1 Introduction
1.1 Proximodistal identity in regeneration
One obvious and important difference between the processes of regeneration in adult animals and embryonic development is that the first has a graded outcome, and the second does not. Consider the example of a complex structure such as the limb. The generation of truncated or partial limbs does not occur in normal development, but as an outcome of some abnormality such as in teratology. In contrast, the regeneration of limbs in various species of salamander occurs from any level of the proximodistal (PD) axis (shoulder to fingertip) so as to regenerate just those structures distal to the plane of amputation. The regenerate is derived from a mound of mesenchymal stem cells called the limb blastema, which arises at the end of the stump as illustrated in Fig. 1 [1]. Thus, the wrist level blastema regenerates just a hand, while the shoulder blastema regenerates an entire arm with a continually graded outcome in between. Although we understand something about how PD identity operates at a cellular and tissue level, its molecular basis and implementation remains largely unknown and is a major focus of current efforts. One significant simplification is that although the blastema derives this property from its level of origin on the limb, it is not subsequently dependent on the adjacent stump for any instructive signals [2]. If a limb blastema is transplanted to the fin or the anterior chamber of the eye, it gives rise to a structure appropriate to its level of origin. In view of this morphogenetic autonomy, the level specific property is sometimes referred to as positional memory.
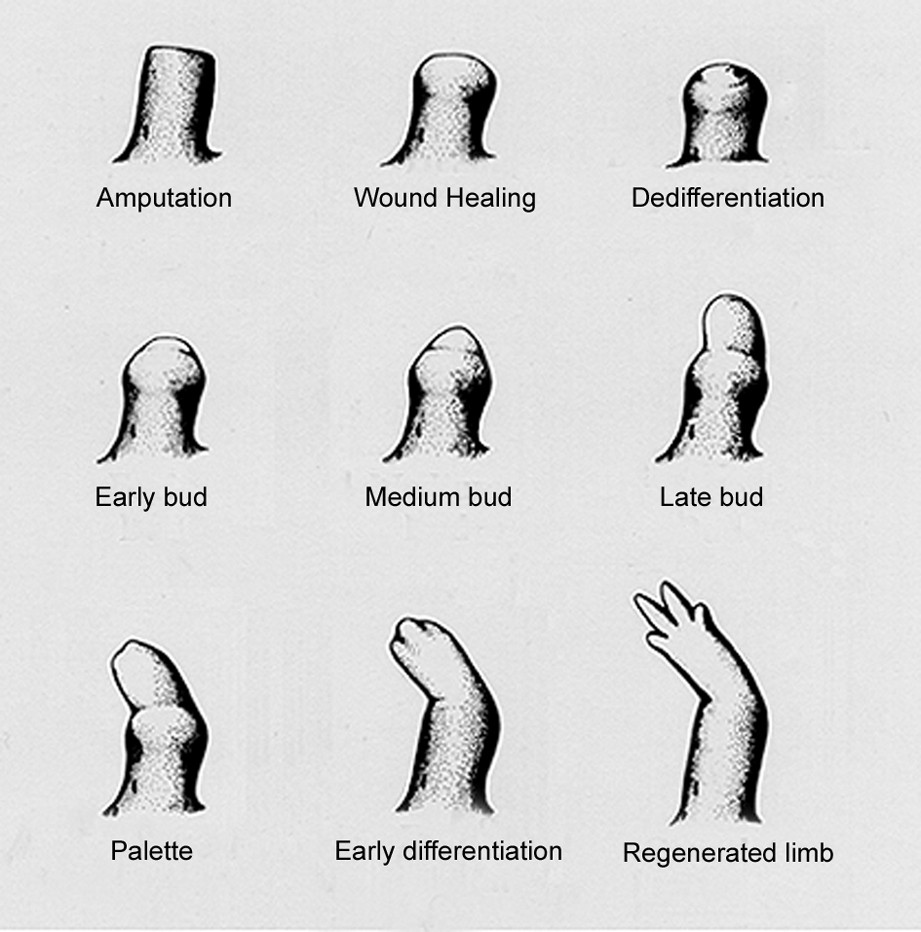
The stages of limb regeneration in an adult newt. Reproduced with permission from Ref. [1].
