1 Introduction
The disease of human immunodeficiency virus (HIV) infection has become a worldwide pandemic [1,2]. UNAIDS reports about an estimated number of 34–46 million people living with HIV worldwide by the end of 2003, and 5 million new infections in 2003 show that it is still growing rapidly. The disease is a major problem first of all in the developing countries of Sub-Saharan Africa. This region accounts for 25.0–28.2 million people infected with HIV by the end of 2003 and 4.2–5.8 million new infections in 2003 (source: UNAIDS http://www.unaids.org/).
Very effective treatments for HIV infected individuals have been developed, and drug prices dropped significantly the last years according to UNAIDS (http://www.unaids.org/). Therefore, they may be widely distributed, not only in the industrial, but also in the developing countries. Thus, it is worth studying the effects that the wide use of antiretroviral can have on the spread of the disease.
HIV predominantly infects CD
Several groups of anti-HIV drugs have been developed that interfere with several processes in the replication cycle of HIV. The most important ones among those are reverse transcriptase inhibitors and protease inhibitors, antiretroviral drugs that inhibit the process of reverse transcription and the viral maturation process, respectively. To interfere with the integration process, integrase is used as another target for the development of anti-HIV drugs. Multiple coreceptors for different types of HIV strains have been identified that probably play an important role in the process of binding to the host cell. These coreceptors are also promising targets for anti-HIV drugs. Starting with highly active antiretroviral therapy (HAART), an anti-HIV combination therapy that includes one protease inhibitor and two reverse transcriptase inhibitors, combination therapy has been used to reduce resistance effects. In many cases, combination therapy leads to a delayed progression of HIV disease. If initiating therapy is used and if dose reductions and inconsistent therapy are avoided, a long-term suppression of the virus below the limit of detection is possible. However, recent studies have shown that HIV persists in a replication-competent form in resting CD
The influence that antiretroviral therapy has on the battle between the human immunodeficiency virus and the human immune system has widely been analysed, see e.g. [6–12]. The spread of HIV/AIDS amongst a population or among interacting communities without treatment has also been investigated [13–19]. Some mathematical and statistical models deal with the spread of HIV/AIDS amongst a population including treatment and/or change of behaviour [8,17,20–25]. For more details on the mathematical modelling of the dynamics of infectious diseases, see [15,26–30].
In [31], the authors formulated and analysed a model for HIV transmission for a homosexual population of varying size with recruitment into the susceptible class proportional to the active population size and with stages of progression to AIDS in [31]. In [32–34], the authors modified this ordinary differential-equation model by incorporating treatment effects. The underlying assumption in [32,33] is that of a worst-case scenario, where infected individuals – no matter if they are treated or not – do not change their behaviour despite their knowledge of the risks. In [32], a general staged progression model with n infectious stages, including anti-HIV drug treatment, was formulated and analysed in detail. A similar model with staged progression based on clinical stages was discussed in detail and simulations were shown in [23].
Although there are lots of research going on in the field of modelling the disease of HIV infection, many effects that antiretroviral treatment has on the spread of HIV infection in a population remain unknown. First of all, it has not been investigated, how the delayed onset of positive treatment effects influences the spread of the disease. Secondly, it is worth studying the possible effects of the latency period, although it is assumed to be very short in the case of HIV (in the range of a few days, see [5]).
In the following, we will study the dynamics of the spread of the disease of HIV infection in a population depending on this treatment-induced delay. We will present an ordinary differential-equation model based on clinical stages as given in [32,33] and the main results in Section 2. In Section 3, we will then modify it by introducing two time delays λ and τ to model the delayed onset of treatment effects (τ) and the latency period (λ). As there is no data on the size of τ, we will show simulations for different scenarios according to the size of this treatment-induced delay. A detailed discussion of the results is given in Section 4.
