1. Introduction
Cell migration, the active and directed movement of cells from one location to another in response to a signal, is a fundamental and intricate process that plays a critical role in various biological phenomena, ranging from embryonic development and tissue repair to immune responses and cancer metastasis. It is essential for shaping tissues and organs during development as well as maintaining tissue homeostasis.
Some cells migrate as individual cells that navigate their environment. However, many cells migrate collectively, as part of cell groups [1]. The term “collective migration” has received different definitions. Historically, it was first used to describe the movement of epithelial cells which are closely adhesive to one another, and therefore necessarily move collectively [2, 3]. However, the definition was more recently widened to all migrations in which the movement of one cell is modulated by the other migrating cells and where the group of cells acts as a cooperative unit [4]. Experimentally, this can be revealed by isolating cells which are then unable to exhibit the migratory behavior they display in the group.
In this article, we briefly review the different cellular behaviors that ensure collective migration, in particular the “contact regulation of locomotion” mechanisms organizing mesenchymal cells, with a focus on Xenopus neural crest cell migration. In a second time, we highlight the recent discovery of “guidance by followers”, a behavior ensuring collective cell migration and coordination of the movement of different tissues.
2. Collective cell migration and contact regulation of locomotion
During collective epithelial cell migration, the collective nature of the movement mainly stems from the fact that cells are tightly attached to each other. Such migration has been extensively described, in particular using epithelial monolayers in a so-called wound healing assay [5, 3]. A gap is formed in a confluent epithelial monolayer, usually by scratching the monolayer or by removing a stencil, and cell movements are recorded. Although the details of the cellular behavior vary depending on the cell type, the migration of epithelial monolayers overall relies on the formation of leader cells facing the empty space. These cells adopt migratory features, forming a large lamellipodium and dragging surrounding cells in a finger-like structure. The rest of the cells then adopt a follower morphology, either being passively dragged or forming small, cryptic lamellipodia contributing to local movement [6, 7, 3].
Although epithelial tissues in vivo usually display more complex structures, their collective migration also relies on an organization in which leaders set the movement and followers are dragged along [8, 9, 10]. During zebrafish development, the primordium of the posterior lateral line forms a small cluster of cells migrating along a track of chemoattractant. This cluster is set in motion by a small group of leader cells detecting a local gradient of a chemoattractant, which is generated by the follower cells, that are then dragged by the leading cells [11, 12, 13, 9, 14]. Separating leaders and followers [15] or changing their identity [16, 12, 9, 17] largely disrupts primordium migration, showing the need for cooperation between cells with distinct leader and follower roles.
While mesenchymal cells are not strongly attached to their neighbors and sometimes migrate individually [18], some of them organize in a migrating group. In the group, cells all display a migratory behavior forming actin rich protrusions, adopting a loose order with only transient contacts and often changing neighbors. Such organization can be the result of the concomitant movement of cells migrating independently from each other, for example immune cells migrating towards the site of a wound [19]. In other instances, cooperation between cells is actually required for proper group migration, thus constituting a bona fide collective cell migration. In these cases, isolated cells behave differently than in the group and are unable to migrate efficiently [20, 21].
Given the loose nature of mesenchymal tissues, cooperative processes leading to collective migration differ compared to epithelial tissues. One of the most extensively described instances of mesenchymal collective migration is that of Xenopus neural crest cells [22, 23]. These cells display a surprisingly large diversity of migratory behaviors (durotaxis, chemotaxis, electrotaxis, etc.) [24, 25, 26, 27, 28], but their migration is mainly driven by two phenomena: contact inhibition of locomotion [29] and co-attraction [30, 31]. Contact inhibition of locomotion was described a century ago [32] and corresponds to the tendency of two colliding cells to stop and then reorient their movement away from the collision site (Figure 1A) [33, 34]. Without additional mechanisms, this would lead to cell dispersal, as cells tend to flee contact. Co-attraction corresponds to the expression of both a chemoattractant and its receptor. Thus, cells attract each other, coalescing into a cluster [30]. The combination of these two properties in the neural crest cells generates a complex behavior where cells tend to move away from a cluster because of contact inhibition of locomotion while at the same time reforming a cluster thanks to co-attraction. This leads to persistent displacement in a laterally constrained environment [35].