There are many steps that intervene between the undifferentiated blastemal cells and the patterned tissue of a hand or arm, and the analysis of positional identity and memory has been assisted by more simple assays of cellular activity. If blastemal cells from non-contiguous levels of the PD axis are confronted by experimental manipulation, this activates cell movement, division, and adhesion, predominantly in the more proximal partner [3]. Positional identity operates by detection of disparity between neighbouring cells and it operates on a hierarchical basis. Elbow level cells, for example, behave as the proximal partner next to wrist cells, and as the distal partner to shoulder cells. These sorts of phenomena have also been observed in other contexts of regeneration, such as insect limb regeneration [4]. The importance of intercellular recognition has lead to the suggestion that a critical determinant for PD identity may be expressed at the cell surface. It remains a challenge to extend the principles of positional confrontations to the generation of the entire distal part of the axis. One possibility is that the end of the limb stump, a specialised and transient tissue called the wound epidermis, may act as a distal limit against which division of more proximal cells may occur [3]. Another requirement is that some of the progeny need to take up an intermediate identity to fill in between the amputation plane and the distal limit. It is also important to remember that the PD axis is just one of the limb axes, and the circumferential or transverse axes have been studied extensively, and also exhibit similar phenomena [5]. Furthermore, the transverse and PD axes are interrelated in that PD regeneration depends critically on the presence of a complete representation of the circumferential identity [6]. In order to make progress with these problems at the level of molecular cell biology it seems critical to identify gene products that are directly implicated in the cellular mechanisms. Although various Hox genes, in particular the Meis genes, are strong candidates as determinants for PD identity [7], the transcriptional regulators do not provide a direct insight to understand how the cellular confrontations are mediated.
1.2 Identification of Prod 1/CD59
One important manipulation that acts to respecify PD identity is to expose and early blastema to retinoic acid (RA), precursor retinoids such as Vitamin A, or synthetic retinoids. This converts distal to proximal in a concentration dependent manner. The concentration range for this effect is about 2.5 fold with both Vitamin A and synthetic retinoids, a surprisingly narrow range [8,9]; this encourages the view that the primary specification of blastemal cells depends on a graded level of expression, possibly of a critical surface component. In view of these considerations, we conducted a differential screen on newt distal limb blastemal cDNAs that was designed to identify (1) sequences that are up-regulated or down regulated by RA, (2) the subset of 1 that are co-ordinately regulated by the PD location of the blastema such that RA up, or RA down, , (3) the subset of 2 that are expressed at the cell surface. We identified approximately 300 different examples of (1), but only six of (2), one of which was a small protein expressed at the cell surface with a GPI glycolipid anchor [10]. This is referred to as Prod 1, although as discussed below it has now been definitively identified as the newt ortholog of the mammalian protein called CD59.
The protein sequence revealed significant similarity with members of the Ly6 family, in particular the conserved family motif between residues 77–84 as well as eight conserved cys residues. The presence of the GPI anchor was verified by expression of Prod 1 or Prod 1/GFP fusion proteins after transfection of mouse cells, followed by release of the protein following digestion with the specific phospholipase that cleaves the anchor [10]. The amino acid sequence of CD59 varies markedly between different species and analysis of protein structure has been critical in evaluating the newt sequence. The structure of human CD59 contains one a-helical region and five stretches of β-strand, and all are present in bacterially expressed Prod 1 as determined by solution NMR, including the critical helical region, which is only found in CD59 when compared to other Ly6 family members (A. Garza and P. Driscoll, personal communication).
1.3 Functional evidence for Prod 1 as a determinant of PD identity
If limb blastemas from two different PD levels are confronted in culture, they adhere and then the P engulfs the D over the next 3–5 days, whereas two P explants or two D explants remain with a stable boundary. This assay, originally established by Nardi and Stocum [11], is quite robust and provides a clear test for cellular recognition of PD identity. If the enzyme PIPLC is introduced into the culture medium after adhesion, then PD conjugates behave like PP or DD [10]. This result is consistent with a functional role for a GPI anchored protein, but does not identify which one. Two affinity purified rabbit anti-peptide antibodies to Prod 1 sequences were introduced in the same context, and both gave dose and time dependent inhibition of the engulfment response in PD conjugates, while a control rabbit antibody to the blastemal cell surface protein tomoregulin had no activity [10]. It could be argued that Prod 1 was concerned with the effector mechanism, for example, the engulfment and cell movement, rather than the recognition of PD disparity itself, but taken together with its graded expression and RA responsiveness, the antibody experiments are supportive of a direct role in PD identity.
This has received strong support from experiments of Echeverri and Tanaka [12], who have developed a system for focal electroporation of plasmids into the limb blastema of the larval axolotl. If the distal cells of the blastema are electroporated with a plasmid expressing GFP, then the labelled cells maintain a distal location during subsequent regeneration, and contribute to tissues in the hand. If the contralateral blastema is electroporated with plasmids expressing RFP and Prod 1, the labelled cells relocate and contribute to the more proximal structures. Some cells are even found in stump tissue proximal to the amputation plane. These experiments show that elevated expression of Prod 1 is sufficient to convert distal blastemal cells to proximal cells. These findings have been confirmed in our laboratory (J. Godwin et al., unpublished).
