1 Introduction
Introduction of “exotic” (i.e., non native from the area considered) species in ecosystems may have several results: (1) ecological consequences such as competitive exclusion, introduction of parasites or diseases, predation, modification of trophic structure, etc. (e.g. [1–3]); or, (2) genetic consequences. Among them, hybridization between exotic and autochthonous species has been documented (e.g. [4–6]). When hybrids are sterile, hybridization represents a cost as parental species “fail” in producing a successful offspring [7]. Such a cost is amplified in species close to extinction because the number of effective breeders is low and critical with regard to population persistence. Moreover, in hybrid zones, processes of introgression often occur [8]. In a context of hybridization induced by man's activity, introgression may be viewed as a genetic invasion of exotic genes from one species to another, a process that has been called “genetic pollution” [9–11]. Such a process is a threat for the genetic integrity of native genomes [12] and thus represents a model for conservation genetics studies. We want to underline that genetic pollution does not only affect distinct species that are involved in introgression but also affects, within a species, distinct allopatric populations that are genetically different or locally adapted. Because of anthropic translocation, allopatry is broken and these populations may be involved in gene exchanges.
Invasive species, mainly resulting from introductions by man, are often implied in perturbations of ecosystems [2]. As an example, with their range distribution increasing, hybridization and introgression with localized species become possible. Thus, genetic pollution may be important for invasive species. European water frogs provide a good model to study such processes. Such frogs are subject to multiple and recurrent introductions [13–16]. It is especially the case in France because of accidental introductions of the species R. ridibunda that are imported mainly from Egypt, Turkey and Balkanic countries for culinary purposes (ca. 700 t of frogs; [14,17]). As a consequence, Rana ridibunda that was previously located to a narrow part of France (Fig. 1) may be considered as an invasive species because its range is now extending to many other parts of France [16]. Several other European water frog species that occur naturally in the Balkanic region (Rana kurtmuelleri), the Near East (Rana bedriagae) or the northern part of Africa (Rana saharica) may also have been introduced in France, although they have not yet been identified [18]. Thus these frogs may be affected by genetic pollution because of translocations.

Range distribution area of R. ridibunda in France. The grey area corresponds to the distribution mapped by Graf & Polls Pelaz [23]. The arrows represent range expansion such as that recorded recently (see [16]).
Moreover, these frogs exhibit a peculiar situation regarding animal hybridisation. They constitute “kleptons” [19,20], i.e. unusual biological “species” which have a peculiar mode of reproduction such as gynogenesis or “hybridogenesis” [21–23]. Especially interesting in this respect are “zygokleptons” [19], i.e. forms of hybrid origin that are fertile, discard in germinal cells one parental genome and produce clonal (non recombining) gametes allowing the persistence of hybrid lineages through “sexual parasitism” of the parental species to re-establish at each generation the genome lost through meiosis (so-called “hybridogenesis” or creditogenesis; see [24]). Usually, such forms are expected to be prevented from genetic pollution because recombination is not expected to occur. However, occasional recombination has been suspected in natural populations of hybridogenetic European water frogs [25,26]. Thus, the integrity of genomes needs to be specified in the taxa implied in such hybridogenetic complexes.
In this context, our study aims at (1) identifying genetic markers that allow one to evidence introductions in R. ridibunda populations; (2) quantifying the proportion of introduced specimens in these natural populations; (3) surveying the “integrity” of their genomes; (4) identifying places were allopatry is broken and co-occurrence (assemblages) arise between different water frog species studied in France; and (5) check if introgression may occur. Moreover, we would like to propose a theoretical scenario for the “genetic pollution hypothesis” and discuss its biological consequences.
2 Materials and methods
2.1 Rana ridibunda survey
European water frogs (n=254) were collected in 11 distinct populations from eastern France in 1997 (along the French Rhone river from North to South; see [16]). As introductions mainly originated from frog importation for culinary purposes, two additional samples were studied, coming from a fish store that imports such frog for restaurants. These frogs are known to originate from Turkey and Egypt, the main exportating countries for these living frogs [17].
Genetic variability was studied on 11 presumptive structural gene loci by starch gel electrophoresis, using continuous buffer systems (Tris-citrate pH 6, Tris-citrate pH 8 and Tris-EDTA-borate pH 8). Slices were stained following standard protocols [27,28]. Loci successfully resolved were: ACO-2 (4.2.1.3), GDA, α-GDH-1 and α-GDH-2, LDH-1 and LDH-2 (1.1.1.27), MPI (5.3.1.8), 6PGDH (1.1.1.44), PGM-1 and PGM-2 (2.7.5.1), SOD (1.15.1.1).
A multivariate analysis (FCA) was performed on the 11 loci in order to see if autochthonous and exotic frogs could be distinguished.