2 ODE model with two infectious stages: model formulation and main results
In the following transmission via sexual contact is assumed. Thus, we consider a sexually active population of varying size N. AIDS patients are assumed to be too sick to be sexually active. Thus, the total population can be divided into a susceptible class of size S and an infectious class before the onset of AIDS. As the infectious period is very long (⩾10 years), it is further divided into several stages. The widely-used CDC-classification suggests 2–6 stages of infection before AIDS with the classes defined by either viral load or CD
See Table 1 for an overview of the parameters and variables used in the formulation of the model equations. Here also the parameter values used in the simulations below are given. The values for the disease progression rates
Parameters, variables and parameter values
| S | number, proportion of susceptibles |
| number, proportion of infectives in stage j | |
| initial numbers of infectives (in thousands) | |
|
|
total active population size |
|
|
initial population size (in thousands) |
| A | number of AIDS cases |
| b=0.001 | ‘birth’ rate constant (number of people becoming sexually active per unit of time) |
| d=0.0006 | natural death rate constant |
| c=0.3 | average number of contacts of an individual per unit of time |
| β=0.5 | probability of disease transmission per contact by an infective in the first stage |
| a β=0.6 β | probability of disease transmission per contact by an infective in the second stage |
|
|
transfer rate constant |
| α=0.1 or 0.2 | transfer rate constant |
| average number of new infections per unit of time (standard incidence) |
Let
| (1) |
The treatment reproduction number R is used to define a threshold condition for the existence and stability of equilibrium points. It can be interpreted as the average number of infections a typical infective causes in a completely susceptible population. In [32], a formula for R was derived for a very general model with n infectious stages. The complexity of this formula increases significantly with increasing n. In the case of two infectious compartments, it yields the following expression for R:
| (2) |
| (3) |
| (4) |
| (5) |
To discuss the local stability of the endemic steady state
| (6) |
| (7) |
| (8) |
| (9) |
| (10) |
| (11) |
| (12) |
By using the Routh–Hurwitz criterion [35], it follows that all eigenvalues of the characteristic equation (9) have negative real parts if and only if the following condition is satisfied:
| (13) |
Theorem 1
The endemic population steady state
For the parameter values as given in Table 1 and
| (14) |
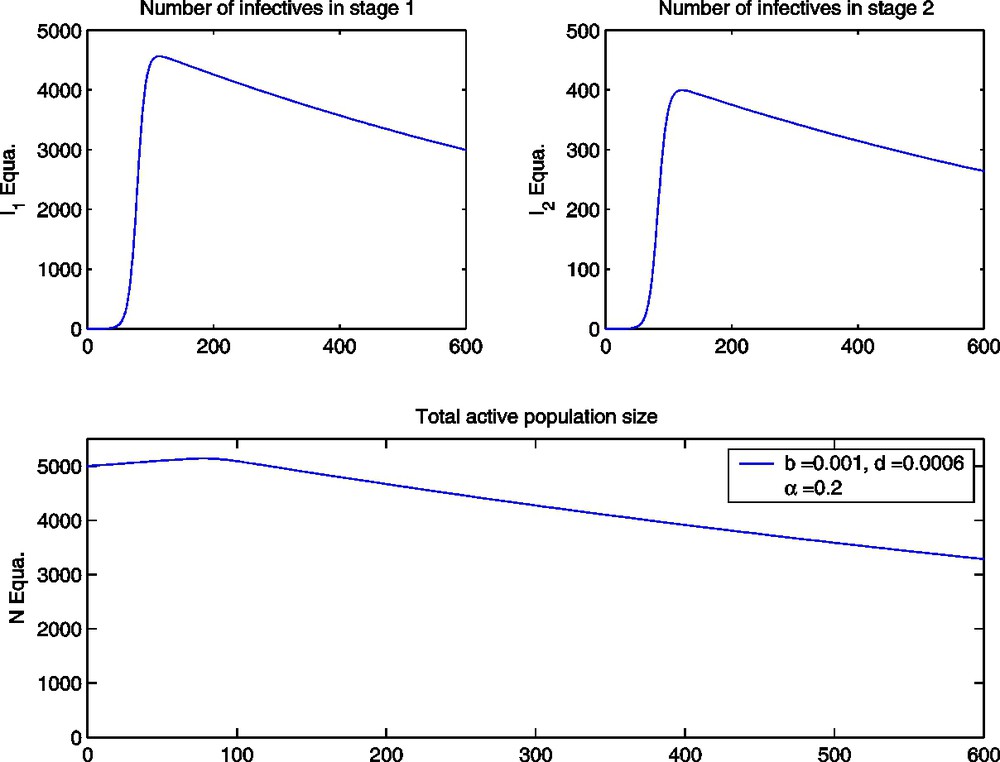
The ODE model with high level treatment (α=0.2) and all parameter values as given in Table 1.
