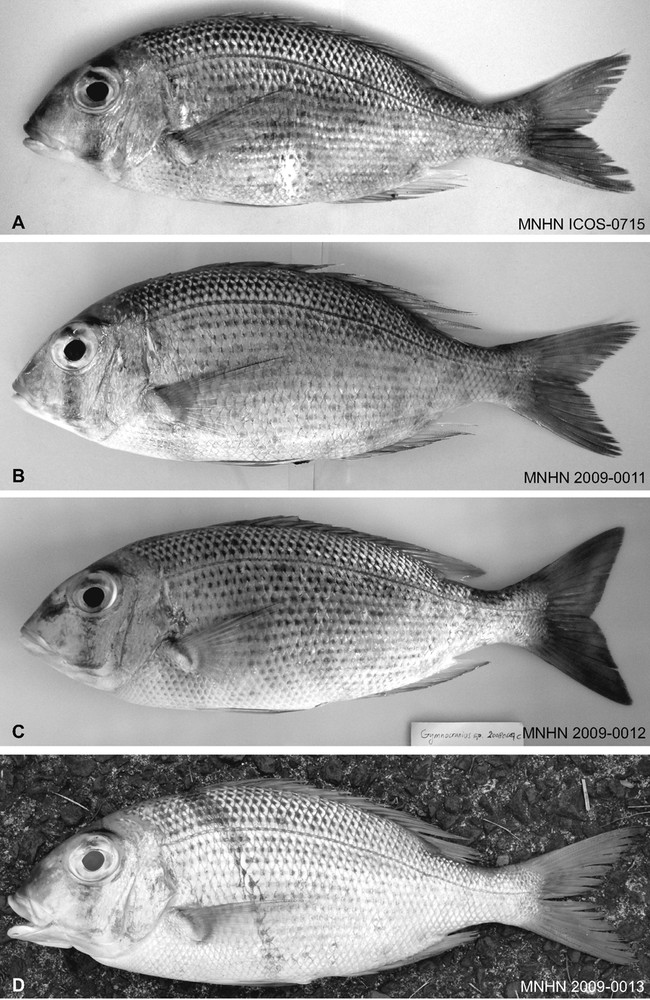
Gymnocranius sp. B specimens chosen as paratypes of G. superciliosus sp. nov. A. Specimen from New Caledonia, standard length (SL) 265 mm; skeleton preserved in the ichthyological collections of the Muséum national d’histoire naturelle, Paris as MNHN ICOS-00715. B. MNHN 2009–0011, southern lagoon of New Caledonia's Grande Terre, SL 220 mm. C. MNHN 2009–0012, New Caledonia, SL 311 mm. D. MNHN 2009–0013, Viti Levu, SL 260 mm.
In the present article, we describe Gymnocranius sp. B and Gymnocranius sp. C as, respectively, G. superciliosus sp. nov. and G. satoi sp. nov. on the basis of morphology, colour patterns, and cytochrome b nucleotide sequences.
2 Materials and methods
The following specimens of Gymnocranius sp. B and Gymnocranius sp. C were collected and examined: Institut de recherche pour le développement, Nouméa (IRDN) No. IRDN-z179 (sp. B, from the northern lagoon of New Caledonia, collected 21 December 2004 at the Nouméa fish market); Muséum national d’histoire naturelle, Paris (MNHN) No. MNHN 2009–0010 (sp. B, captured using a demersal gillnet, New Caledonia, 21 January 2005); MNHN 2009–0011 (sp. B, demersal gillnet, southern lagoon of New Caledonia, 2 March 2005); MNHN 2009–0012 (sp. B, demersal gillnet, New Caledonia, 7 June 2008); MNHN 2009–0013 (sp. B, demersal gillnet, Viti Levu, Fiji, 19 September 2008); and MNHN 2011–0103 (sp. C, reportedly from the Lesser Sunda islands, November 2009). Measurements on these specimens were made to the nearest mm using a Vernier calliper. Eight other specimens were examined by either P. Borsa, P. Béarez, S.P., F. Giancarlo (BioKor, Indonesia and West Papua), or J.-L. Justine (IRD, Nouméa) at the time of collection and subsequently re-examined and measured from photographs: IRDN-20071124-G3 (sp. C, from Raja Ampat, West Papua, 24 November 2007); IRDN-20080131-A (sp. C, reportedly from the Lesser Sunda islands, collected at the Kedonganan fish market, 31 January 2008); IRDN-20080426 (sp. B, spearfished off Pindaï, north-western lagoon of New Caledonia, 21°21′S 164°57′E, depth 7 m, coral sandy bottom, 26 April 2008); IRDN-20081022 (sp. B, spearfished off Loop islet, Chesterfield Islands, 19°58′S 158°28′E, depth 5 m, coral sandy bottom, 22 October 2008); J.-L. Justine's (pers. comm.) catalogue No. JNC-583 (sp. C, baited handline, Sournois reef, New Caledonia); JNC-2912 (sp. B, baited handline, Tomboo Pass, New Caledonia, 21 April 2009); JNC-3055 (sp. C, baited handline, pass south of Tomboo reef, New Caledonia, 16 September 2009); MNHN ICOS-00715 (sp. B, New Caledonia, 24 August 2002).
