1 Introduction
Soil salinity is one among the several environmental stresses causing drastic changes in the growth, physiology, and metabolism of plants, and threatening the cultivation of plants around the globe. Salt accumulation in irrigated soils is one of the main factors that diminish crop productivity, since most of the plants are not halophytic [1]. Salt stress induces various biochemical and physiological responses in plants and affects almost all plant processes [2]. Salinity can cause hyperionic and hyperosmotic effects on plants, leading to membrane disorganization, increase in reactive oxygen species (ROS) levels, and metabolic toxicity [3]. Salinity also induces water deficit, even in well-watered soils by decreasing the osmotic potential of soil solutes, thus making it difficult for roots to extract water from their surrounding media [4]. Excessive sodium (Na+) inhibits the growth of many salt-sensitive plants and glycophytes, which include most crop plants.
Mechanisms of salt tolerance, not yet clear, can be to some extent explained by stress-adaptation effectors that mediate ion homeostasis, osmolyte biosynthesis, toxic radical scavenging, water transport, and long-distance response coordination [3]. The typical first response of all plants to salt stress is osmotic adjustment. Compatible solutes accumulation in the cytoplasm is considered as a mechanism to contribute salt tolerance [5]. To counter with salt stress, plants increase the osmotic potential of their cells by synthesizing and accumulating compatible osmolytes such as proline (PRO) and glycine betaine (GB), which participates in the osmotic adjustment [6]. PRO and GB are thought to function as osmoprotectants for proteins [7]. Accumulation of PRO and GB provide an environment compatible with the macromolecular structure and function and helps to adapt the salinity injury [5]. Protein hydrolysis under salt-stressed plants is associated with increased PRO content [8]. Proline oxidase (PROX) and γ-glutamyl kinase (γ-GK) play an important role in controlling the level of PRO, PROX catalyzes the conversion of PRO to glutamate, and γ-GK plays an important role in the synthesis of PRO [4]. The enzymes γ-GK and γ-glutamyl phosphate reductase (γ-GPR) are regarded as an enzyme complex called pyrroline-5-carboxylate (P5C) synthetase because the resulting product, glutamic γ-semialdehyde is non-enzymatically converted to P5C. From there, the P5C is converted into PRO by -pyrroline-5-carboxylate reductase (P5CR). The regulation of PRO synthesis is probably controlled by the activity of P5C synthase [9].
In addition to this, an important consequence of salinity stress in plants is the excessive generation of ROS such as superoxide anion (), H2O2, and the hydroxyl radicals, particularly in chloroplasts and mitochondria [10,11]. Generation of ROS causes rapid cell damage by triggering off a chain reaction [12]. In order to survive under stress conditions, plants are equipped with oxygen radical detoxifying enzymes, such as superoxide dismutase (SOD), ascorbate peroxidase (APX), catalase (CAT), and antioxidant molecules, like ascorbic acid (AA), α-tocopherol, and reduced glutathione (GSH) [13]. ROS scavenging depends on the detoxification mechanism provided by an integrated system of non-enzymatic reduced molecules like AA and glutathione and enzymatic antioxidants [14]. Antioxidant mechanisms may provide a strategy to enhance salt tolerance in plants.
Chemical treatment and agronomical crop management practices have been tried to alleviate the salinity effects without much success, and a widespread practice to reduce salt content in soils is leaching. With the rising cost of water, this method may not continue to be a feasible method for the future. In addition, economic pressures on water supplies may force agriculture to use greater amounts of water of lesser quality with respect to salt concentration level [15]. Improving plant resistance to salt, although not a final solution, may provide field stability in subsistence agriculture. A possible alternative is to induce the capability within plants to face successfully the detrimental situation by treatment with growth regulators. Application of growth regulators has been reported to mitigate the adverse effects of salinity [14]. The use of plant growth regulators results in a significant increase in the growth and yield of many crops under stress conditions [16]. One possible approach to reducing the effect of salinity on plant productivity is through the addition of calcium supplements to irrigation water [17]. Supplementing the medium with Ca2+ alleviates growth inhibition by salt in glycophyte plants [18]. Ca2+ sustains K+ transport and K+/Na+ selectivity in Na+ challenged plants. The interaction of Na+ and Ca2+ on plant growth and ion relations is well-established [19].
H2O2 is an endogenous signalling molecule involved in plant responses to abiotic and biotic stresses such as extremes of temperature, light intensity, drought, pathogen, salinity, as well as stimuli such as plant hormones and gravity [11]. Accumulation of H2O2 will also lead to enhance potential for production of hydroxyl radicals, which leads to lipid peroxidation (LPO) and membrane deterioration. Under saline environments, the plant lipid metabolism is interrupted because of oxidative damage to membrane lipids by active oxygen species (AOS) and LPO [20,21]. LPO can also be initiated enzymatically by lipoxygenases (LOX) [22], and this enzyme incorporates molecular oxygen into linoleic and linolenic acids, to form lipid hydroperoxides [23].