2.2 General water frog survey
Altogether 608 frogs were randomly sampled in 32 aquatic sites. In order to determine the taxonomic composition at each site, each frog was identified using a combination of specific allozymic markers, specifically LDH-B, αGDH (E.C. 1.1.1.8), AHH (E.C. 3.3.1.1), MPI, and PGM-2.
3 Results
3.1 R. ridibunda survey
Among the 11 loci, all were polymorphs except the SOD (data not shown). As revealed by allelic frequencies (Table 1), three loci appeared as genetic markers of introduction from Turkey and Egypt: GDA, ACO-2 and MPI. The allele GDA-100 was common in the Egyptian frogs analysed (20%) but very rare in autochthonous frogs (1.4%; 4 frogs among which 2 were homozygote 100/100). The allele 120 of ACO-2 reached 33.33% in the Turkish sample and 61.11% in the Egyptian one. Although this allele was scarce in French populations (2.76%), all 6 frogs bearing this allele were homozygote 120-120. The MPI-135 allele was rare in France (0.63%) and more common in the Egyptian sample (12.5%).
Allelic frequencies at 3 loci. Underlined numbers represent the (suspected) markers of allochtonous origin
| Locus | Allele | France | Turkey | Egypt |
| N=254 | N=9 | N=10 | ||
| GDA | – | |||
| 110 | 0.2990 | 0.6111 | 0.8000 | |
| 120 | 0.6765 | 0.3889 | – | |
| – | – | |||
| ACO-2 | 100 | 0.3088 | 0.1111 | 0.0556 |
| 105 | 0.0069 | – | – | |
| 110 | 0.6567 | 0.5556 | 0.3333 | |
| MPI | 90 | 0.0042 | – | – |
| 100 | 0.4937 | 0.2000 | – | |
| 119 | 0.4958 | 0.8000 | 0.80 | |
| – |
The multivariate analysis performed on the whole set of loci showed that (1) there is a genetic difference between the autochthonous and exotic frogs (genetic distance d=0.33 for Egyptian frogs, d=0.115 for Turkish ones; [29]); (2) this difference is mainly due to ACO, GDA-100 and GDA-130 (axis 1, 3, and 2 of the analysis, respectively); and (3) the “French” frogs that bear alleles GDA-100 and ACO(2)-120 are very close to exotic populations and different from the other French populations, suggesting that such frogs are, in fact, exotic ones and originated from introductions (Fig. 2). The frogs bearing the GDA-130 allele were also close to exotic frogs because they also show the ACO(2)-120 allele.
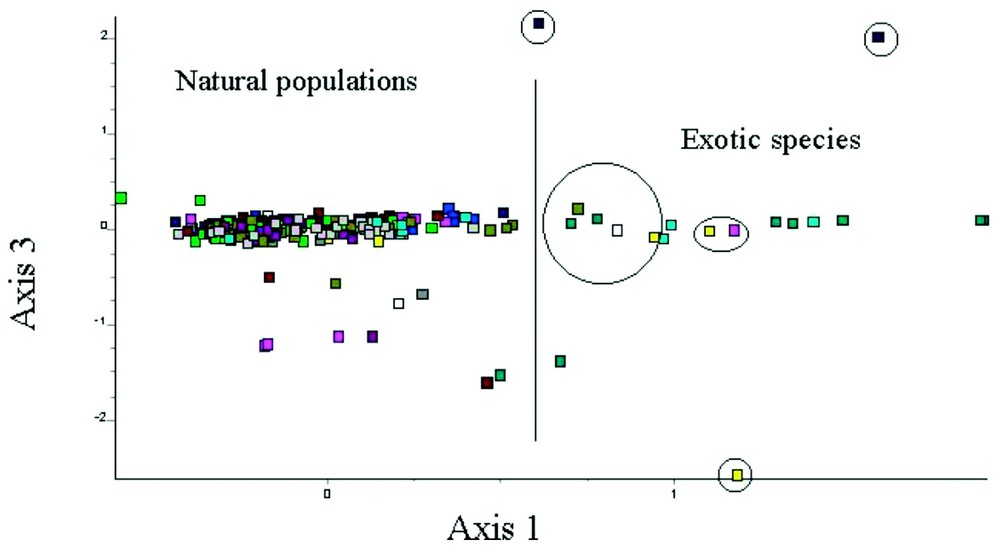
Factorial map performed on the multilocus genotypes of 11 populations of R. ridibunda from France and 2 samples from Egypt and Turkey. Individuals on the right part of the map are from samples obtained from a fish store (blue-sky and blue-green are respectively from Turkey and Egypt). The other individuals surrounded by a circle were sampled in French natural populations and are suspected to represent exotic frogs i.e. introductions.
Based on the genotype and the factorial map, we can estimate the introduced individuals to at least 10 frogs (3.94%) from 6 distinct populations. Thus, 54.5% of the populations were affected by such introductions.