Photographs of the specimens examined are presented on Figs. 1–3 and in Supplementary material, Fig. S1. Specimens MNHN 2009–0010, MNHN 2009–0011, MNHN 2009–0012, MNHN 2009–0013, and MNHN 2011–0103 have been preserved whole. Specimen MNHN ICOS-00715 has been preserved as skeleton. For specimens IRDN-z179, IRDN-20080426, and JNC-2912, only the neurocranium has been preserved. Muscle-tissue or fin-clip samples of the foregoing except MNHN ICOS-00715 have been preserved in 95% ethanol.

Holotype of Gymnocranius superciliosus sp. nov., registered at Muséum national d’histoire naturelle, Paris, No. MNHN 2009–0010; SL 323 mm. Scale bar: 5 cm.

Holotype of Gymnocranius satoi sp. nov., registered at Muséum national d’histoire naturelle, Paris, under No. MNHN 2011–0103; SL 397 mm. Scale bar: 5 cm.
Whole genomic DNA was extracted from fin-clips of individuals Nos. MNHN 2009–0010 (Gymnocranius sp. B) and IRDN-20071124-G3 (Gymnocranius sp. C) using the Qiagen DNeasy extraction kit (Qiagen, Valencia CA, USA) according to the manufacturer's instructions. These tissue samples and DNA extracts are nos. Let 20 and Let 19, respectively, in the collections of the Marine Phylogenomics Laboratory at the Institute of Oceanography, National Taiwan University, Taipei. Oligonucleotides Mono14695F (5′- A A G C C A C C G T T G T T A T T C A A C T A -3′) and Mono15971R (5′- G A A T G T T A G C T T T G G G A G C T T T T -3′) were designed by W.J.C. to specifically amplify the complete cytochrome b gene in Monotaxinae. Conditions for polymerase-chain amplification reaction (PCR) were as follows: 0.5 units GoTaq® Flexi DNA polymerase (Promega, Madison WI, USA), 1× reaction buffer, 2 mM of MgCl2, 200 μM of each dNTP, 0.2 μM of each primer, and 20–50 ng of genomic DNA in a 25 μL of final reaction volume. Thermocycling conditions for PCR were: initial denaturing step at 95 °C for 4 min followed by 35 cycles of 95 °C (for 40 s), annealing Tm of 55 °C (for 40 s), and 72 °C (for 1–1.5 min. depending on size of fragments), and then a final extension step of 72 °C (for 7 min) before a 4 °C soak. Finally, the PCR products were cleaned up following the AMPure magnetic bead cleanup protocol (Agencourt Bioscience Corp., Beverly MA, USA) and resuspended in 30 μL sterile water. Sequences were then determined by Macrogen Inc. (Seoul, South Korea) using an ABI 3730xl analyzer (Applied Biosystems, Foster City CA, USA).
The sequences of these two individuals were aligned with the 315-bp fragments of the cytochrome b gene of G. elongatus, G. euanus, G. grandoculis, G. oblongus, Gymnocranius sp. B, and Gymnocranius sp. C that have been produced previously [8,16], using BioEdit [17].
3 Gymnocranius superciliosus sp. nov.
G. superciliosus sp. nov. (Table 1; Figs. 1 and 2). Previously referred to as G. griseus (non Temminck and Schlegel 1844 [3]) [4]; Gymnocranius sp. [7,18,19]; and Gymnocranius sp. B [8–12].