Different modes of contact regulation of locomotion. (A) Contact inhibition of locomotion corresponds to the active repolarization of two colliding cells away from the contact point. (B) Contact enhancement of locomotion is the stabilization of cell migration direction upon contact with another cell. (C) Contact following of locomotion is the alignment of a cell contacting the posterior side of a leading cell, resulting in the formation of a small train of cells. Cells are color-coded according to their identity.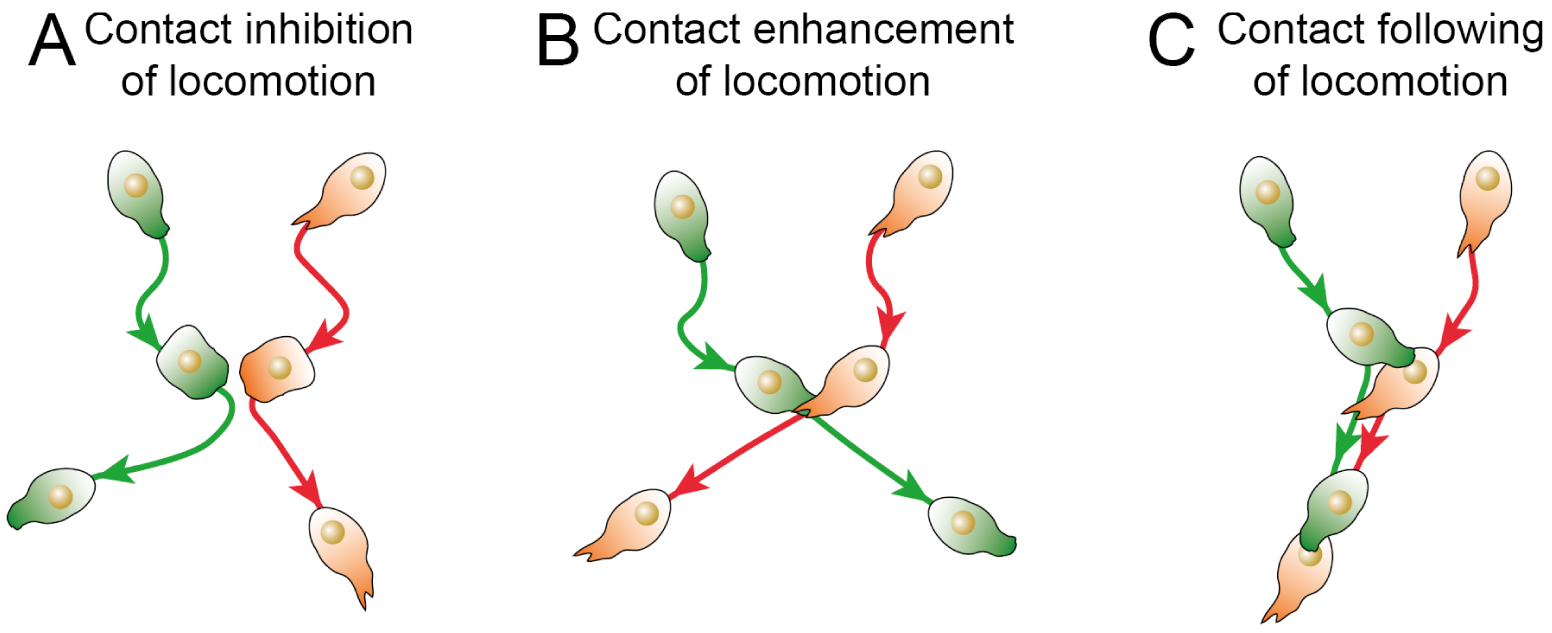
Two types of contact inhibition are reported depending on what happens after cell collision [34]. In type-I contact inhibition of locomotion, colliding cells actively migrate away from each other, forming migratory structures at the opposite side compared to the point of collision. In type-II contact inhibition of locomotion, cells simply reorient their migration after colliding with another cell, although there is a debate about whether this is actually an active process or simply the result of steric hindrance.