If considering that “exotic-like” frogs are to be discarded from the French populations to evaluate allelic frequencies, thus GDA-130 and ACO-120 become absent in France i.e. represent specific markers between French populations and allochtonous ones (Table 2). GDA-100 and MPI-135 remain present although scarce because few frogs bear these alleles at an heterozygous state. It suggests cases of genetic pollution i.e. invasion of exotic alleles in the genetic pool of Western populations.
Allelic frequencies at 3 loci. The third column represents allelic frequencies calculated on the whole sample of frog from French natural populations while the fourth column represents allelic frequencies after discarding from the sample the individuals suspected to be exotic. As underlined numbers represent the (suspected) markers of allochtonous origin., the fifth column may represent the cases of genetic pollution. The allele with (GDA-130) represents a potential additional marker because it is linked with ACO(2)-120 in our samples
| Locus | Allele | All frogs | Sample without | Remaining frogs |
| from France | “exotic-like” frogs | with exotic allele | ||
| N=254 | N=244 | |||
| GDA | ||||
| 110 | 0.2990 | 0.2944 | ||
| 120 | 0.6765 | 0.6954 | ||
| ACO-2 | 100 | 0.3088 | 0.3190 | |
| 105 | 0.0069 | 0.0071 | ||
| 110 | 0.6567 | 0.6738 | ||
| MPI | 90 | 0.0042 | 0.0043 | |
| 100 | 0.4937 | 0.4935 | ||
| 119 | 0.4958 | 0.4957 | ||
3.2 General water frog survey
The 608 individuals belong to Rana ridibunda (n=355 frogs; 58.38%); R. kl. esculenta (n=178; 29.28%); and R. lessonae (n=75; 12.33%).
Among the 32 sites, the taxonomic composition recorded was:
- 1. Pure R. ridibunda population in 14 ponds (43.75%).
- 2. L-E assemblage (R. lessonae+R. kl. esculenta) in six ponds (18.75%).
- 3. Pure R. kl. esculenta population in five ponds (15.63%).
- 4. Assemblage involving R. ridibunda+another unexpected water frog species in three ponds (9.38%).
- 5. R-E assemblage (R. ridibunda+R. kl. esculenta) in two ponds (6.25%).
- 6. Pure R. lessonae population in one pond (2.27%).
- 7. Assemblage involving R. lessonae+another unexpected water frog species in one pond (2.27%).
Genotypic frequencies recorded at three loci. In bold, genotypes characteristics of R. kl. esculenta; underlined, those characteristics of R. lessonae (see e.g. [27]). Asterics represent suspected introgression cases
| Taxon | N | LDH-B | MPI | PGM-2 | |||||||
| Ae | ce | ah | ch | dd | cd | ||||||
| R. kl. esculenta | 23 | 12 | 11 | 13 | 10 | 16 | |||||
| R. lessonae |
4 Discussion
Our study highlights the difference in genetic structure (allelic composition, genetic distance) between Oriental populations (Egypt and Turkey) and Occidental ones. However, in France, several frogs caught in natural populations are, in fact, genetically close to frogs originating from Egypt and Turkey, the two main sources for frog import (Fig. 2). In addition, such individuals bear alleles, in an homozygote condition, that are rare in France but very common or even dominant in Oriental populations. This strongly suggests that they are in fact exotic frogs resulting from recent introductions.
This hypothesis is confirmed by the fact that the elimination of the ten “exotic-like” individuals from the sample of French frogs modifies the allelic frequencies so that the alleles GDA-130 and ACO(2)-120 appear then absent from French natural populations (i.e. specific markers of allochtonous origin). The ACO(2)-120 allele appears without doubt as a marker of Oriental origin.
The GDA-130 allele was not found in the exotic individuals studied probably because of our limited sampling size but it seems also to be a marker of Oriental populations as it is linked with ACO(2)-120 in our sample. The MPI-135 allele has already been suspected to reveal an exotic origin as it is present in populations from Anatolia [17]. These results point to the need for an extensive genetic study of Oriental frogs in order: (1) to identify with more accuracy the alleles that can be considered reliable markers of exotic origin; (2) to be able to establish more precisely which frogs are introduced and how many populations are affected by introductions; and (3) to begin to measure the biological and genetic (e.g. introgression) effects of such introductions.
Our study strongly suggests that introductions have been multiple as they affect at least 6 populations several hundred kilometres distant (near Camargue, Southern France and near Leman lake, Central-Eastern France). Indeed, 54.5% of the studied populations, seem to contain, at least, one exotic individual while a sample from a population from Southern France disclosed at least three introduced frogs (16.6% of the sample). These observations highlight the potential importance of introductions on the biology of natural populations.