Measurements on specimens of Gymnocranius superciliosus sp. nov., listed by increasing size.
| Parameter | Specimen no. | ||||||||
| MNHN ICOS-00715a | MNHN 2009–0011a | MNHN 2009–0013a | MNHN 2009–0012a | MNHN 2009–0010b | JNC-2912 | IRDN-20081022 | IRDN-z179 | IRDN-20080426 | |
| SL (mm) | 214 | 220 | 260 | 311 | 323 | 344 | 387 | 419 | 430 |
| BDd (mm) | 79 | 77 | 98 | 106 | 118 | 127 | 142 | 137 | 161 |
| Ratio of SL to BDd | 2.71 | 2.86 | 2.65 | 2.93 | 2.74 | 2.71 | 2.73 | 3.06 | 2.67 |
| BDa (mm) | 75 | 77 | 92 | 99 | 110 | 114 | 122 | 129 | 148 |
| Head length (mm) | 63 | 70 | 86 | 96 | 105 | 101 | 132 | 120 | 135 |
| Snout length (mm) | 24 | 30 | 35 | 42 | 48 | 45 | 66 | 54 | 60 |
| Eye diameter (mm) | 21 | 22 | 27 | 27 | 29 | 30 | 37 | 33.5 | 30 |
| Inter-orbital width (mm) | - | 25 | 30 | 36 | 36 | - | - | 45 | - |
| Predorsal length (mm) | 76 | 79 | 90 | 113 | 118 | 120 | 148 | 136 | 153 |
| Prepelvic length (mm) | 72 | 76 | 91 | 107 | 114 | 129 | 157 | 155 | 153 |
| Preanal length (mm) | 128 | 137 | 166 | 196 | 202 | 219 | 255 | 248 | 279 |
| Pored scales, lateral line | 48 | 48 | 48 | 49 | 48 | 49 | 48 | 49 | 48 |
a Paratype.
b Holotype.
3.1 Vouchers and type material
Material examined (Table 1; Figs. 1 and 2; Supplementary material, Fig. S1): IRDN-z179 (neurocranium preserved); IRDN-20080426 (neurocranium preserved); IRDN-20081022 (fin-clip preserved); JNC-2912 (photo voucher); MNHN 2009–0010 (holotype); MNHN 2009–0011 (paratype); MNHN 2009–0012 (paratype); MNHN 2009–0013 (paratype); MNHN ICOS-00715 (skeleton; paratype).
3.2 Diagnostic description
The following diagnosis of G. superciliosus sp. nov. is based on the nine specimens whose measurements are given in Table 1. Morphology: a slender Gymnocranius, with ratio of standard length to body depth between 2.65 and 3.06; forehead bumpy; lower edge of eye well above axis of body; caudal fin moderately forked, its lobes slightly rounded. Number of scales rows above lateral line: 6. Pored scales on lateral line: 48 (for 6/9 specimens examined) or 49 (3/9). Colour: flanks silvery; scales above lateral line with a dark-grey basal patch forming longitudinal rows; scales with similar dark-grey basal patch on a more or less extended portion of flank below lateral line; up to two dozens or more pale-blue speckles against bronze background on snout and cheeks, distinctive on fresh, larger individuals, faint on smaller individuals; pale-blue band joining the nostrils on forehead and reaching the eyes, bright in freshly captured, larger individuals; area immediately above eye (supraorbital shelf) forming a distinctive blackish eyebrow; more or less conspicuous vertical dark bar crossing the eye; upper lip reddish to red, lower lip white; dorsal, pectoral, anal and caudal fins reddish to red.
