1 Introduction
Mountain hydrographic systems are characterized by steep slopes and low water temperatures. Compared to lowland rivers, the impact of sporadic pollutions on the benthic fauna in mountain streams follows a different pattern, mainly because dissolved oxygen is not a limiting factor. Moreover, pollution is usually rapidly washed downstream and diluted by tributary supplies [1].
Several works have been carried out on the impact of man-induced disturbances on benthic macrofauna in alpine streams, i.e. in the Austrian torrents [2,3], and in the Pyrenees [4], however few specifically on the seasonal impact of tourism. In 1975, Lafont [5] studied the impact of the wastewater discharge from the ski stations on streams of the French Alps and Jura Mountains. In Andorra, two studies were conducted on the higher course of the Ariège River to assess the impact of the ski activities and the impact of the sewage treatment plant of the city Pas de la Case on Diatoms [6], and on Chironomid populations [7]. Prat et al. [8,9] conducted an extensive survey of the physicochemical composition of the Andorran streams with special attention to eutrophication processes. Data on the ecology of the Plecoptera and the Ephemeroptera and on the factors determining the distribution of some Diptera within the Andorran hydrographic network are also available [10,11].
The Andorran economy is mainly based on the seasonal tourism, ski in winter and mountain hiking in summer. The permanent population of Andorra, mainly urban, is of 65 877 inhabitants (population census 2000). However, the Andorran population is characterized by a regular increase (4.3% between 1990 and 2000) and a strong seasonal variability (11 million tourists in 2000; source: tourist information of Andorra). In winter, the frequentation of the snow sport stations generates an increase in wastewater discharges (about 336 438 tourists in February 1999 at the city Pas de la Case, corresponding to the maximum number tourists for the period of winter school holidays) and in summer, mountain hiking, hydrothermal curing and commercial tourism due to the duty free zone status of Andorra also strongly increase the production of wastewater (644 267 tourists in August at Pas de la Case in 1999, corresponding to the maximum number tourists for the summer period, and 179 508 tourists in November at Pas de la Case in 1999, corresponding to the minimum number tourists for the other periods). As a consequence, the ecological functioning of the Andorran rivers is disturbed.
This study aims to describe the longitudinal and seasonal changes of the water chemistry composition of the Andorran streams and to identify the impacts of these changes on the benthic macroinvertebrate assemblages. Results are discussed taking into consideration the seasonal tourism activities.
2 Methods
2.1 Studied sites
Andorra is a small landlocked country between France and Spain (Fig. 1). Its altitude ranges between 840 m and 2946 m. The Andorran hydrographic network is organized around two main rivers located on the Spanish slope: the Riu Valira d'Orient and the Riu Valira Del Nord (Fig. 1). The first crosses Andorra from east to west, the second from north to south. They join in Andorra la Vieilla, the main city of Andorra, and become the Riu Gran Valira, a tributary of the Rio Segre (Ebro's catchment). Twelve out of the 13 sampling sites were located on this hydrographic unit. The site of Pas de la Case is atypical, because located on the Pyrenees French slope on the Riu Arieja and downstream the first wastewater treatment plant built in Andorra. Geographical and morphological characteristics of the studied sites are given in Table 1 with additional information about the type of anthropogenic impact affecting each site. Sites located in the upper course of the streams are exposed to ski activities. Most of the sites located in the mid and low courses of the streams are exposed to the discharge of domestic sewages (cities, ski resorts and camping areas) and to mechanical disturbances caused by the development of the tourism infrastructures.
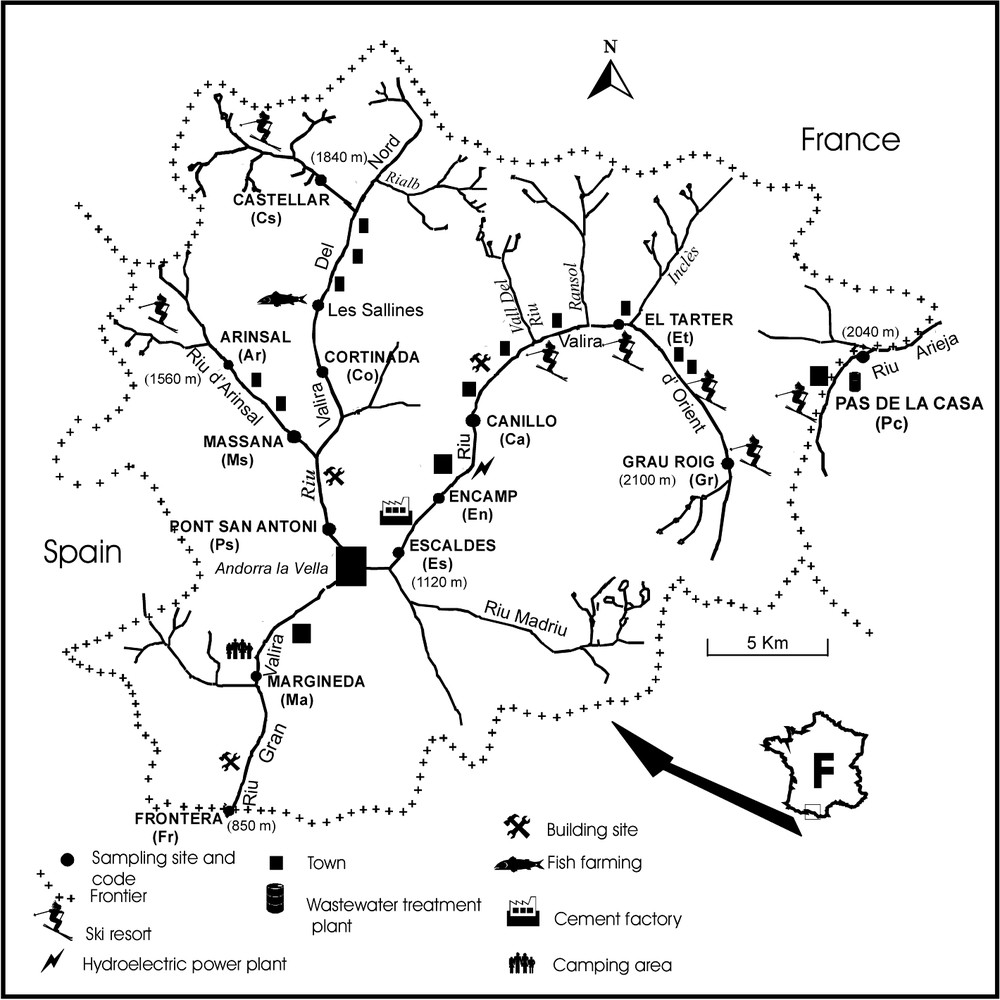
Map of the hydrographic network of Andorra and location of the sampling sites.