Our study validates the hypothesis of multiple introductions that was invoked to account for the expansion of the distribution of R. ridibunda in most parts of France ([16] and Fig. 1). Thus, R. ridibunda may be considered as an invasive species in France. However, the presence of ancient native populations cannot be fully discarded nor the hypothesis of more ancient introductions from other countries such as those recorded in Switzerland [30]. All biological consequences of these introductions are to be studied. Because of their importance in France, one major risk is the extinction of remaining natural populations of R. ridibunda if they still exist. Another risk is to modify species distribution, favouring unexpected sympatries and give rise to new hybridisation opportunities [31] that is the basis for processes of genetic pollution. As an example, we recorded 3 sites with unexpected assemblages likely because of translocations of R. ridibunda in a region where it was expected previously to be absent.
The scarce presence of exotic (Egyptian) alleles GDA-100 and MPI-135 in the French population even after discarding “exotic-like” individuals suggests an introgression of exotic genes in French populations (i.e. genetic pollution). This would mean a first way for genetic pollution, at the intra-specific level, through matings between Oriental and Western populations of R. ridibunda (Fig. 3a). This may have important biological consequences, as it is known that Oriental populations of this species exhibit strong differences in genetic structure and biology: in particular, they are hybridogenetic-resistant [32], and no hybrids are known to be present in these Oriental populations.
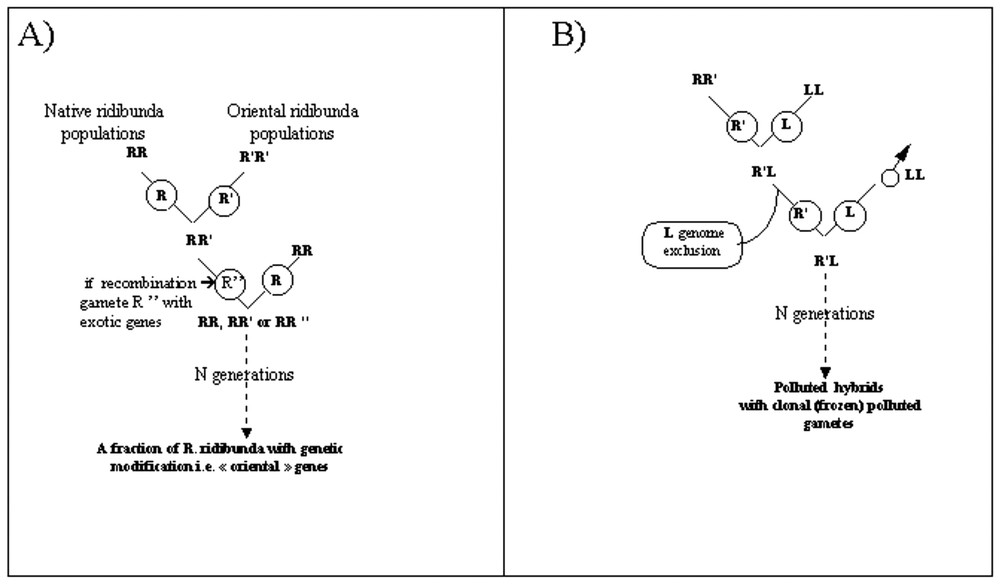
Theoretical scenario of genetic pollution. (A) Within the R. ridibunda species. (B) Transmission of exotic alleles to another taxon (a hybrid taxon; R. kl. esculenta) through natural hybridization between a polluted R. ridibunda and R. lessonae.
A second way for genetic pollution may occur at the inter-specific level through (natural) hybridization between a “polluted” R. ridibunda and a R. lessonae (Fig. 3b). Such matings would result in “polluted hybrids”. As, in European water frogs, hybrids are characterized by fertility, hybridogenesis (i.e. hemiclonal reproduction through genome exclusion of the lessonae genome; see e.g. [23]) and long-term persistence through matings at each generation with R. lessonae, such hybrid may produce “polluted gametes” bearing exotic genes. In such cases, the polluted genome is persistent in hybrids through generations. A genetic investigation of hybrids using markers of allochtonous origin remains to be performed in order to establish the existence of polluted hybrids. Such a way does not appears purely hypothetical, as our results suggested occasional recombination and introgression within water frogs.
5 Conclusions
Considering water frogs, studies are still needed to test the validity of the genetic pollution models proposed and to establish their biological consequences, because our work is preliminary. However, we aim to underline that the genetic pollution model has a more general value as introgression is often caused by introduction of exotic populations or exotic species. Studies on genetic pollution have been too much neglected in conservation biology [10,11] because it is not clear if it may represent a direct risk for species survival. Nevertheless, it clearly affects the genetic integrity of natural populations and cannot be without consequences on their fate. Thus, it represents a problem to be taken into account in future conservation genetics studies.
Acknowledgements
We want to thank F. Renaud for assistance in performing protein electrophoresis.