G. superciliosus sp. nov. is here described by the nucleotide sequence of its cytochrome b gene (from holotype, MNHN 2009–0010): 5′- A T G G C C A G T C T C C G A A A A A C C C A C C C C C T C C T A A A A A T T G C A A A T G A T G C A C T A G T C G A C C T A C C G G C C C C A A C A A A C A T T T C T G C C T G A T G A A A T T T T G G C T C T C T A C T A G G T C T C T G C T T A A T C G C C C A A A T C C T T A C T G G C C T A T T T C T C G C C A T A C A T T A C A C C T C T G A T A T C G C C A C A G C A T T C T C C T C C G T G G C C C A C A T T T G C C G A G A C G T A A A C T T C G G A T G A C T C A T T C G T A A C C T C C A T G C C A A T G G G G C C T C A T T T T T C T T C A T C T G T A T T T A T C T C C A T A T T G G C C G A G G A T T A T A C T A C G G C T C C T A C C T G T A C A A A G A G A C C T G A A A T A T C G G A G T A G T C C T G C T T C T C C T A G T A A T A A T A A C A G C T T T C G T A G G C T A T G T T C T C C C C T G G G G A C A A A T A T C T T T T T G A G G T G C C A C C G T C A T C A C T A A C C T C C T C T C C G C A G T G C C A T A T G T A G G A A A C A C T C T T G T C C A A T G A A T T T G A G G C G G C T T T T C A G T C G A C A A T G C T A C A C T C A C C C G A T T T T T C G C T T T C C A C T T T C T C T T C C C C T T C G T C A T T G C A G C T G C A A C T A T C C T C C A C C T T C T A T T C C T A C A C G A A A C C G G A T C T A A C A A C C C T C T A G G C C T A A A T T C A G A C T C A G A C A A A A T T T C A T T T C A C C C C T A C T T C T C C T A T A A A G A C C T G C T A G G T T T C G C A G C T G T C C T G A T C A C C C T C A C C T G T C T A G C A C T T T T C T C C C C C A A C C T T C T T G G A G A C C C G G A C A A C T T C A C A C C T G C G A A C C C C C T C G T G A C C C C T C C C C A T A T T A A A C C A G A G T G A T A C T T T C T A T T C G C G T A C G C C A T T C T A C G T T C A A T T C C A A A T A A A C T T G G T G G C G T A C T C G C A C T C T T G G C T T C C A T C C T G G T T C T T A T G G T G G T G C C C A T C C T C C A C A C A T C T A A A C A A C G G A G T T T A A C A T T C C G T C C C C T A A C A C A A T T T C T C T T T T G A G T T C T T A T T G C C A A T G T T G C C A T T C T T A C C T G A A T T G G A G G A A T G C C T G T A G A A C A C C C G T T C A T T A T C A T T G G C C A A A T C G C A T C T C T T C T C T A C T T T T C G C T C T T C C T C A T T G C T A T A C C A C T A G C A A G T T G A T G G G A G A A C A A A A C T T T A G G T T G A G C T -3′.
3.3 Etymology
The epithet superciliosus, meaning ‘eyebrowed’, was chosen by reference to the conspicuous dark patch above eye, which evokes an eyebrow. This feature is particularly noticeable when the fish is seen underwater ([4,19]; Supplementary material, Fig. S1F). We propose as vernacular name: eyebrowed large-eye bream.
3.4 Notes on distribution, habitat and ecology
G. superciliosus sp. nov. was collected by spearfishing at 5–7 m depth on coral-sand bottom in the north-western lagoon of New Caledonia's Grande Terre and in the Chesterfield Islands (central Coral Sea) in April 2008 and October 2008, respectively. Samples of this species were also collected using handlines in the southern lagoon of New Caledonia's Grande Terre in March 2006, April 2009 and September 2009 (J.-L. Justine, pers. comm.). It was also sampled from the lagoons of Viti Levu and Gau islands in Fiji in September 2008, and from the northern lagoon of New Caledonia in December 2004. See also [19] for the underwater photograph of an individual from Fiji. A specimen was caught on a bottom line by C.A.J. Duffy (pers. comm.) in the south-western lagoon of Nukufetau atoll, Tuvalu in April 2007, “in 15–20 m depth, on a small patch of reef surrounded by sand”. G. superciliosus sp. nov. has also been reported from Queensland (as “G. griseus”) by Coleman [4]. It was also sighted off Pulau Fam in Raja Ampat (West Papua) in December 2007 (P. Borsa, pers. obs.). This species is not mentioned from the Japanese archipelago [6]. It was not found by us among Gymnocranius spp. samples from the Lesser Sunda islands or from southern Sulawesi, where seven other Gymnocranius species including G. elongatus, G. frenatus, G. grandoculis, G. microdon, G. satoi sp. nov. and two yet undescribed species (Gymnocranius sp. D and Gymnocranius sp. E) have been observed (P. Borsa and W.J.C., pers. obs.). Therefore, the distribution of G. superciliosus sp. nov. may be restricted to the tropical south-western Pacific, but more observations will be necessary to accurately delineate it.