Geographical and morphological characteristics of the study sites. Types of disturbance: (1) anthropogenic wastewater discharges, (2) mechanical disturbances due to the development of the tourism infrastructures
| Sites | Grau Roig | El Tarter | Canillo | Encamp | Escaldes | Margineda | Frontera |
| Code | Gr | Et | Ca | En | Es | Ma | Fr |
| Stream | Riu Valira d'Orient | Riu Valira d'Orient | Riu Valira d'Orient | Riu Valira d'Orient | Riu Valira d'Orient | Riu Gran Valira | Riu Gran Valira |
| Latitude North | |||||||
| Longitude East | |||||||
| Altitude (m) | 2100 | 1700 | 1400 | 1240 | 1120 | 940 | 850 |
| Distance to the source (km) | 3.2 | 9.7 | 16.1 | 20 | 22.9 | 28.9 | 35.1 |
| Stream order | 3 | 4 | 4 | 4 | 4 | 5 | 5 |
| Width (m) | 6 | 9 | 5 | 6 | 5 | 10 | 10 |
| Depth (cm) | 20–30 | 50–60 | 40 | 50–60 | 50–60 | 50–70 | 50–70 |
| Main influencing activities | Ski | Ski | – | Building site/ trade tourism | Cement sewage | Trade tourism | Building site/ trade tourism |
| Types of disturbance | 1 | 1 | 1 | 1 and 2 | 1 | 1 and 2 | 1 and 2 |
| Sites | Castellar | Cortinada | Arinsal | Massana | Pont San Antoni | Pas de la Case | |
| Code | Cs | Co | Ar | Ms | Ps | Pc | |
| Stream | Riera de Tristana | Riu Valira Del Nord | Riera d'Arinsal | Riera d'Arinsal | Riu Valira Del Nord | Riu Arieja | |
| Latitude North | |||||||
| Longitude East | |||||||
| Altitude (m) | 1960 | 1340 | 1530 | 1380 | 1120 | 2060 | |
| Distance to the source (km) | 4.1 | 12.0 | 4.9 | 8.2 | 18.6 | 2.4 | |
| Stream order | 4 | 5 | 4 | 4 | 5 | 3 | |
| Width (m) | 7 | 11 | 4 | 5 | 9 | 6 | |
| Depth (cm) | 15–20 | 25–30 | 20–30 | 20–30 | 40–50 | 20–30 | |
| Main influencing activities | Mountain hiking | Fish farm/camping areas | Ski | Mountain hiking/building site | Building site | Ski/trade tourism | |
| Types of disturbance | 1 | 1 | 1 | 1 and 2 | 2 | 1 |
2.2 Data collection
Nine physicochemical variables (temperature, pH, conductivity, COD, BOD, nitrate, nitrite, ammonium and phosphate) were measured weekly from September 1998 to December 1999 by the Medi-Ambient Department of Andorra. Except for temperature, measured in situ, the other physicochemical analyses were conducted at the laboratory according to a normalized protocol.
The benthic macroinvertebrates were sampled in autumn 1998, winter 1998/1999, summer 1999 and autumn 1999 in each sampling sites. At each site, eight subsamples were taken using a Surber net (200 μm mesh size, sampling area of 1/20 m2), according to the microhabitat diversity). All the collected specimens were counted and the family level was retained for this study.
2.3 Statistical analysis
Three approaches were combined in order to analyse the following data.
(1) An analysis of the inter-date inertia (between-analysis [12]) was conducted on both physicochemical and biological datasets. This analysis allowed us to focus on differences between sampling dates without excluding the spatial dimension [13,14]. The matrix of physicochemical parameters was built using the data recorded in the four weeks prior to the invertebrate sampling, in order to detect the modifications of the structure of the faunal assemblages due to prior alteration of the water quality. The four single records were averaged. The final matrix was of 52 samples by nine parameters. The matrix of benthic macroinvertebrates was built using densities of taxa. Taxa represented by fewer than 10 specimens recorded over the study were previously removed from the matrix in order to overcome the strong dependence of ordination methods on single outlier species [15]. Data were standardized by total sample density in order to allow comparability between samples. The working matrix was of 416 samples by 43 taxa. Between-analyses required a pre-ordination of the sites by an ordination method [13,14]. A standardized principal component analysis (sPCA) was performed on the physicochemical variables and a correspondence analysis (CoA) was conducted on the fauna variables.
(2) An analysis of the community structure based on the taxa richness, on the Fisher [16], Shannon [17], Simpson [18], and Margalef [19] diversity indices, and on the Shannon equitability index [17]. A functional feeding group analysis was also performed. The taxa were classified into five functional feeding groups: predators, filtering collectors, gathering collectors, shredders, and scrapers, according to the Cummins's categorisation [20,21].
(3) The third approach was to calculate two biological quality indices expressed in quality classes, i.e. the French indice biologique général normalisé (IBGN) [22], used in routine by the French water agencies in the monitoring programs, and the modified Biological Monitoring Working Party [23], adapted for Spanish rivers.
3 Results
3.1 Ordination of the studied sites
The two inter-dates analyses, performed respectively on the physicochemical and invertebrate datasets, similarly classified the 13 studied sites into four groups: group 1, Castellar–Grau Roig–Arinsal; group 2, Cortinada–El Tarter–Massana; group 3, Canillo–Pont San Antoni–Encamp; group 4, Escaldes–Margineda–Frontera–Pas de la Case (Fig. 2A and B). Only the site El Tarter shifted from group 2 to group 1 and the site Escaldes from group 4 to group 3 in the ordination based on the abiotic variables by comparison to the ordination based on the biotic variables. In both analyses, the total amount of variance explained by the two first axes was more than 70% with more than 55% explained by the first axis and less than 20% by the second axis. Groups 1 and 2 (upstream sites) were separated from group 3 and 4 (mid course and downstream sites) along the F1 axis and groups 1 and 4 were separated from groups 2 and 3 by the second axis (Fig. 2A and B). The projection of the physicochemical variables on the factorial plan of the sPCA showed that the values of all the parameters were higher in the downstream sites (Fig. 2C). Sites from the mid-course of the streams (group 3) exhibited the higher values of organic disturbance (BOD, COD) as well as of reduced forms of nitrogen compounds ( and ). Sites from the lower course (group 4) exhibited the higher values of conductivity, nitrate and phosphate contents (Fig. 2C). The inter-seasons analysis separated on the F1 axis autumn samples from winter and summer samples with 45% of explained variance. Samples from autumn 1998 and summer 1999 were separated on the F2 axis to samples from autumn and winter 99, with 41% of explained variance (Fig. 2D).