Until recently, contact inhibition of locomotion was considered to be the main phenomenon controlling collective behavior and interaction between colliding cells. However, recent studies have described two other cellular behaviors upon cell–cell contact. In a minimalist system where cell collision is controlled, it has been observed that when the front of a cell collides with the rear of another cell, the rear cell tends to stay attached to the front cell and to follow its migration (Figure 1B) [36]. This phenomenon, called “contact following of locomotion”, can lead to the formation of small trains of collectively migrating cells. Such a behavior has recently been observed in colonies of Dictyostelium Discoideum [37]. In the same organism, it has also been described that collision between cells tends to stabilize the migration of both cells [38]. This phenomenon, termed “contact enhancement of locomotion”, tends to accelerate the spreading of colonies (Figure 1C). It has recently been proposed to group these contact-driven behaviors under the name “contact regulation of locomotion” [39], to stress that these behaviors rely on cell signaling and active cell responses, and to distinguish them from purely physical collective phenomena such as plithotaxis, the alignment in the direction of the lowest shear stress [40]. These different instances show that there is a whole repertoire of cellular responses to contact in addition to contact inhibition of locomotion, offering a wide range of mechanisms for coordinating collective migrations.
In a recent paper, in order to better understand how interactions between cells coordinate migration, we studied the elongation of the axial mesoderm of the zebrafish embryo during gastrulation [41]. We discovered another phenomenon of contact regulation of locomotion leading to collective migration in a mesenchymal tissue and its coordination with other tissues. In the anterior axial mesoderm, cells detect the active migration of cells immediately behind them and align their migration accordingly, a behavior we named “guidance by followers”.
3. A novel process underlying mesenchymal collective migration
During gastrulation, the zebrafish embryo is made of a big spherical yolk cell on top of which sit the embryonic cells, exhibiting three major concomitant cellular movements: epiboly, ingression, and convergence and extension (Figure 2A–D). Epiboly consists of a movement of the embryonic margin towards the vegetal pole of the embryo [42, 43]. Ingression is the movement of cells at the margin towards the yolk cell to form the deep hypoblast below the surface epiblast [44, 45, 46, 43]. Finally, convergence and extension corresponds to a global movement from both hypoblast and epiblast cells of convergence towards the dorsal midline and of extension along the animal-vegetal axis [43, 47].