Little is known of the ecology of G. superciliosus sp. nov., except that is “encountered around sandy lagoons along the outer Great Barrier Reef and cays of the Coral Sea” [4]. It has been captured by spearfishing on sandy bottom of back-reef lagoon and large pools at depths from 5 to 7 m in the north-western lagoon of Grande Terre and in the Chesterfield lagoon. Nouméa fishermen capture G. superciliosus sp. nov. together with other Gymnocranius spp. by setting gillnets on sandy bottom in the southern part of the southern lagoon of Grande Terre close to Kouaré pass, at depths between 15 m and 30 m. Individual No. JNC-2612 was captured by hand line baited with squid meat on the sandy bottom of Boulari Pass (depth 20 m). The stomach of specimen IRDN-20080426 contained broken bivalve shells; that of specimen JNC-2912 contained scraps of urchin shell. Its predatory behaviour has been briefly described by Coleman [4]: “[…] usually a solitary animal [which] swims a few meters from the bottom, making short forays to the sandy sea floor to investigate any movement which might indicate the presence of prey […]”. An isolated individual was seen and photographed by P. Borsa, about 1 m above the sandy bottom at 22 m depth in the pass west of Pulau Fam, West Papua (Supplementary material, Fig. S1F).
4 Gymnocranius satoi sp. nov.
G. satoi sp. nov. (Table 2; Fig. 3). Previously referred to as G. lethrinoides (non Bleeker 1850 [5]) [6]; Gymnocranius sp. [1,2,20,21]; Gymnocranius sp. C [8–10,12].
Measurements on specimens of Gymnocranius satoi sp. nov., ranked by increasing size (except specimen JNC-583).
| Parameter | Specimen no. | ||||
| IRDN-20080131-A | JNC-3055 | MNHN 2011–0103a | IRDN-20071124-G3 | JNC-583 | |
| SL (mm) | 303 | 344 | 397 | 422 | - |
| BDd (mm) | 127 | 142 | 166 | 172 | - |
| Ratio of SL to BDd | 2.39 | 2.42 | 2.39 | 2.45 | 2.40 |
| BDa (mm) | 114 | 123 | 148 | 153 | - |
| Head length (mm) | 100 | 106 | 119 | 130 | - |
| Snout length (mm) | 37 | 49 | 48 | 57 | - |
| Eye diameter (mm) | 31 | 36 | 40 | 38 | - |
| Inter-orbital width (mm) | - | - | 46 | - | - |
| Predorsal length (mm) | 114 | 127 | 140 | 162 | - |
| Prepelvic length (mm) | 109 | 124 | 145 | 163 | - |
| Preanal length (mm) | 197 | 215 | 247 | 271 | - |
| Pored scales on lateral line | 47 | 49 | 48 | 49 | 49 |
a Holotype.
4.1 Vouchers and holotype
Material examined (Table 2; Fig. 3; Supplementary material, Fig. S1): IRDN-20071124-G3; IRDN-20080131-A; JNC-583; JNC-3055; MNHN 2011–0103 (see below).
We chose as holotype specimen MNHN 2011–0103. This specimen was purchased at the Kedonganan (Bali) fish market. Vendors at the market claimed the fish originated from Lombok or West Sumbawa (F. Giancarlo, pers. comm.). However, subsequent interviews with fish vendors in Bali and Lombok have yielded inconsistent and therefore unreliable indications on the origin of the catches in some instances (P. Borsa, pers. obs.; A. Sembiring, pers. comm.). One should therefore consider this specimen to originate from a region of several hundred kilometres radius centred in southern Bali, which is the range reported for the fishing boats that land their catch in Kedonganan [22]. Measurements on the holotype are reported in Table 2.