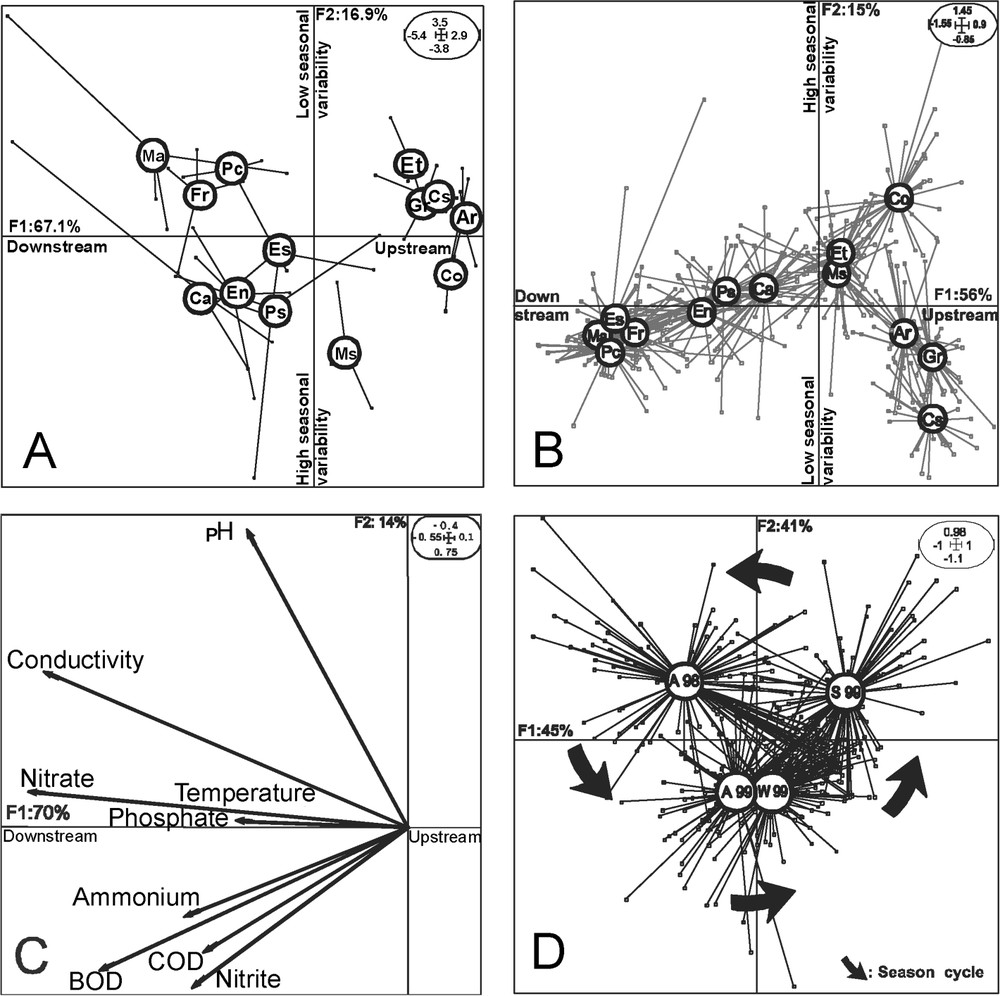
Factorial map of the inter-date analyses (A) based on the physicochemical variables, (B) based on the faunistic variables (full names of the sites are provided in Table 1), (C) projection of the physicochemical variables on the factorial map of the standardized PCA, (D) factorial map of the inter-season analysis based on the faunistic data. A: Autumn, W: winter, S: summer.
The analysis of the physicochemical data showed increasing values for all the parameters from upstream sites to downstream sites (Table 2).
Average values, SEM (standard error on the mean), minimum and maximum values for the nine physicochemical parameters measured. Groups according to the between analysis: (1) = upstream nonpolluted sites, (2) = little polluted sites, (3) = downstream polluted site, (4) = strongly polluted sites
| Sites (group) | Temperature (°C) | pH (pH unit) | Conductivity (μS cm−1) | COD (mg O2 l−1) | BOD (mg O2 l−1) | Nitrate (mg N–NO3 l−1) | Nitrite (μg N–NO2 l−1) | Ammonium (μg N–NH4 l−1) | Phosphate (mg P–PO4 l−1) | |
| Gr (1) | Average value | 5.8 | 7.49 | 49 | 4.3 | 0.5 | 1.91 | 9 | 90 | 0.14 |
| S.E.M. | 0.56 | 0.03 | 2 | 0.6 | 0.2 | 0.19 | 2 | 23 | 0.02 | |
| Minimum value | 1.0 | 7.6 | 39 | 3.0 | 0.1 | 0.1 | 5 | 50 | 0.1 | |
| Maximum value | 11.3 | 7.9 | 82 | 5.0 | 0.1 | 2.2 | 5 | 100 | 0.1 | |
| Et (1) | Average value | 5.2 | 7.36 | 69 | 8.1 | 1.7 | 1.13 | 15 | 373 | 0.37 |
| S.E.M. | 0.47 | 0.03 | 4 | 1.0 | 0.4 | 0.16 | 3 | 115 | 0.098 | |
| Minimum value | 2.3 | 7.1 | 40 | 7.0 | 0.1 | 1.3 | 5 | 100 | 0.1 | |
| Maximum value | 10.6 | 7.6 | 93 | 14.0 | 5.0 | 1.3 | 20 | 800 | 0.6 | |
| Cs (1) | Average value | 5.7 | 7.41 | 55 | 4.3 | 0.7 | 0.93 | 12 | 147 | 0.17 |
| S.E.M. | 0.51 | 0.03 | 2 | 0.9 | 0.3 | 0.11 | 4 | 34 | 0.024 | |
| Minimum value | 4.0 | 7.5 | 44 | 3.0 | 0.1 | 1.3 | 5 | 50 | 0.1 | |
| Maximum value | 12.5 | 7.6 | 66 | 8.0 | 0.1 | 1.8 | 5 | 1600 | 0.2 | |
| Ar (1) | Average value | 8.3 | 8.02 | 67 | 4.9 | 0.2 | 0.36 | 23 | 66 | 0.12 |
| S.E.M. | 0.48 | 0.04 | 4 | 1.9 | 0.1 | 0.08 | 3 | 10 | 0.019 | |
| Minimum value | 6.6 | 8.1 | 32 | 2.0 | 0.1 | 0.1 | 10 | 50 | 0.1 | |
| Maximum value | 15.8 | 8.6 | 93 | 5.0 | 0.1 | 0.1 | 50 | 50 | 0.1 | |
| Co (2) | Average value | 7.0 | 7.59 | 73 | 2.5 | 1.7 | 1.16 | 27 | 54 | 0.19 |
| S.E.M. | 0.50 | 0.03 | 2 | 0.4 | 0.9 | 0.10 | 17 | 2 | 0.045 | |
| Minimum value | 4.7 | 7.9 | 42 | 0.1 | 0.1 | 0.1 | 5 | 50 | 0.1 | |
| Maximum value | 13.5 | 8 | 85 | 5.0 | 0.1 | 1.3 | 5 | 50 | 0.2 | |
| Ms (2) | Average value | 8.3 | 8.02 | 164 | 4.0 | 1.2 | 1.83 | 16 | 181 | 0.20 |
| S.E.M. | 0.48 | 0.04 | 6 | 0.8 | 0.5 | 0.11 | 5 | 51 | 0.042 | |
| Minimum value | 6.6 | 8.1 | 36 | 0.1 | 0.1 | 0.1 | 5 | 50 | 0.1 | |
| Maximum value | 15.8 | 8.6 | 202 | 7.0 | 0.1 | 1.8 | 5 | 50 | 1.0 | |
| Ca (3) | Average value | 6.8 | 7.97 | 207 | 5.4 | 2.4 | 3.50 | 51 | 493 | 0.82 |
| S.E.M. | 0.45 | 0.05 | 10 | 0.6 | 0.7 | 0.18 | 9 | 79 | 0.359 | |
| Minimum value | 4.3 | 8.1 | 82 | 12.0 | 3.0 | 4.4 | 20 | 500 | 0.3 | |