In [32], it was shown in detail that
3 Models with time delay: latency period and treatment induced delay
Let constant τ (in months) be the time delay from the start of treatment in the symptomatic stage
We incorporate these two delays into our ordinary differential-equation model (1) and use the same parameters as in Table 1. We then get the following delay differential-equation model:
| (15) |
| (16) |
The characteristic equation of (16) is given by:
| (17) |
Definition 1
The steady state
The characteristic equation (17) of the linearised system at the endemic steady state
Here we have (again with
Remark 1
We have
The location of the roots of Eq. (17) has been studied by many authors (see [38–46]). The following result, giving necessary and sufficient conditions for the absolute stability of (16), was proved by Hale et al. in [37,47].
Lemma 1
The delay differential-equation system(16)is absolutely stable if and only if:
- (1)
- (2)
To study the delay case, we consider the ordinary differential-equation model (1) first (with
Let
If, on the other hand, the characteristic equation (17) has a pair of purely imaginary roots iω,
To be able to find a condition for all eigenvalues to have negative real parts, we will determine if (17) has purely imaginary roots.
Since the positive equilibrium
If
| (18) |
| (19) |
The resulting differential-equation model including delayed onset of treatment effects but no latency period is formulated as follows:
| (20) |
| (21) |
| (22) |
| (23) |
Remark 2
If
Notice that in the case of a population with high-level treatment (
Again, let
| (24) |
| (25) |
Then the real part of
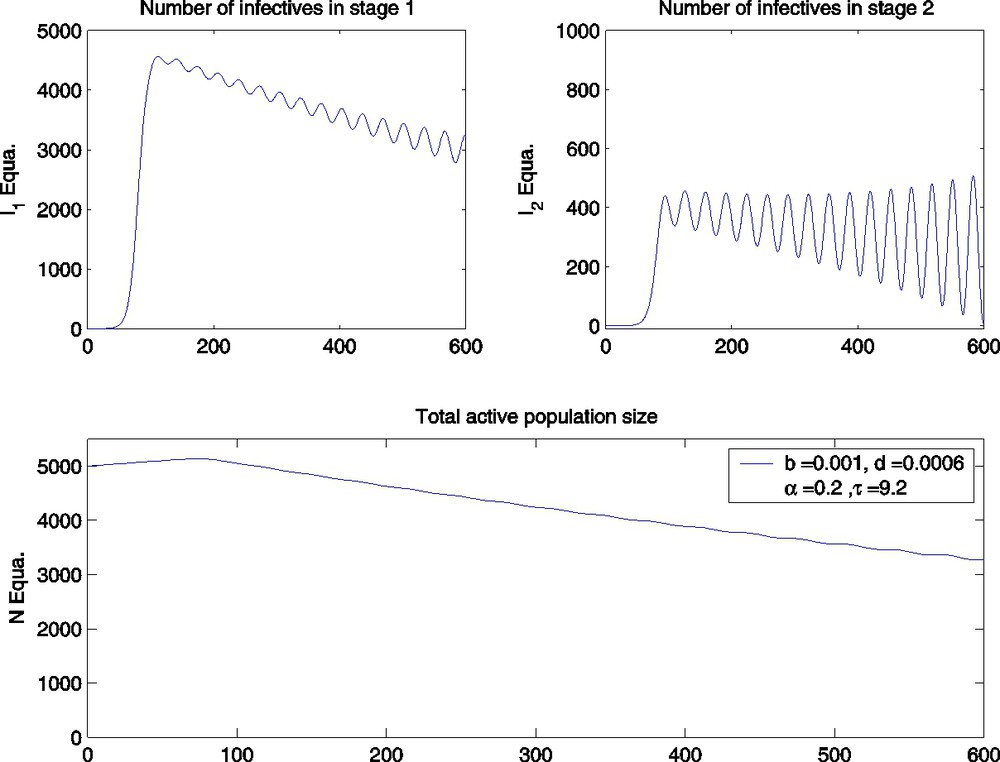
The DDE model with α=0.2,τ=9.2 and parameter values as given in Table 1.