4.2 Diagnostic description
The following description of G. satoi sp. nov. is based on the four specimens whose measurements are given in Table 2. Morphology: a high-bodied Gymnocranius, with ratio of standard length to body depth between 2.39 and 2.45 (Table 2); forehead bumpy; lower edge of eye well above axis of body; caudal fin shallowly forked, its lobes convex on inner side. Number of scale rows above lateral line: 6. Pored scales on lateral line: 47–50 (Table 2). Colour: flanks silvery; scales above the lateral line with a dark-grey basal patch forming longitudinal rows; scales in the three rows below lateral line in middle of flank similarly forming darker rows, but basal patch not as dark as above lateral line; blue speckles against bronze background on snout an cheeks, distinctive on fresh, larger individuals, faint on smaller individuals; blue band joining the nostrils on forehead; area immediately above eye (supraorbital shelf) forming a distinctive brownish to blackish eyebrow; more or less conspicuous vertical dark bar crossing the eye; dorsal, pectoral, anal and caudal fins reddish to bright vermilion red; upper lip reddish to red, lower lip white.
G. satoi sp. nov. is here described by the nucleotide sequence of its cytochrome b gene (from specimen IRDN-20071124-G3): 5′- A T G G C C A G C C T T C G A A A A A C T C A C C C T C T T C T A A A A A T T G C G A A C G A T G C A C T A G T C G A C C T A C C A G C C C C A A C A A A C A T T T C C G C T T G A T G A A A T T T T G G C T C C C T A C T A G G T C T C T G C T T A A T C G C A C A A A T C C T T A C T G G C C T C T T C C T C G C T A T A C A C T A C A C C T C T G A T A T C G C T A C A G C A T T C T C C T C C G T A G C A C A C A T T T G C C G A G A T G T A A A C T T C G G A T G G C T T A T T C G T A A C C T C C A T G C C A A C G G A G C C T C A T T T T T C T T C A T C T G T A T C T A T C T C C A T A T T G G C C G A G G A C T G T A C T A C G G C T C C T A C C T A T A C A A A G A A A C C T G A A A T A T C G G A G T A G T C C T G C T T C T C C T A G T A A T G A T A A C A G C T T T C G T G G G C T A T G T T C T C C C C T G A G G A C A A A T A T C C T T T T G A G G C G C C A C T G T C A T C A C C A A C C T C C T C T C T G C A G T A C C A T A T G T A G G G A A C A C C C T A G T C C A A T G A A T T T G A G G C G G C T T C T C A G T C G A C A A T G C C A C A C T C A C C C G A T T C T T C G C C T T C C A C T T C C T C T T C C C C T T C G T C A T T G C A G C T G C A A C C A T C C T C C A C C T T C T G T T C C T A C A C G A A A C C G G A T C C A A C A A C C C T C T A G G C C T A A A T T C A G A C T C A G A C A A A A T T T C A T T C C A C C C C T A C T T C T C G T A C A A A G A C C T T C T A G G C T T T G C A G C C G T C C T G A T C A C C C T C A C C T G T C T C G C G C T T T T C T C C C C C A A C C T T C T T G G G G A C C C A G A T A A C T T C A C A C C C G C C A A C C C C C T C G T A A C A C C T C C C C A T A T T A A A C C A G A A T G A T A C T T T C T A T T C G C G T A T G C A A T C C T A C G C T C A A T T C C A A A T A A A C T T G G C G G A G T A C T C G C A C T C C T A G C T T C C A T C C T G G T T C T C A T G G T A G T G C C T A T C C T C C A C A C A T C T A A A C A A C G A A G C T T G A C A T T C C G T C C C A T A A C A C A A T T T C T C T T T T G A G T T C T T A T T G C C A A T G T A G C C A T T C T T A C C T G A A T T G G A G G A A T G C C T G T A G A A C A C C C G T T C A T T A T C A T T G G C C A G G T T G C A T C T C T T C T C T A C T T T T C A C T C T T C C T C G T T G C C A T G C C G C T G G C A A G T T G A T G G G A G A A C A A A A A T C T A G G T T G A G C T -3′.
4.3 Etymology
Named in the honour of Torao Sato, a Japanese ichthyologist who contributed to the taxonomy of Lethrinidae and who recognized the red-finned Gymnocranius presented in [6] (as “G. lethrinoides”) as “distinct from the other Gymnocranius species” [1]. The vernacular name of this species should be Sato's large-eye bream, in replacement of, or in addition to Blacknape large-eye bream, the name previously coined by [2].