| Maximum value | 11.0 | 8.3 | 284 | 14.0 | 27.0 | 5.7 | 30 | 2600 | 0.8 | |
| En (3) | Average value | 6.8 | 7.93 | 196 | 8.8 | 4.3 | 3.37 | 43 | 553 | 0.46 |
| S.E.M. | 0.51 | 0.04 | 9 | 1.0 | 0.7 | 0.18 | 6 | 132 | 0.062 | |
| En (3) | Minimum value | 6.4 | 7.8 | 92 | 14.0 | 0.1 | 5.7 | 60 | 100 | 0.2 |
| Maximum value | 12.5 | 8.3 | 259 | 6.0 | 3.0 | 5.7 | 140 | 800 | 0.3 | |
| Ps (3) | Average value | 8.4 | 7.87 | 149 | 9.8 | 2.7 | 2.38 | 44 | 430 | 0.34 |
| S.E.M. | 0.48 | 0.10 | 6 | 1.0 | 0.4 | 0.11 | 7 | 58 | 0.031 | |
| Minimum value | 8.3 | 7.3 | 96 | 8.0 | 0.1 | 1.8 | 5 | 50 | 0.3 | |
| Maximum value | 15.0 | 8.2 | 190 | 13.0 | 7.0 | 2.6 | 120 | 800 | 0.6 | |
| Es (4) | Average value | 6.6 | 7.85 | 110 | 6.4 | 1.0 | 2.10 | 63 | 525 | 0.43 |
| S.E.M. | 0.56 | 0.06 | 8 | 0.6 | 0.2 | 0.17 | 13 | 155 | 0.106 | |
| Minimum value | 4.4 | 7.7 | 93 | 7.0 | 0.1 | 2.2 | 60 | 200 | 0.2 | |
| Maximum value | 13.9 | 8.7 | 326 | 17.0 | 2.0 | 4.4 | 60 | 500 | 2.5 | |
| Ma (4) | Average value | 8.3 | 7.55 | 152 | 15.4 | 6.0 | 2.87 | 154 | 1503 | 0.71 |
| S.E.M. | 0.52 | 0.03 | 7 | 1.2 | 1.0 | 0.18 | 36 | 252 | 0.069 | |
| Minimum value | 4.0 | 7.4 | 116 | 7.0 | 4.0 | 2.2 | 140 | 900 | 0.9 | |
| Maximum value | 14.7 | 7.7 | 175 | 44.0 | 26.0 | 4.0 | 160 | 4200 | 1.0 | |
| Fr (4) | Average value | 8.7 | 7.65 | 155 | 13.4 | 4.0 | 3.15 | 142 | 798 | 0.51 |
| S.E.M. | 0.53 | 0.04 | 5 | 2.5 | 0.5 | 0.17 | 18 | 118 | 0.040 | |
| Minimum value | 6.7 | 7.6 | 105 | 7.0 | 0.0 | 3.1 | 20 | 50 | 0.4 | |
| Maximum value | 13.6 | 8.2 | 205 | 23.0 | 2.0 | 3.5 | 330 | 1300 | 0.6 | |
| Pc (4) | Average value | 6.4 | 7.42 | 268 | 16.7 | 7.0 | 2.65 | 82 | 10 379 | 0.97 |
| S.E.M. | 0.45 | 0.04 | 21 | 2.3 | 1.2 | 0.25 | 14 | 2042 | 0.579 | |
| Minimum value | 0.3 | 6.1 | 67 | 0.1 | 0.1 | 0.1 | 5 | 50 | 0.1 | |
| Maximum value | 13.5 | 7.9 | 706 | 67.0 | 44.0 | 14.5 | 560 | 67 700 | 33 |
Mean values of conductivity ranged from 49 to 69 μS cm−1 in sites from group 1 and from 110 to 268 μS cm−1 in sites from group 4. The same pattern was observed for the organic compounds with for example mean values of BOD ranging from 0.2 to 0.7 mg l−1 O2 in sites from group 1 and from 1 to 4 mg l−1 O2 in sites from group 4. For the nitrogen compounds, means values of ammonium ranged, for example, from 66 to 147 μg l−1 in sites of group 1, from 54 to 373 μg l−1 in sites of group 2, from 430 to 553 μg l−1 in sites of group 3, and from 525 to 67 700 μg l−1 in sites of group 4. Similarly, mean values of phosphates ranged from 0.12 to 0.17 mg l−1 in sites from group 1, 0.19 to 0.37 mg l−1 in sites from group 2, 0.34 to 0.82 mg l−1 in sites from group 3 and 0.43 to 0.97 mg l−1 in sites from group 4. The standard errors on the mean (SEM), calculated on the weekly measurements of the physicochemical variables, were higher for all the parameters in the sites from group 4, especially in Pas de la Case (Table 2). Sites of group 3 exhibited the highest SEM values for conductivity, BOD and ammonium, followed by those of groups 2 and 1.