Axial mesoderm during zebrafish gastrulation. (A–D) Pictures of Gsc:GFP embryo, a transgenic line labelling axial mesoderm, during gastrulation and corresponding schematics. (A,B) Dorsal view. (C,D) Side view. (A,C) Purple and white dashed lines respectively delimit polster and posterior axial mesoderm, both labelled in green in this transgenic line; red dashed line marks the embryonic margin. epi: epiblast; po: polster; pm: posterior mesoderm. (B,D) Epiboly, convergence and extension and ingression movements are depicted by red, grey and purple arrows respectively. A: animal; Veg: vegetal; R: right; L: left; D: dorsal; V: ventral. (E,F) Pictures of Gsc:GFP transgenic axial mesoderm during gastrulation in side and dorsal view respectively. Axial mesodermal cells are labelled in green and membranes in red. Purple and white dashed lines respectively delimit polster and posterior axial mesoderm. All scale bars are 50 μm long. Adapted from [41, 48], all rights reserved.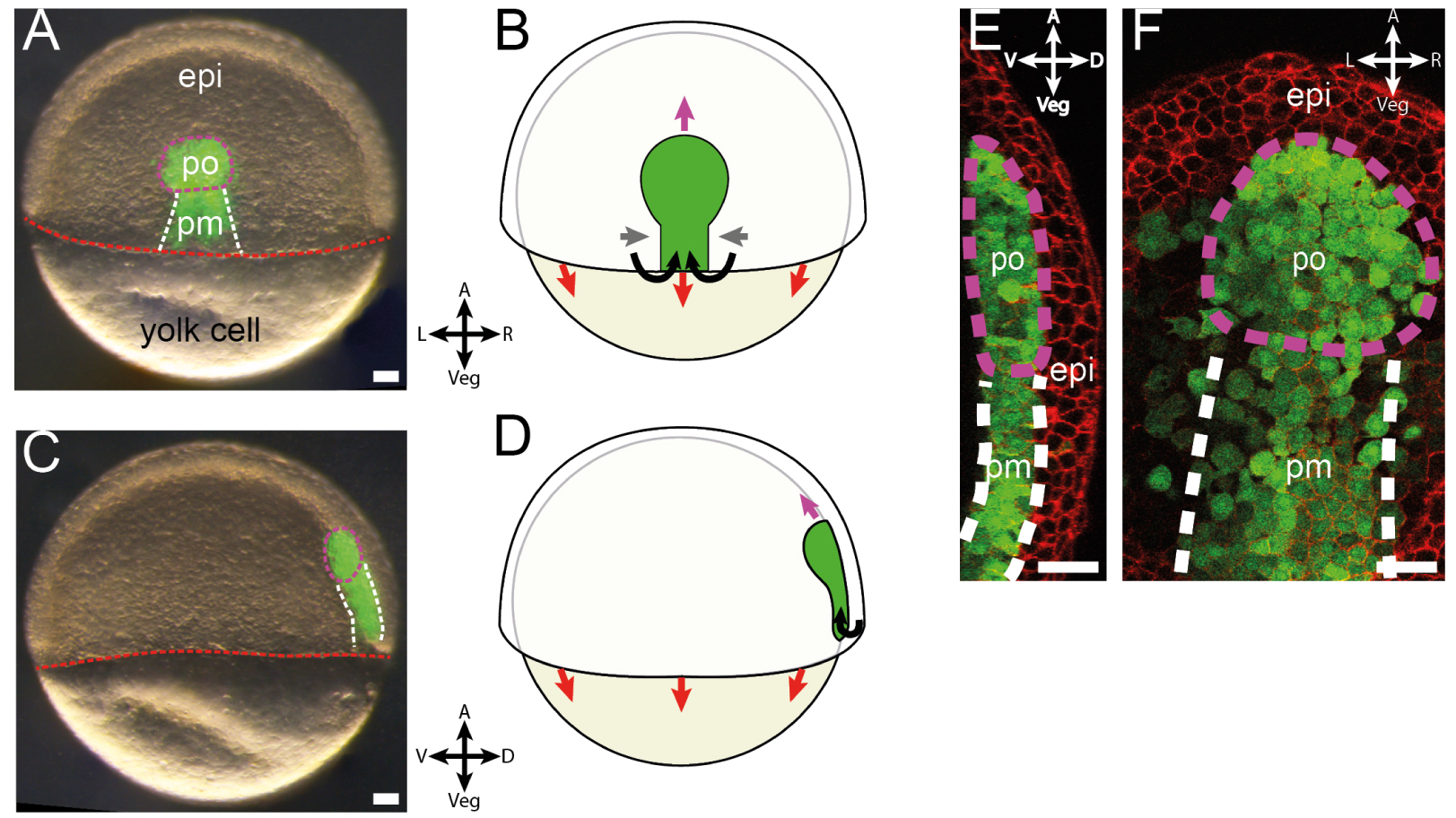
The axial mesoderm is a structure that elongates during gastrulation from the dorsal margin towards the animal pole of the embryo and is composed of two tissues (Figure 2E, F). The polster, closest to the animal pole, is a mesenchymal tissue composed of roughly 200 cells in a loose, three-dimensional arrangement [49, 50, 51]. Polster cells display active migratory behavior oriented towards the animal pole of the embryo. They are followed by the posterior axial mesoderm, which undergoes convergence and extension, maintaining contact with the polster during its migration [52, 50].
Because all polster cells display similar oriented migratory behavior, it has initially been thought that they were migrating independently from each other [53]. However, transplantation experiments, have shown that single cells, or small groups of cells, when isolated from the rest of the polster, would remain motile but lose their orientation towards the animal pole [49]. Conversely, these isolated cells recovered their orientation upon contact with the polster. These experiments established polster migration as a bona fide instance of collective cell migration and suggested that the directional information that guides polster cell migration is contained within the polster and is transmitted between cells via cell–cell contacts by an unknown mechanism.
In order to understand the guidance of polster migration, we sought to locate the information of direction in the tissue by removing or isolating portions of the tissue and observing which parts are necessary to drive migration. For this purpose, we developed spatially confined three-dimensional laser ablations using a pulsed laser, to ablate precisely individual polster cells in the deep hypoblast, without affecting neighboring tissues (Figure 3A) [54]. We then performed various cuts in the polster and first noticed that removal of the first rows of cells does not affect polster migration, suggesting that there is no specific leader role for these cells [55]. More importantly, we observed that the polster, when separated from the posterior mesoderm, is unable to orient its migration autonomously. However, when contact with the posterior mesoderm is preserved, migration is oriented, suggesting that interaction with the posterior mesoderm is actually required for directionality. We confirmed this observation using microsurgery and cell transplantations [56]. It thus appeared that, contrary to our expectations, the directional information guiding the polster is not self-contained in the polster, but is in fact provided by the following tissue.