4.4 Notes on distribution and habitat
G. satoi sp. nov. specimens were collected from the southern lagoon of New Caledonia and from Raja Ampat in western West Papua (present work). This species’ distribution also includes southern Japan [6], and possibly Australia's Great Barrier Reef, the Solomon Sea, the Bismarck Sea and the whole Pacific-Ocean coast of New Guinea [2]. Additional specimens including the holotype of the species reportedly originate from the Lesser Sunda islands, Indonesia, but we were unable to independently confirm this. From the limited data available from New Caledonia (J.-L. Justine, pers. comm.), it seems that this species dwells on the coral sandy bottom at depths between 20 m and 40 m in the vicinity of coral-reefs.
5 Discussion
The two species described here are reproductively isolated from each other [8,10] hence they fit the definition of biological species [23]. While colour patterns are similar between the two species, they mainly differ by the ratio of standard length to body depth and by the shape of the caudal fin.
In his brief description of Gymnocranius sp. [1], T. Sato mentioned a “caudal fin shallowly forked, its lobes rounded”: this feature points to G. satoi sp. nov. and much less so to G. superciliosus sp. nov., which has a moderately forked caudal fin and moderately curved and relatively elongated lobes. The specimen photographed by Masuda et al. [6] (as “G. lethrinoides”) and the description and drawing of Gymnocranius sp. by Carpenter and Allen [2] both correspond to G. satoi sp. nov. Although Sato [1] included Coleman's [4] “G. griseus” specimen in his definition of Gymnocranius sp., the latter is different from Masuda et al.’s [6] “G. lethrinoides” and instead matches the present description of G. superciliosus sp. nov. by the relative elongation of its body and by the shape of its caudal fin, which are two characters diagnostic of the latter. An underwater picture of G. superciliosus sp. nov. (from Fiji) has also been published by [19] under “Gymnocranius species”. The authors redirect the reader to Carpenter and Allen's [2] identification sheet of “Gymnocranius sp.”, although the description and drawing provided in the latter designate another species (the one here described as G. satoi sp. nov.). Thus, two distinct species (namely, G.superciliosus sp. nov. and G. satoi sp. nov.) have long been confused in the specialized literature under the single term “Gymnocranius sp.”. We hope that the present work offers the clarification needed to resolve the apparent mismatch between Coleman's [4] and Allen and Erdmann's [19] illustrations on the one side, and the descriptions of Gymnocranius sp. provided by Sato [1] and Carpenter and Allen [2] on the other side.
G. superciliosus sp. nov. and G. satoi sp. nov. can be distinguished from all other known Gymnocranius species (except G. euanus) by their reddish pectoral, dorsal, anal and caudal fins. They are distinct from G. euanus by their general body shape and by their pigmentation patterns: G. euanus has apparently randomly distributed blackish scales on flanks [2], a feature that is not present in either G. superciliosus sp. nov. or G. satoi sp. nov. The two latter can also be distinguished from G. elongatus, G. euanus, G. grandoculis and G. oblongus from the Coral Sea and adjacent regions by diagnostic nucleotides along the 315-bp fragment of the cytochrome b gene sequenced by [8] (Fig. 4).

Polymorphic nucleotide sites at the cytochrome b locus in six Gymnocranius species (G. elongatus, G. euanus, G. grandoculis, G. oblongus, G. superciliosus sp. nov., G. satoi sp. nov.) from the Coral Sea and adjacent regions. Point mutations diagnostic of G. superciliosus sp. nov. and G. satoi sp. nov. relative to the other species are highlighted. The sequence used as reference is that of an uncatalogued specimen of G. elongatus lodged at the Australian Museum, Sydney (AMS), GenBank accession No. AF381260 [16]; numbering of nucleotide sites starts from the first nucleotide of the cytochrome b gene. IRDN: Institut de recherche pour le développement, Nouméa; MNHN: Muséum national d’histoire naturelle, Paris. Sequence of individual IRDN-20071124-G3 from present survey; all other sequences from [8,16].
Monogenean parasites usually are specific to a fish species [12]. Assuming this, it is intriguing to observe that G. superciliosus sp. nov. is parasitized by the same monogenean species (Lamellodiscus magnicornis, L. parvicornis and L. tubulicornis) as those encountered in up to three other sympatric Gymnocranius spp. [12]. This may indicate genetic divergence between the four Gymnocranius species too recent to have lead to the ecological specialization and speciation of their Lamellodiscus spp. parasites. Nevertheless, one cannot discard the hypothesis that the Lamellodiscus spp. found on Gymnocranius spp. are actually host-specific, but include a number of morphologically cryptic species.