If Prod 1 is a direct determinant of PD identity, how does the confrontation of cells expressing different levels lead to activation (or inhibition) of activities such as movement, adhesion or division? We have suggested a simple model whereby molecules on opposing cell surfaces interact by homophilic adhesion, thereby allowing neighbouring cells to ‘titrate’ their expression [10]. In PD conjugates this would leave spare Prod 1 molecules on P cells at the cellular interface, and these could serve as receptors for either a cell bound or diffusible ligand. A similar model has recently been proposed for the regulation of cell division by morphogens in Drosophila imaginal discs. It may be possible to test the model in cell culture and this is considered later. An obvious requirement for the model is the existence of diffusible or cellular ligands, and this is one focus of our current efforts.
1.4 Expression of Prod 1/CD59 in the normal and regenerating limb
We have recently established conditions for reproducible detection of Prod 1 by immunohistochemical staining of sections of the limb. In order to preserve the immunogenicity of the Prod 1 epitopes we use a procedure originally developed for another GPI linked protein, the prion protein PrP [13]. This employs glutaraldehyde fixation and low temperature wax embedding, along with horseradish peroxidase based detection. This manuscript is concerned with some results obtained with this method, which are chosen because they contribute to some long-standing questions about PD identity.
2 Results
2.1 Graded expression in an adult limb
In Fig. 2a, the expression of Prod 1 in an adult newt limb has been analysed by preparing RNA from five sections along the PD axis, followed by normalised RNAse protection analysis. The x axis is expressed as a percentage of the entire PD axis, as determined by measuring its extent in a group of adult newts. The most distal section of the limb, which has the lowest level of expression, has been assigned a normalised value of unity. Most genes show a distribution over the axis that is not significantly different from uniform, whereas a few show a gradient that is in the opposite direction, i.e., distal high. Prod 1 shows a proximal high gradient, and it should be noted that this was not part of the screen leading to its identification. The gradient appears not to be linear and the data points fall on a single exponential with a maximum proximal value of approximately 2.5 (Fig. 1b).
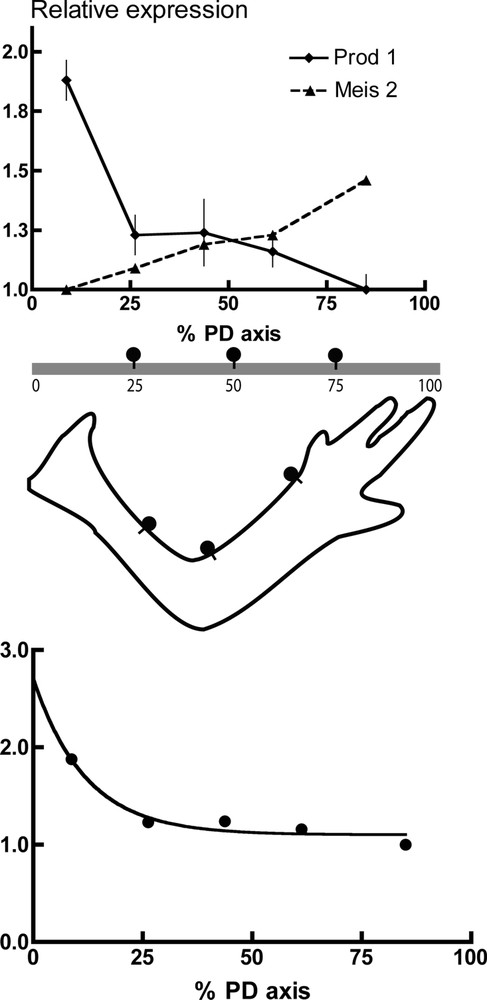
Gradients of gene expression in the adult newt limb. The adult newt limb was cut into five approximately equal pieces at mid-humerus, elbow, mid-radius/ulna and wrist. RNA was extracted and analysed by RNAse protection analysis normalised to an EF-1α probe. (a) The values on the Y axis were adjusted for Prod 1 to a value of unity for the hand, the most distal segment. For Meis 2, the values are adjusted to the most proximal segment, which was the lowest. The x axis is plotted as the % total extent of the PD axis as determined by averaging the relative length to the elbow and wrist for a set of five limbs. Each of the five points is located in the middle of the segment analysed, for example, segment 1 extends from 0 to 17.5% of the axis, and hence the point is at 8.75%. The values for Prod 1 are the mean ± standard deviation for seven independent determinations on two sets of limb samples. For Meis 2 the values are the mean of two determinations. (b) The values of Fig. 2a were plotted according to a single exponential, which gives a 50% value at approximately 10% of the PD extent.