Experimental demonstration of guidance by followers. A schematic, a representative image and simplified results are presented for each experiment. Compasses and barred compasses respectively indicate oriented and misoriented migration or migratory behavior. (A) Deep, three dimensional and spatially restrained laser ablations. Red lines on the microscopy image and on the small axial mesoderm schematics indicate position of the cut, purple and white dashed lines respectively delimit polster and posterior axial mesoderm. (B) Transplantation of a WT polster in a host where the posterior axial mesoderm is slowed. Transplanted polster cells are labelled with red nuclei on the microscopy image. White and purple dashed lines respectively indicate the host posterior axial mesoderm and the transplanted polster. (C) Isolation of WT polster cells from a WT axial mesoderm by migration deficient buffer cells. WT transplanted cells, buffer cells and host are respectively labelled in red, with blue nuclei or in plain green. Purple dashed line indicates the front of host axial mesoderm. (D) Transplantation in a WT host of perturbed polster cells overexpressing or depleted for specific proteins. Transplanted polster cells are labelled in red. Purple and white dashed lines respectively delimit polster and posterior axial mesoderm. All scale bars are 50 μm long. Adapted from [41], all rights reserved.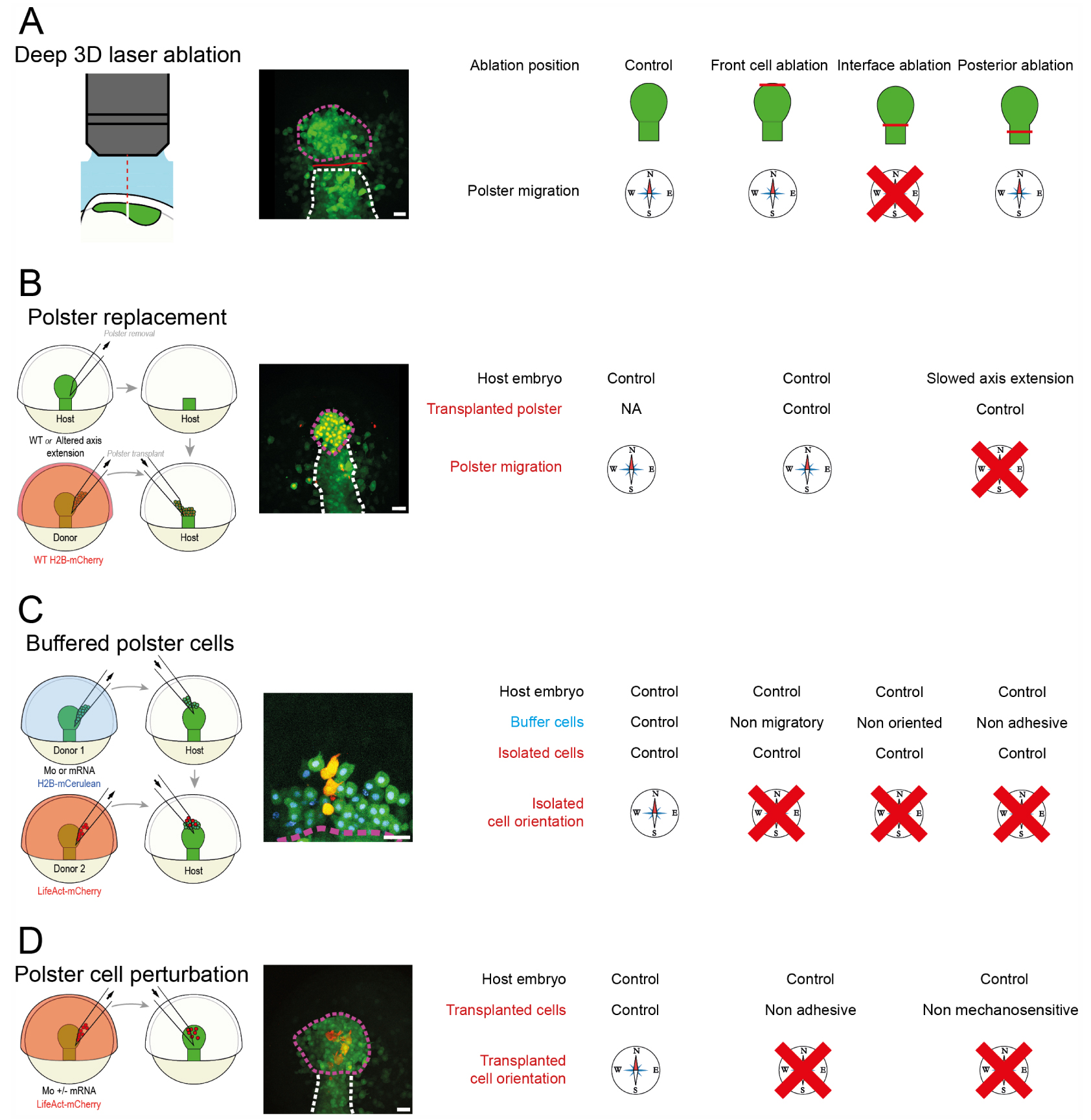
We then sought to understand how interaction with the posterior mesoderm drives polster migration. In particular, since the posterior mesoderm elongates during gastrulation, we tested whether extension is required for polster guidance (Figure 3B). Using microsurgery approaches and functional genetics, we specifically manipulated the extension speed of the posterior mesoderm. It turned out that the orientation of a wild-type polster is lost when the following posterior mesoderm elongates more slowly. This shows that proper elongation of the posterior tissue is required for polster guidance.
To explain how the movement of the following tissue could orient the polster, we considered two hypotheses. Polster cells could orient either by detecting the fact of being pushed, for example by feeling an induced stress, or by detecting an oriented signal from the following cells, like their active migration. Using multiple microsurgery and cell transplantation, we isolated some polster cells from the rest of the tissue by introducing a row of buffer cells whose migration was perturbed, being either non-protrusive, non-oriented or non-adhesive (Figure 3C). Despite being pushed by axis extension, such isolated cells are not oriented, showing that polster cells detect the active, oriented migration of their neighbors and align with it.