3.2 Structure of the communities
Values of taxa richness, diversity and equitability indices decreased downstream (Table 3). The sites located upstream, i.e. Grau Roig, Castellar, and Arinsal, sheltered respectively 25, 22 and 27 families, whereas only 19 families were recorded at Frontera and only 12 below the wastewater treatment plant of Pas de la Case. The sites of group 1 had values of indices of diversity ranging between 0.14 and 4.53; between 0.42 and 0.74 for the indices of structure, between 13 and 15 for IBGN index and between 73 and 139 for the index. The values for the sites of the group 2 were ranging between 0.12 and 4.05 for indices of diversity, between 0.19 and 0.79 for the indices of structure, between 11 and 16 for the IBGN index, and between 105 and 126 for the index. The values for the sites of the third group were ranging between 0.06 and 2.94 for indices of diversity, between 0.02 and 0.48 for the indices of structure, between 2 and 14 for the IBGN index and between 16 and 188 for the index. The values for the sites of the group 4 were ranging between 0.02 and 1.53 for indices of diversity, between 0.01 and 0.20 for the indices of structure, between 2 and 11 for the IBGN index, and between 7 and 62 for the index.
Number of taxa, Fisher, Shannon, Simpson, and Margalef indices of diversity, equability and biological index values at each site and each season. (Water quality classes are indicated into brackets after the biological index values)
| Sampling sites | Dates | Taxa no. | Fisher index | Shannon index | Simpson index | Margalef index | Equitability | IBGN | |
| Pas de la Case (Pc) | Autumn-98 | 4 | 0.53 | 0.16 | 0.96 | 0.15 | 0.08 | 2 (HC) | 7 (V) |
| Winter-99 | 5 | 0.68 | 0.04 | 0.99 | 0.04 | 0.02 | 2 (HC) | 13 (V) | |
| Summer-99 | 8 | 0.90 | 0.07 | 0.99 | 0.07 | 0.02 | 3 (HC) | 28 (IV) | |
| Autumn-99 | 5 | 0.49 | 0.05 | 0.99 | 0.04 | 0.02 | 2 (HC) | 21 (IV) | |
| Grau Roig (Gr) | Autumn-98 | 14 | 2.01 | 2.66 | 2.11 | 2.64 | 0.70 | 13 (1B) | 73 (II) |
| Winter-99 | 16 | 2.69 | 2.94 | 0.16 | 2.89 | 0.74 | 14 (1B) | 99 (II) | |
| Summer-99 | 20 | 3.50 | 2.53 | 0.34 | 2.47 | 0.58 | 15 (1B) | 132 (I) | |
| Autumn-99 | 18 | 2.96 | 2.75 | 0.22 | 2.70 | 0.66 | 14 (1B) | 114 (I) | |
| El Tarter (Et) | Autumn-98 | 21 | 2.92 | 2.51 | 0.32 | 2.48 | 0.57 | 15 (1B) | 111 (I) |
| Winter-99 | 17 | 2.72 | 2.00 | 0.38 | 1.96 | 0.49 | 13 (1B) | 79 (II) | |
| Summer-99 | 23 | 3.50 | 1.89 | 0.38 | 1.86 | 0.42 | 13 (1B) | 119 (I) | |
| Autumn-99 | 24 | 3.62 | 2.00 | 0.44 | 1.98 | 0.44 | 15 (1B) | 133 (I) | |
| Canillo (Ca) | Autumn-98 | 13 | 1.64 | 0.95 | 0.74 | 0.94 | 0.26 | 13 (1B) | 61 (II) |
| Winter-99 | 15 | 2.26 | 1.20 | 0.60 | 1.18 | 0.31 | 13 (1B) | 61 (II) | |
| Summer-99 | 16 | 2.75 | 1.91 | 0.38 | 1.86 | 0.48 | 10 (2) | 77 (II) | |
| Autumn-99 | 17 | 2.32 | 0.62 | 0.82 | 0.61 | 0.15 | 13 (1B) | 81 (II) | |
| Encamp (En) | Autumn-98 | 11 | 1.61 | 1.49 | 0.48 | 1.47 | 0.43 | 12 (2) | 48 (III) |
| Winter-99 | 11 | 1.34 | 0.34 | 0.91 | 0.33 | 0.10 | 5 (3) | 50 (III) | |
| Summer-99 | 6 | 0.67 | 0.23 | 0.94 | 0.22 | 0.09 | 2 (HC) | 16 (IV) | |
| Autumn-99 | 13 | 1.67 | 0.63 | 0.81 | 0.62 | 0.17 | 12 (2) | 57 (III) | |
| Escaldes (Es) | Autumn-98 | 9 | 1.59 | 0.62 | 0.84 | 0.58 | 0.20 | 4 (HC) | 35 (IV) |
| Winter-99 | 4 | 0.55 | 0.07 | 0.99 | 0.06 | 0.03 | 2 (HC) | 7 (V) | |
| Summer-99 | 9 | 0.67 | 0.18 | 0.94 | 0.17 | 0.06 | 3 (HC) | 31 (IV) | |
| Autumn-99 | 4 | 0.51 | 0.12 | 0.97 | 0.12 | 0.06 | 2 (HC) | 12 (V) | |
| Margineda (Ma) | Autumn-98 | 6 | 0.72 | 0.09 | 0.99 | 0.08 | 0.03 | 11 (2) | 26 (IV) |
| Winter-99 | 7 | 1.02 | 0.14 | 0.97 | 0.13 | 0.05 | 3 (HC) | 20 (IV) | |
| Summer-99 | 7 | 0.73 | 0.05 | 0.99 | 0.05 | 0.02 | 3 (HC) | 16 (IV) | |
| Autumn-99 | 8 | 1.01 | 0.17 | 0.96 | 0.16 | 0.06 | 5 (3) | 36 (III) | |
| Frontera (Fr) | Autumn-98 | 14 | 1.65 | 0.27 | 0.94 | 0.27 | 0.07 | 5 (3) | 62 (II) |
| Winter-99 | 6 | 0.66 | 0.03 | 1.00 | 0.02 | 0.01 | 2 (HC) | 13 (V) | |
| Summer-99 | 7 | 0.77 | 0.12 | 0.98 | 0.11 | 0.04 | 4 (HC) | 16 (IV) | |
| Autumn-99 | 9 | 1.06 | 0.23 | 0.94 | 0.23 | 0.07 | 4 (HC) | 35 (IV) | |
| Casteliar (Cs) | Autumn-98 | 18 | 3.23 | 2.81 | 0.19 | 2.75 | 0.67 | 14 (1B) | 103 (I) |