The Nouméa fish market boasts coral-reef fishes captured in the southern, the north-western, and the northern reefs and lagoons of New Caledonia. Five Gymnocranius species (G. euanus, G. grandoculis, G. oblongus, G. satoi sp. nov. and G. superciliosus sp. nov.) are regularly seen on the stalls there, with the first two species being the most frequently occurring (A. Collet and P. Borsa, pers. obs.). Nearly every month from 2002 to 2010, our IRD colleague J.-L. Justine has used handlines above the reef and coral-reef sandy bottom between 20 and 40 m in the passes of the southern lagoon of New Caledonia, to collect fishes. Gymnocranius spp. catches were distributed as following: 75% G. euanus, 15% G. grandoculis, 4% G. oblongus, 2% G. satoi sp. nov., and 4% G. superciliosus sp. nov (N = 100) (from the photograph files of J.-L. Justine, pers. comm.). During two expeditions to the Chesterfield islands in October 2008 and May 2012, handline fishing and spearfishing on sandy bottom at 5–25 m in the southern lagoon yielded 95% G. euanus and 5% G. superciliosus sp. nov. (N = 42) (P. Borsa, pers. obs.). From these observations, it appears that the three Gymnocranius species that we recently described, namely G. oblongus [10], G. satoi sp. nov. (this study) and G. superciliosus sp. nov. (this study), are less abundant than G. euanus and G. grandoculis in the catches. Their inferred relative rarity may explain why they have long remained overlooked despite a number of underwater visual surveys of shore fishes in the Coral Sea and adjacent regions [24–29].
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.
Acknowledgements
We are grateful to A. Collet (IRD, Nouméa) for help with sampling, photographing and preserving two of the type specimens, and for excellent assistance with genetic analyses; to A. Cheype (IRD, Nouméa), D. Deschamps (fisherman and fish merchant at the Nouméa fish market), J.-L. Justine (MNHN/IRD, Nouméa), the Li-Khau family (Nouméa fish market), L. Olonde (Nouméa fish market), J. Palene (Marine nationale, Nouméa) and anonymous West Papuan fishermen at the Sorong fish market for donating specimens and/or tissue samples; to C. Cristofoli, F. Giancarlo, and A. Wulandari (U. Seblas Maret, Surakarta) for purchasing fishes at the Kedonganan fish market and for details concerning the possible origin of specimen MNHN 2011–0103; to R. Causse and P. Pruvost (MNHN) for taking care of the fish specimens under their responsibility; to C. Sand (Institut d’archéologie de la Nouvelle-Calédonie et du Pacifique, Nouméa), D. Ponton (IRD), A. Rivaton (Adecal, Nouméa) and P. Chavance (ZoNéCo, Nouméa) for support. J.-L. Justine also provided excellent photographs of the specimens in his catalogue, encouraged us to finalize the present work, and provided helpful comments to the final version of the manuscript. P. Borsa's underwater observations of G. superciliosus sp. nov. in Raja Ampat (West Papua) were made during KR Baruna Jaya VIII Ekspedisi Widya Nusantara of LIPI (Jakarta, Indonesia) in partnership with M. Adrim (LIPI-P2O, Ancol, Indonesia). C.A.J. Duffy's (Department of Conservation, Auckland, New Zealand) capture of a G. superciliosus sp. nov. specimen in Tuvalu was part of an NZAid-funded marine biodiversity survey. Sampling in Fiji was during a workshop funded by Agence française de développement, with the assistance of D. Lecchini (IRD) and S. Bala (University of the South Pacific, Suva, Fiji). Funded by IRD-UR 128/UR 227, a Fonds pacifique pour le développement grant to P. Borsa, ZoNéCo–Opération “Jeunes poissons” grants to P. Borsa and D. Ponton, Taiwan National Science Council grant No. NSC 101–2611-M-002–016-MY3 to W.J.C., and by personal contributions from P. Borsa, C. Cristofoli, F. Giancarlo, and S.P. All experiments reported here complied with the current laws and regulations of New Caledonia and West Papua. Designed the study: P. Béarez, P. Borsa, W.J.C.; contributed reagents or materials or analysis tools: P. Borsa, W.J.C., S.P.; analyzed and interpreted the data: P. Béarez, P. Borsa, W.J.C.; wrote the paper: P. Borsa.