We also performed the RNAse protection analysis on the expression of the newt homeobox gene called Meis 2, which has been implicated in PD identity during limb regeneration [7]. In a comparison of expression in proximal and distal blastemas in parallel with the analysis of Prod 1, the difference was observed to be approximately two fold (Fig. 1c). The expression along the PD axis in normal limb was quite different from Prod 1, however, since it was a shallow gradient (Fig. 1c).
It is difficult to investigate the expression of Prod 1 at the proximal limit by RNAse protection for technical reasons and we have used the immunohistochemical procedure instead. Rather than trying to examine the different tissues of the limb at each level, we focussed on the major branches of peripheral nerve that run from the proximal limit of the axis to more distal regions of the limb. Fig. 3 illustrates sections taken at different levels of a single nerve branch, which were cut and processed together on the same slide. The nerve axons do not express the antigen but it is expressed by the cells of the nerve sheath, principally Schwann cells. It can be seen that the most proximal section, taken at about 5% of the axis, is clearly stronger than the others are, and in general, the most distal section is the weakest. We have analysed five nerves from different animals in this way and although the staining of the middle three sections is somewhat variable, all examples are broadly consistent with the distributions outlined in Fig. 2a and b.
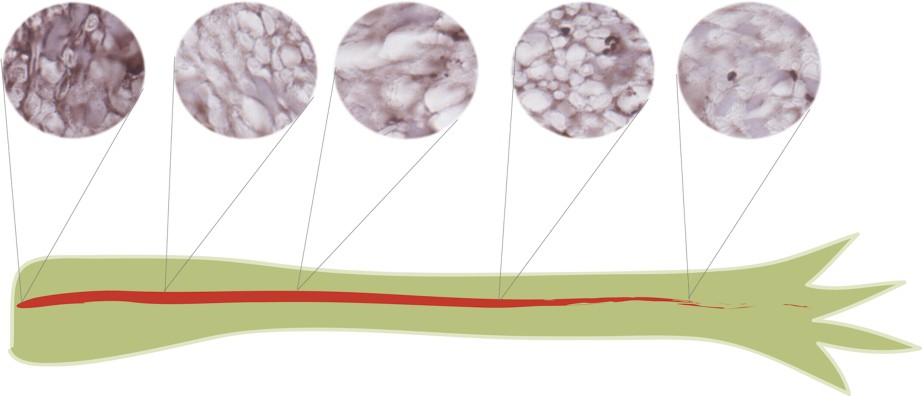
Graded expression of Prod 1 along the PD axis of a single peripheral nerve branch in the adult newt limb. Sections of a limb were mounted and processed together for Prod 1 staining. The plane of sectioning is approximately normal to the PD axis, but is more oblique in some sections. The nerve axons are negative for Prod 1, but the nerve sheath cells, principally Schwann cells, are positive for Prod 1 and clearly strongest at the most proximal part of the nerve. Their appearance can be compared to the graded distributions of Fig. 2a and b. Comparable results have been obtained on five nerves processed independently in this way.
2.2 Expression after RA treatment
Prod 1 was isolated in part, because it is markedly up-regulated by a proximalising dose of RA. These analyses were performed at the RNA level, and in order to study its regulation at a tissue level, newts with distal limb blastemas were injected either with RA or with its vehicle, dimethylsulfoxide (DMSO), prior to analysis of Prod 1 in sections. The RA-treated distal blastemas showed a clear upregulation of Prod 1 expression throughout the mesenchymal tissues relative to the parallel samples from DMSO-treated animals (Fig. 4a and b). In observing these samples, our attention was drawn to the appearance of the RA-treated distal dermis of the regenerate. The effect of RA was particularly marked in up-regulating Prod 1 in this tissue as shown at higher power (Fig. 4c and d). The dermis is recognised as a particularly important source of cells for tissue patterning during regeneration as discussed below. These examples of antibody staining on tissue sections illustrate the utility for understanding pattern formation in a complex tissue architecture such as that of the limb.