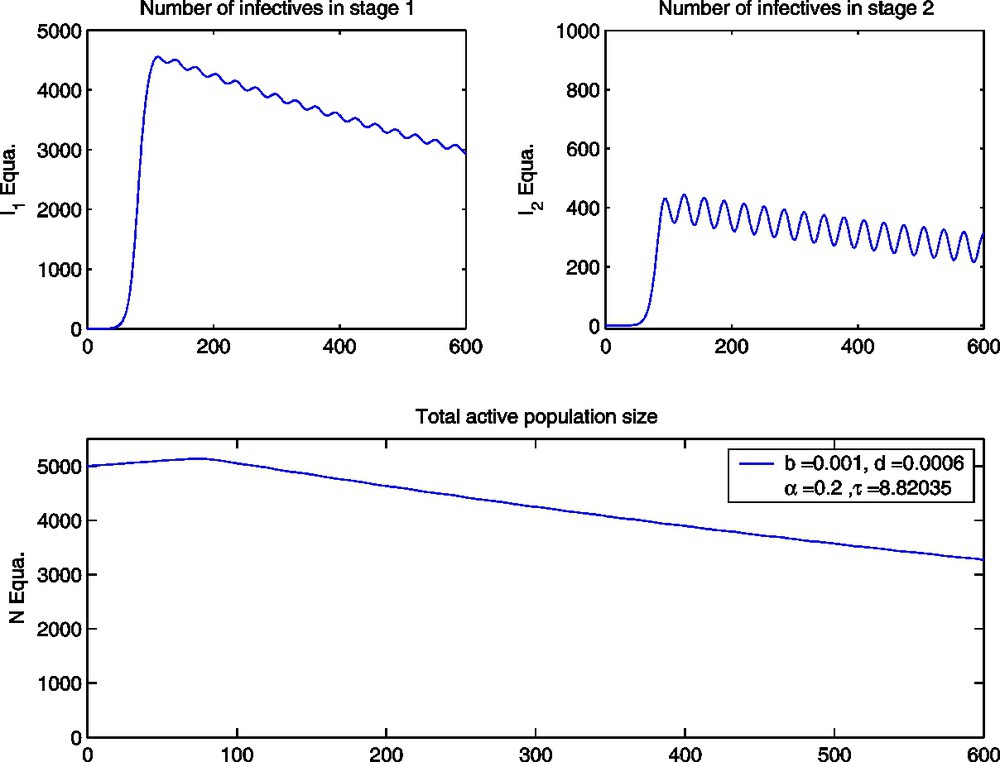
The DDE model with α=0.2,τ≅8.82035 and parameter values as given in Table 1.