We then dissected the molecular mechanism underlying the detection of neighbors’ migration using gene loss of function (Figure 3D). We identified that the adherens junction complex E-Cadherin/α-Catenin/Vinculin is required for polster orientation. Interestingly, α-Catenin is a mechanosensitive protein, able to trigger cell signaling and recruit the adapter protein Vinculin in response to a mechanical force [57, 58, 59, 60]. We showed that the mechanosensitive domain of α-Catenin is required for proper distribution of PI3K, a kinase controlling the formation of protrusions and cell orientation [51]. We then quantified the cellular distribution of α-Catenin and observed that it accumulates mainly at the posterior side of the cells, where there is contact with protrusions formed by follower cells. Using laser ablation, we observed that these protrusions are indeed under tension, and that this tension requires the adherens junction protein E-Cadherin.
Thus, we showed that polster cells are able to detect the migratory activity of a following cell and align with it and proposed to name this phenomenon “guidance by followers”. Using numerical simulations, we verified that guidance by followers is sufficient to account for the collective guidance of a group of cells (the polster) by oriented following cells. Importantly, varying the speed of the following cells in the simulations, we observed that the polster would always match its migration speed to the elongation of the posterior mesoderm thanks to this guidance mechanism. We could prove experimentally that this prediction is correct, suggesting that guidance by followers, on top of ensuring collective guidance of the polster, also guarantees robust elongation of the axial mesoderm, preventing separation of the two tissues during gastrulation.
Based on these observations, we proposed a mechanistic model for polster migration and coordination with posterior mesoderm extension during axial mesoderm elongation (Figure 4). During gastrulation, the posterior mesoderm undergoes convergence and extension. At the contact between the two tissues, a cell from the front row of posterior mesodermal cells forms a protrusion that contacts a polster cell. This protrusion forms an adherens junction then builds up tension that recruits and opens α-Catenin in the polster cell. This provokes redistribution of PI3K activity in this cell at the opposite side compared to the contact, leading to the alignment of polster cell polarity with those of the posterior cell. In turn, this polster cell will form protrusions and orient a cell from the next row and so on until the whole tissue is oriented away from the posterior mesoderm and migrates efficiently towards the animal pole of the embryo.