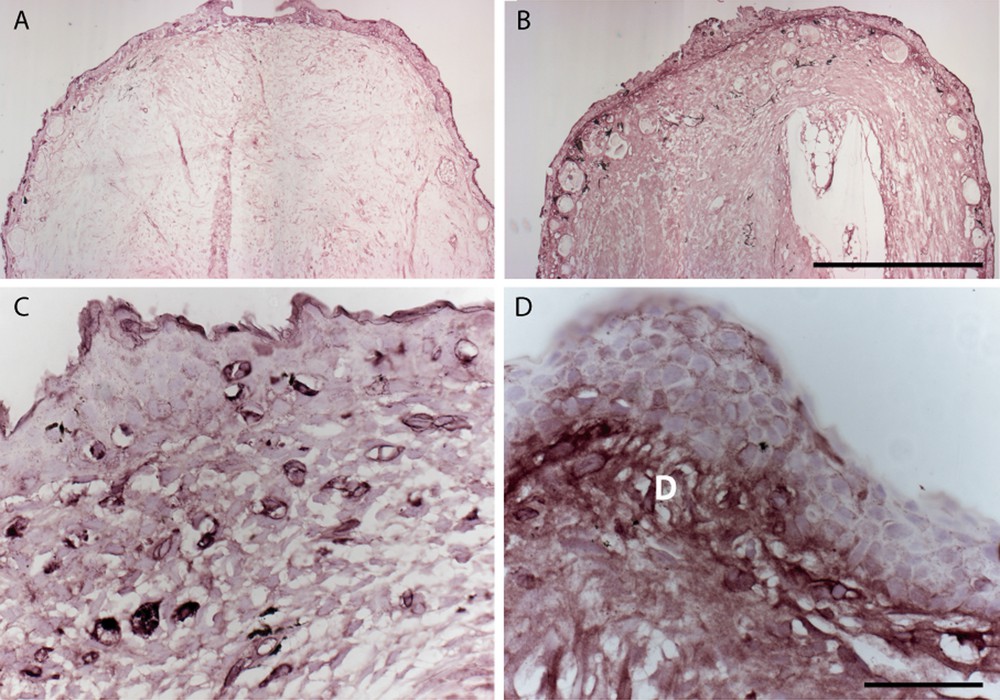
Expression of Prod 1 in distal limb blastemas and limb dermis after proximalisation with RA. Newts were amputated at mid-radius/ulna and injected with RA or DMSO (vehicle) at 14d post-amputation. Blastemas were taken five days later, sectioned and stained for Prod 1 expression. (A) Appearance of blastema from DMSO injected animal or (B) RA injected. Note the higher expression in the RA-treated case, as well as the appearance of more glands in the skin. (C) High-power view of dermis in the regenerating limbs treated with DMSO or (D) treated with RA. Note the marked induction of expression in the dermis (D). Scale bar: A, B, 100 μm; C, D, 50 μm.
3 Discussion
The data presently available support the hypothesis that the level of expression of Prod 1 is a critical determinant of PD identity. It becomes important to ask how blastemal cells arising on the PD axis derive their level of expression after amputation. There are a number of possibilities but we will consider two contrasting models. One is that the cells experience different concentrations of RA along the axis, and this sets the appropriate initial level of Prod 1. It is unlikely that this would operate as a gradient over the dimensions of an adult salamander limb, but it is possible that it could be locally released from the wound epidermis to act on the underlying mesenchyme. There is evidence that RA is released from the wound epidermis in culture, but the evidence that this property varies according to the level of amputation on the PD axis is equivocal [14]. The second model is that Prod 1 is expressed in a stable gradient along the axis in the cells of the adult limb that are precursors of blastemal cells, and that after amputation the blastemal cells inherit this level of expression. It is obviously significant in this context that we observe a gradient of expression in the adult limb, although in itself this is not sufficient evidence to establish the model. It is interesting that the Meis 2 gene shows greater expression in a proximal than a distal blastema, but does not show a gradient of expression in the adult limb. It may be a ‘downstream’ determinant of PD identity, rather than an upstream determinant derived from the precursor cells of the adult limb. The question of the relationship between Prod 1 and Meis 1 and 2 will need to be addressed further by altering the expression of these genes.
The effect of RA on elevating the expression of Prod 1 is readily detectable at the tissue level, but the effect on the dermal cells is particularly noteworthy from the viewpoint of pattern formation. The dermal fibroblasts carry the information for the pattern as shown by classical experiments in which skin (epidermis + dermis) is grafted to an irradiated limb [6]. Although the irradiated muscle and cartilage are not able to contribute to the regenerate, the normal dermal fibroblasts give rise to blastemal cells that are able to organise the pattern in the absence of muscle. It is clear that RA has a marked effect on Prod 1 expression by these cells, and the familiar proximalisation of the pattern may follow from this. The properties of newt dermal fibroblasts are a focus of our current efforts.