| Winter-99 | 14 | 2.25 | 2.77 | 0.20 | 2.73 | 0.73 | 13 (1B) | 85 (I) | |
| Summer-99 | 19 | 2.91 | 1.52 | 0.60 | 1.49 | 0.36 | 14 (1B) | 114 (I) | |
| Autumn-99 | 20 | 3.01 | 2.83 | 0.19 | 2.80 | 0.66 | 14 (1B) | 114 (I) | |
| Cortinada (Co) | Autumn-98 | 22 | 4.05 | 3.51 | 0.12 | 3.43 | 0.79 | 15 (1B) | 124 (I) |
| Winter-99 | 24 | 3.59 | 1.98 | 0.42 | 1.95 | 0.43 | 16 (1B) | 140 (I) | |
| Summer-99 | 20 | 3.24 | 2.39 | 0.28 | 2.35 | 0.55 | 15 (1B) | 105 (I) | |
| Autumn-99 | 24 | 3.21 | 2.46 | 0.31 | 2.45 | 0.54 | 16 (1B) | '160 (I) | |
| Pont San Antoni (Ps) | Autumn-98 | 18 | 2.84 | 1.96 | 0.39 | 1.93 | 0.47 | 14 (1B) | '188 (I) |
| Winter-99 | 7 | 0.78 | 0.06 | 0.99 | 0.06 | 0.02 | 3 (HC) | 28 (IV) | |
| Summer-99 | 16 | 3.34 | 1.36 | 0.63 | 1.28 | 0.34 | 9 (2) | 80 (II) | |
| Autumn-99 | 16 | 1.99 | 0.26 | 0.94 | 0.26 | 0.07 | 13 (1B) | 90 (II) | |
| Arinsal (Ar) | Autumn-98 | 20 | 2.83 | 1.78 | 0.46 | 1.79 | 0.42 | 15 (1B) | 132 (I) |
| Winter-99 | 19 | 3.10 | 2.51 | 0.25 | 2.47 | 0.59 | 14 (1B) | 99 (II) | |
| Summer-99 | 22 | 4.53 | 3.23 | 0.14 | 3.13 | 0.72 | 15 (1B) | 139 (I) | |
| Autumn-99 | 20 | 3.64 | 3.07 | 0.17 | 3.00 | 0.71 | 14 (1B) | 114 (I) | |
| Massana (Ms) | Autumn-98 | 16 | 3.10 | 2.21 | 0.34 | 2.14 | 0.55 | 11 (2) | 94 (II) |
| Winter-99 | 18 | 2.80 | 0.78 | 0.80 | 0.76 | 0.19 | 13 (1B) | 97 (II) | |
| Summer-99 | 16 | 2.54 | 2.58 | 0.21 | 2.67 | 0.66 | 11 (2) | 96 (II) | |
| Autumn-99 | 21 | 3.57 | 2.61 | 0.26 | 2.56 | 0.59 | 15 (1B) | 126 (I) |
Regarding the seasonal variability of the faunistic indices, no seasonal trends were observed for the sites located in upper (group 1) and extreme low course (Frontera and Pas de la Case) of the streams, while diversity and equitability indices were lower in summer and winter in the sites located in the middle course (group 3 and Massana in group 2) and in the low course (Margineda and Escaldes) of the streams (Table 3). Pollutant-sensitive taxa, such as Athericidae and Rhyacophilidae, were present at the upstream sites: Grau Roig, El Tarter, Castellar, Cortinada and Arinsal, and disappeared completely in the downstream sites (Table 4). The Plecoptera Perlidae, Perlodidae, Leuctridae and Nemouridae, and the Trichoptera Brachycentridae followed this pattern too.
Occurrence of the macroinvertebrate taxa recorded in the sampling sites
| Taxa | Pc | Gr | Et | Ca | En | Es | Ma | Fr | Cs | Co | Ar | Ms | Ps | |
| TURBELLARIA | Planariidae | + | + | + | + | + | + | + | + | |||||
| NEMATODA | + | + | + | + | + | |||||||||
| OLIGOCHAETA | + | + | + | + | + | + | + | + | + | + | + | + | + | |
| HIRUDINEA | Glossiphoniidae | + | + | + | + | + | + | + | + | + | ||||
| MOLLUSCA | Ancylidae | + | + | + | + | + | + | + | + | + | + | + | ||
| Bythinellidae | + | + | + | + | + | + | + | + | ||||||
| Lymnaeidae | + | + | + | |||||||||||
| Neritidae | + | |||||||||||||
| Valvatidae | + | |||||||||||||
| HYDRACARINA | + | + | + | + | + | + | + | + | + | + | + | |||
| EPHEMEROPTERA | Baetidae | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Ephemerellidae | + | + | + | + | + | |||||||||
| Heptageniidae | + | + | + | + | + | + | + | + | + | |||||
| Leptophlebiidae | + | + | ||||||||||||
| PLECOPTERA | Leuctridae | + | + | + | + | + | + | + | + | + | + | + | + | |
| Nemouridae | + | + | + | + | + | + | + | + | + | + | + | |||
| Perlidae | + | + | + | + | + | |||||||||
| Perlodidae | + | + | + | + | + | + | + | + | + | + | + | + | ||
| Taeniopterygidae | + | |||||||||||||
| COLEOPTERA | Dryopidae | + | ||||||||||||
| Elmidae | + | + | + | + | + | + | + | + | + | |||||
| Hydraenidae | + | + | ||||||||||||
| TRICHOPTERA | Brachycentridae | + | + | + | + | + | + | + | + | + | + | + | ||
| Glossossomatidae | + | + | + | |||||||||||
| Goeridae | + | + | + | |||||||||||
| Hydropsychidae | + | + | + | + | + | + | + | + | + | |||||
| Limnephilidae | + | + | + | + | + | + | + | + | ||||||
| Psychomyiidae | + | + | + | |||||||||||
| Rhyacophilidae | + | + | + | + | + | + | + | + | ||||||
| Sericostomatidae | + | + | + | |||||||||||
| DIPTERA | Athericidae | + | + | + | + | + | + | |||||||
| Blephariceridae | + | + | + | + | + | + | ||||||||
| Ceratopogonidae | + | + | + | + | ||||||||||