Mechanistic model for guidance by followers organizing polster collective migration. A polster cell contacted by a following cell detects tension generated by the active migration of the follower through mechanosensation. This orients the migration of the contacted cell which, in turn, orients the cell in front of it so that the directional information propagates through the entire group. Adapted from [41], all rights reserved.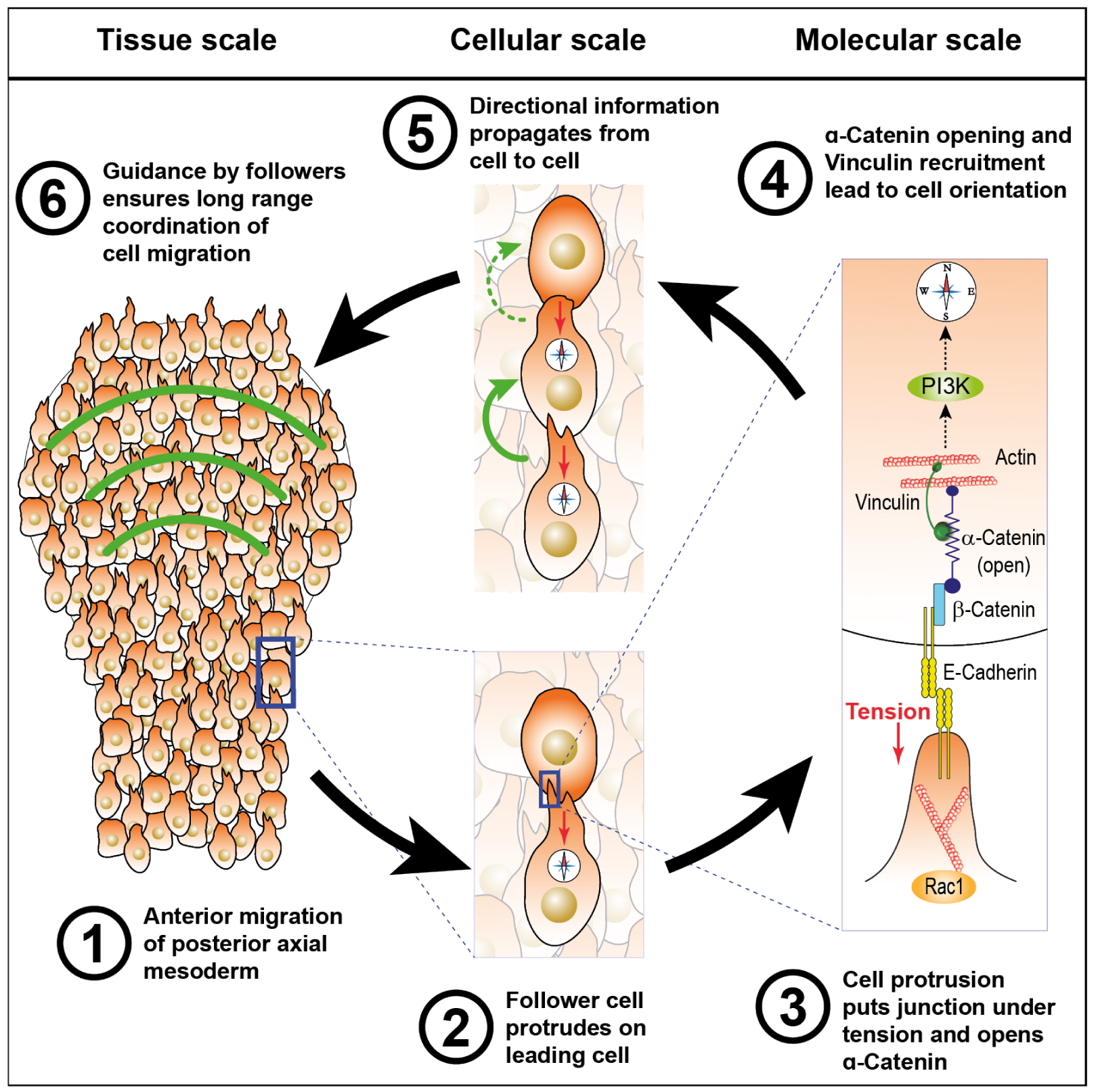
4. Perspectives
The full mechanism that leads to guidance by followers is not yet elucidated, but several leads are open for understanding this phenomenon in more detail. In a recent article, we studied what might be the outcome of conflicting information on polster collective migration [61]. We proposed a development of the simulations, testing different implementations of guidance by follower response upon multiple collisions. Although the different models performed almost equally well, the model proposing that leading cells align with the cell presenting the largest surface of contact seems to fit the experimental data slightly better. Controlled interaction between cells may provide more experimental input to understand how a cell integrates multiple stimuli.