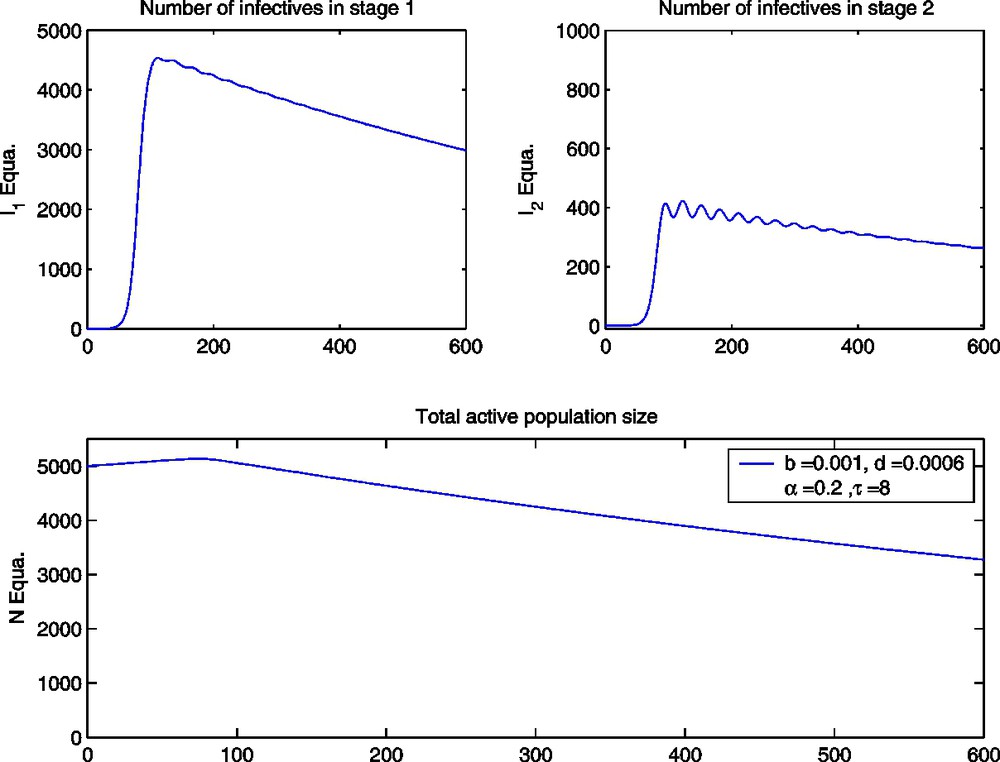
The ODE model with α=0.2,τ=8 and parameter values as given in Table 1.
Theorem 2
The infected steady state
If
Biologically, this means that there is a critical value for the treatment-induced delay
4 Discussion
The underlying assumption in the present paper is that people who are infected with HIV do not change their behaviour despite the knowledge of the risks. In [50], S. Blower states that incidence rates of HIV will fall as more HIV-positive individuals gain access to treatment (HAART), but that this public health benefit will only occur if the levels of risky behaviour do not increase. Computer simulations based on a simple ordinary differential-equation model for an HIV epidemic (see [33]), on the contrary, show that treatment without reduction of risky behaviour may even increase the proportion of infected individuals. This effects can also be seen in the delay differential-equation models presented in the present paper. The simulations for the ODE model in [33] show that the combination of reduction of risky behaviour together with antiretroviral drug treatment is a very promising strategy in fighting the epidemic of HIV infection. This has to be taken into account, when anti-HIV regimens are distributed in the poorest countries of the world, where behaviour change is hard to achieve because of low educational levels or cultural reasons.
In our delay differential-equation models we do have two threshold parameters: The treatment reproduction number R serves as a threshold for the existence of an endemic equilibrium point, and the size of the treatment-induced delay τ has enormous effects on the dynamics of the spread of the disease of HIV infection in the population. This treatment-induced delay τ can be seen as the minimum time span that patients have to be under medical treatment until positive treatment effects occur. If and only if the treatment reproduction number R is above one, there is a positive steady state. If in this case, the treatment induced delay τ is below a critical value, the total sexually active population will reach the infected equilibrium point (see Fig. 2). Note that at this positive steady state there are still people that have not been infected with HIV. The proportion of these susceptibles is given by
The present work wants to be understood as a starting point for further research on analysing treatment effects including time delay on the spread of an HIV epidemic. The next step will be to analyse the general model with n infected stages. We also have to point out that we assumed exponential growth for the total population in the absence of the disease, what is a very restrictive assumption. The simulations with logistic growth for the ODE model given in [32] show that the disease of HIV infection also could lower the carrying capacity or give rise to a second positive steady state below the carrying capacity. It remains to analyse the delay differential-equation model with other than exponential population growth and the role of treatment and behaviour change under these circumstances.
Acknowledgments
The first author was supported by the Fonds zur Förderung der wissenschaftliche Forschung under SFB F003, ‘Optimierung und Kontrolle’, and by EC programme Centers of Excellence of State in phase of pre-accession, No. ICA1-CT-2000-70024.