| Chironomidae | + | + | + | + | + | + | + | + | + | + | + | + | + | |
| Dixidae | + | |||||||||||||
| Dolichopodidae | + | |||||||||||||
| Empididae | + | + | + | |||||||||||
| Limoniidae | + | + | + | + | + | + | + | + | + | + | + | + | ||
| Psychodidae | + | + | + | + | + | + | + | + | + | + | + | + | ||
| Simuliidae | + | + | + | + | + | + | + | + | + | + | + | + | ||
| Thaumaleidae | + | + | + | + | + | + | + | + | + | + | + | |||
| Tipulidae | + | + | ||||||||||||
| Taxa number | Total taxa = 42 | 12 | 25 | 30 | 19 | 18 | 14 | 15 | 19 | 22 | 31 | 27 | 25 | 26 |
A progressive reduction in the density of some taxa along the longitudinal gradient until their disappearance in the downstream sites was also observed. This was the case for the Baetidae, which were present in the upstream reaches with a density of more than 4080 ind m−2 in Grau Roig, Castellar, Arinsal, El Tarter, Cortinada, a density from 520 to 21 040 ind m−2 in the mid-elevation sites (Massana, Canillo, Encamp), of less than 6860 ind m−2 in the downstream sites (Pont San Antoni, Escaldes) and disappeared at Margineda, Frontera and Pas de la Case. The same pattern was observed to a lesser extent for Chironomidae, as well as for the Heptageniidae, Simuliidae, Limoniidae, and Thaumaleidae, which almost disappeared in the Riu Gran Valira (Table 4). In contrast, some taxa increased in density from the upstream to the downstream sites, however disappeared in the highly disturbed sites. Psychodidae typically followed this pattern, with respectively densities of 4 and 8 ind m−2 in Grau Roig and Cortinada, and 24 and 34 ind m−2 in Encamp and Pont San Antoni (Table 4). In the highly polluted sites Pas de la Case and Frontera, densities decreased again (14 ind m−2 at Frontera and 2 ind m−2 at Pas de la Case). Strongly disturbed sites such as Margineda, Frontera and Pas de la Case were numerically dominated by Oligochaeta (respectively 11 175, 16 192 and 12 908 ind m−2 – annual density) and, to a lesser extent, by Nematodes and Glossiphoniidae (Table 4).
The functional feeding group analysis showed a modification of the trophic structure of the communities along the longitudinal gradient. In the upper sites, Grau Roig, El Tarter, Castellar, Cortinada, and Arinsal, the five trophic categories were represented, while in the mid-course sites, Canillo, Encamp, Escaldes, Margineda, Massana and Pont San Antoni, predators and filtering collectors disappeared (Fig. 3). In the downstream sites, Pas de la Case and Frontera, all the trophic categories disappeared, except gathering collectors, largely represented by Oligochaeta, whose proportion increased downstream parallel to the degradation of the water quality. They dominated the communities at Pont San Antoni, Escaldes, Margineda, Frontera and Pas de la Case where they represented more than 95% of the invertebrates (Fig. 3).
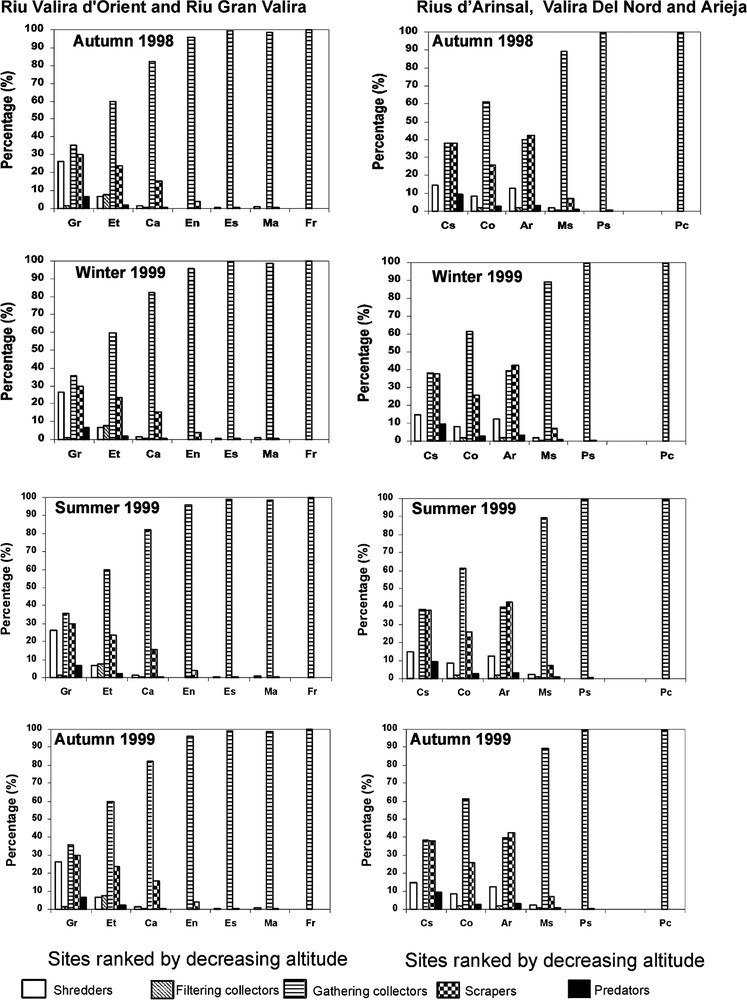
Variations of the trophic structure of the invertebrate assemblages.
Replacement of pollutant-sensitive taxa by pollutant-tolerant taxa within a trophic group was also observed. This was the case in the Rius d'Arinsal and Del Nord at the mid-elevation sites (cites ones) where, for example, Perlodidae, Rhyacophilidae, and Planariidae (pollutant-sensitive predators) were progressively replaced downstream by Glossiphoniidae (pollutant-tolerant predators) (Fig. 3, Table 4) – except accidents related to the drift. No seasonal changes were observed in the trophic structure of the benthic macroinvertebrate assemblages of the studied sites.
3.3 Biological indices
The calculation of the IBGN and scores led to classify the studied sites in three groups differing in their seasonal patterns of biological quality (Table 3): (1) sites with a good biological quality throughout the year (Class 1 of IBGN – Class I of ), namely Grau Roig, El Tarter, Castellar, Cortinada, and Arinsal; (2) sites whose biological quality remained bad throughout the year (classes 3 and HC of IBGN, classes IV and V of BMWP), namely Escaldes, Frontera and Pas de la Case; (3) sites whose biological quality changed seasonally (Canillo, Encamp, Massana, Pont San Antoni, Margineda).