We have shown that mechanosensation of neighboring cell protrusions is required for polster cell orientation. However, the distribution of forces in the polster is still unknown, as is their amplitude. To better understand how cells orient in response to forces applied by neighbors, it would be necessary to describe the mechanical state of the tissue at different scales. Preliminary data using small deformable PDMS beads as tissue scale force sensors suggest that there is a front-to-rear gradient of anisotropic tension and that the maximal tension seems to be aligned with the direction of migration [48]. However, a finer resolution of forces might be necessary to understand the force applied and experienced by the cells for example by using molecular FRET tension sensor modules [62].
From a molecular point of view, more functional analyses would be necessary to understand how cells are applying forces through their protrusions and how the opening of α-Catenin triggers orientation of cells. Indeed, several signaling pathways are known to be required for polster cell orientation, like Wnt/Planar cell polarity (PCP), PI3K, and E-Cadherin [49, 63, 51, 64]. In Medaka, another fish model organism, FGF signaling has also been reported as necessary for proper polster migration [65]. How all these pathways are interrelated is still an open question, but a first interesting observation is that several of them are common to those described in other instances of contact regulation of locomotion. For instance, contact inhibition of locomotion described in neural crest cells relies on the formation of N-Cadherin adherens junction and activation of the Wnt/PCP pathway that locally activates the small GTPase RhoA, leading to the collapse of the protrusion and the formation of another protrusion at the opposite side of the cell [34]. Similarly, contact following of locomotion relies on the Wnt/PCP pathway as its loss of function abrogates collective behavior [36].
Despite these areas of uncertainty, the newly identified mechanism, guidance by followers, represents a significant step forward in understanding collective cell migration. It adds to our insight into how mesenchymal cells can communicate and how they organize to migrate as a group. Moreover, given its proximity to other instances of contact regulation of locomotion, it suggests the existence of a toolbox that cells use to communicate at contact sites, which involves pathways such as the Wnt/PCP pathway and Cadherin-mediated junctions.
Furthermore, this discovery helps to understand how different tissues coordinate and orient their movement in the complex environment of a developing embryo. Guiding a tissue with a chemotactic gradient over long distances in a dynamic environment could prove very complex. Similarly, ensuring the relative motion of different tissues that move by different mechanisms could be a challenging task. Guidance by followers allows one tissue to guide another, which is an efficient solution for robustly coordinating tissue movement.
Finally, guidance by followers might provide insight into how groups of cells manage to migrate without apparent external guidance cues, such as streams of metastatic cells leaving a tumor. With guidance by leaders, metastatic cells would require an external guidance cue to efficiently migrate. On the contrary, if cells are oriented by their immediate followers, then an arbitrarily long column of cells could theoretically be guided by an initial asymmetry at one end. Adding new cells exiting the tumor would thus orient the last row of the stream, then orient the next row, and so on. Further work might thus identify mechanisms similar to guidance by followers in other systems.
Declaration of interests
The authors do not work for, advise, own shares in, or receive funds from any organization that could benefit from this article, and have declared no affiliations other than their research organizations.
Acknowledgements
I thank Sophie Escot, Amélie Elouin, Diego Jahn, Sebastián González-Tirado, Jörn Starruß, Lutz Brusch for the help, and Nicolas B. David for the supervision on the work described in this article. I am also grateful to Nicolas B. David, Manon Valet, Anaïs Bailles, Roudaina Boukheloua and Giulia Serafini who critically read the manuscript. This work was supported by a fellowship from the Alexander von Humboldt foundation.




 CC-BY 4.0
CC-BY 4.0