This pattern was similar to that observed for the diversity indices with sites located in the upper course of the streams (groups 1 and 2) exhibiting a good biological quality throughout the year, sites located in the middle section of the streams (group 3, Massana and Margineda) exhibiting seasonal variations of the biological quality and sites located downstream (group 4) exhibiting a bad biological quality throughout the year.
4 Discussion
Results showed a degradation of the physicochemical and biological qualities of the Andorran streams as altitude decreased. In addition, sites from the mid-section of the streams exhibited a degradation of the physicochemical and biological qualities during summer and winter by comparison to the autumn period.
The deterioration of the ecological quality of the Andorran streams had to be related to the strong increase in the tourists' number for the last decades. If the ecological degradation of the mid- and low-altitude sections of the streams was initiated by the concentration and intensification of human settlements in these zones, the sites of high altitude undergo for their part seasonal disturbances due to seasonal tourism activities. Tourism increases locally the organic load and the phosphate and nitrogen release. Increase in fine particle load directly downstream of the ski stations was already mentioned by Molles and Gosz [1]. Although the organic load was high in most of the studied sites located in the upper sections, the impact on the fauna was not so strong because of a continuous and important renewal of the water mass due to high current velocities and dilution by water from unpolluted tributaries. As a result, the exposition of the fauna to the pollutants was not long enough to deeply affect the benthic macroinvertebrate communities and the pollution was transported downstream, where it was added to local pollution. In the downstream sites the pollution increased progressively due to the combination of local and upstream sources. This strong charge in pollutants was partially assimilated by the streams in the mid-elevation sites as shown by the high concentrations of reduced compounds in these sites however not assimilated in the lower sites because the temperature remains unfavourable for the aerobic microbial activity and consequently for the mineralization of the organic matter. In addition, the increase of suspended matter loads due to the construction of new tourism infrastructures (stabilisation of stream banks, enlargement of the roads, and construction of new buildings) in the cities located downstream and to the multiplication of wastewater discharges caused by tourist activities [24] scraped the biofilm, also reducing the primary production and the nutrients assimilation.
Modifications of the benthic macroinvertebrate assemblages were mainly characterized by a reduction of the taxa richness and a progressive dominance of a limited number of specialised pollutant-tolerant taxa.
The results obtained here confirm those found on the Lladure [4], another Pyrenean torrent, where four reaction types of the fauna to organic disturbance were described: (1) loss of sensitive taxa due to slight organic pollution, (2) progressive reduction in the density of certain taxa and their disappearance at the most polluted sites, (3) increase in the overall density of certain taxa for weak organic loads, (4) proliferation of pollutant-tolerant taxa straight at the sewage point.
In addition to the simplification of the faunal assemblages resulting from polluting impacts, the trophic structure of the communities also changed, reflecting the modifications of the nature of the food resources available. The modification of the trophic community structure has to be related to a continuous accumulation of fine organic deposits used by gathering collectors, and to the development of biofilms used by scrapers. Predators, the more specialised group, appeared to be most sensitive. Their disappearance from the mid- and low-elevation sites was directly related to the rarefaction of the specific preys, and to the change in habitat conditions like streambed becoming covered with filamentous bacteria and algae. The loss of filtering collectors in the downstream sites had to be associated with the increasing load of suspended matter, which clogged filtering systems.
Mid-altitude sites, like Canillo, Encamp, Escaldes, Pon San Antoni, Margineda, showed seasonal fluctuations of the environmental conditions, with higher pollutant loads during winter and summer and a consecutive seasonal adaptation of the benthic macroinvertebrate assemblages. The flow regime had to be considered to explain these fluctuations, with the low water level in summer and winter favouring the concentration of the pollutants and the high water level in spring and autumn favouring conversely the dilution of the pollution and its drift toward downstream. This partially explains why the sites located in the mid reaches present contrasted benthic macroinvertebrate assemblages over the year. The faunistic assemblages were characterized by a simplification of their structure during the winter and summer periods and by a partial restoration to the pristine state in spring when snowmelt washed the superficial polluted sediments downstream and allowed the recolonization by drifting macroinvertebrates. Over the year, two phases can be distinguished: a ‘critical period’ in winter and summer and a ‘period of recovery’ in autumn.
Biomonitoring of mountain streams, based on a four seasons sampling protocol of benthic macroinvertebrates and the use of biological indices, appears satisfying. The standardization of the sampling techniques allows comparisons [25]. There is no ideal and universal index to assess the ecological quality of a stream [26]; however, despite accepted limitations like the low sensibility to slight degradation of the water quality [27,28], the use of biological indices is justified and widely adopted in biological surveillance programs. The biological indices (IBGN and BMWP) used in this study reflected the dynamics of the modifications of the benthic macroinvertebrate assemblages due to chronic disturbance like in Pas de la Case or seasonal disturbance like in Encamp or Margineda. Considering the high variability of the environmental conditions in mountain streams, weekly physicochemical surveys coupled to biological assessment methods appear to be complementary [29,30]. This is especially true because these two approaches survey events operating at different scale of time [31]. Except in the case of acute pollution, long-term modifications of the communities are caused by repeated or chronic expositions to pollutants, whereas changes in physicochemical composition of water are instantaneous.
In Andorra, none of the studied sites got a maximal score. Despite the objectives of good ecological quality of the streams fixed by the environment agency of Andorra, the ecological quality of the streams is getting worse since the assessment made by Prat et al. [9]. Recently, new wastewater treatment plants had been installed in order to improve the ecological stand of the hydrographic network. However, these installations are not sufficient to face the pollution produced by the increasing number of inhabitants, the highly fluctuating seasonal number of tourists and the mechanical disturbances due to the construction of tourism infrastructures. The benthic invertebrate communities in polluted mountain streams show a good adaptation to the widely fluctuating conditions. The rapid resilience of these communities is favoured by the predictable discharges of melted snow, which initiates dilution processes and by the recolonization from unpolluted tributaries. However, this pattern does not apply if pollution becomes chronic.
Recently, a new danger appeared: the increasing use of artificial snow to provide and maintain attractive skiing conditions. Such a practice, influence the natural flow regime of the streams as the water is pumped from the streams to make the artificial snow, reducing the stream flow in winter and increasing floods of melted-snow in spring. Further long-term investigations should be carried on this problem.
Acknowledgements
Many thanks to the Government of Andorra for its financial support and to the Medi Ambient Department for supplying the physicochemical data. We are also greatly indebted to all the people who helped on the field, in particular to Francis Dauba (ENSA, Toulouse). To Julia Hunt, who so kindly improved the English, and to the reviewers for their useful comments, many thanks too.