With increasing realization of health hazards and toxicity associated with the indiscriminate use of synthetic drugs and antibiotics, more and more people are interested in the use of plants and plant-based drugs revived throughout the world. Therefore, exploitation of medicinal plants became more and more popular [24]. For the past several years, several scales of physiology have been applied to study responses to salt-stress tolerance mechanisms and methods to overcome salt stress in field crops [25,26]. However, little information is gained about the physiological basis in terms of PRO metabolism and effects on secondary metabolite content under salt stress in medicinal plants. It seems necessary to do research related to the correlation between medicinal plants and salt stress for the increasing need of medicinal plants. In order to meet the ever-increasing demand for medicinal plants, for the indigenous systems of medicine as well as for the pharmaceutical industry, some medicinal plants need to be cultivated commercially, but the soil salinity and other forms of pollutions pose serious threats to plant production [27]. Therefore, it seems valuable to test the important medicinal plants for their salt-tolerance capacity. Catharanthus roseus (L.) G. Don. (Family: Apocynaceae) is one of the highly exploited and studied medicinal plants. This plant contains alkaloids that are valuable source of antitumour agents like vinblastine and vincristine used in chemotherapy of leukaemia and in the treatment of Hodgkin's disease, and also a popular ornamental plant [28]. C. roseus is classed as a glycophyte. Despite the relative great number of reports on the medicinal aspects [29,30], growth-regulator effects [31], and water-stress studies [32–35] of C. roseus plants, there are only a few attempts to explain the physiological basis of salt effects and osmoregulation and secondary metabolites' accumulation [5,13,14]. To the best of our knowledge, no information on the physiological response in terms of PRO and alkaloid metabolisms of C. roseus to NaCl and CaCl2 application is available. The purpose of this study was to provide additional information on the oxidative damage (LPO and H2O2 contents), osmolyte concentration (GB and PRO contents), PRO metabolizing enzymes (γ-GK and PROX), antioxidant enzymes (SOD, POX and CAT) activities, and indole alkaloid accumulation in C. roseus under individual and combined NaCl and CaCl2 treatments.
2 Materials and methods
2.1 Plant materials and growth
The seeds of C. roseus were collected from the Department of Horticulture, Annamalai University, Tamil Nadu, India. Seeds were then surface sterilized in an aqueous solution of 0.1% HgCl2 for 60 s to prevent fungal attack, and rinsed in several changes of sterile water. The seeds were pre-soaked in 500 ml of deionized water (control), 80 mM NaCl, 80 mM NaCl + 5 mM CaCl2 and 5 mM CaCl2 solutions for 12 h. Seeds were sown in plastic pots filled with soil mixture containing red soil, sand, and farmyard manure (FYM) at 1:1:1 ratio. Before sowing the seeds, the pots were irrigated with the respective treatment solutions and the electrical conductivity (EC) of the soil mixture was measured. Four seeds were sown per pot and the pots were watered to the field capacity with deionized water up to 90 days after sowing (DAS), and every care was taken to avoid leaching. The initial EC level of the soil was maintained by flushing each pot with the required volume of corresponding treatment solution on 45, 60 and 75 DAS.
The position of each pot was randomized at four-day intervals to minimize spatial effects in the greenhouse, where the temperature was 28 °C during the day and 22 °C at night, and the relative humidity (RH) varied between 60 and 70%. The seedlings were thinned to one per pot on 20 DAS. Plants were uprooted randomly on 90 DAS and analysed for estimating the oxidative damage, osmolyte concentration, PRO metabolism, and indole alkaloid accumulation.
2.2 Oxidative damage
2.2.1 H2O2 content
The hydrogen peroxide content was determined according to Velikova et al. [36]. 0.5 g of fresh plant material was homogenized in an ice bath with 5 ml of 0.1% (w/v) trichloroacetic acid (TCA). The homogenate was centrifuged at 12,000 rpm for 15 min, and 0.5 ml of the supernatant was added to 0.5 ml of 10 mM potassium phosphate buffer (pH 7.0) and 1 ml of 1 M potassium iodide (KI). The absorbance of the supernatant was measured at 390 nm in a spectrophotometer (U-2001-Hitachi). The content of H2O2 was calculated by comparison with a standard calibration curve, previously plotted by using different concentrations of H2O2.
2.2.2 Lipid peroxidation
LPO was estimated as thiobarbituric acid reactive substances (TBARS) [37]. A fresh sample (0.5 g) was homogenized in 10 ml of 0.1% TCA, and the homogenate was centrifuged at 15,000 rpm for 15 min. To 1.0 ml aliquot of the supernatant, 4.0 ml of 0.5% thiobarbituric acid (TBA) in 20% TCA were added. The mixture was heated at 95 °C for 30 min in the laboratory electric oven and then cooled in an ice bath. After centrifugation at 10,000 rpm for 10 min, the absorbance of the supernatant was recorded at 532 nm in our spectrophotometer. The TBARS content was calculated according to its extinction coefficient of 155 mM−1cm−1 and expressed in units (U). One ‘U’ is defined as μmole of MDA formed min−1 mg−1 protein.
2.3 Osmolyte concentration
2.3.1 Proline content
The PRO content was estimated by the method of Bates et al. [38]. The plant material was homogenized in 3% aqueous sulfosalicylic acid and the homogenate was centrifuged at 10,000 rpm. The supernatant was used for the estimation of the PRO content. The reaction mixture consisted of 2 ml of acid ninhydrin and 2 ml of glacial acetic acid, which was boiled at 100 °C for 1 h. After termination of reaction in ice bath, the reaction mixture was extracted with 4 ml of toluene, and absorbance was read at 520 nm.
2.3.2 Glycine betaine content
The amount of GB was estimated according to the method of Grieve and Grattan [39]. The plant tissue was finely ground, mechanically shaken with 20 ml deionized water for 24 h at 25 °C. The samples were then filtered and filtrates were diluted to 1:1 with 2 N H2SO4. Aliquots were kept in centrifuge tubes and cooled in ice water for 1 h. Cold KI–I2 reagent was added and the reactants were gently stirred with a vortex mixture. The tubes were stored at 4 °C for 16 h and then centrifuged at 10,000 rpm for 15 min at 0 °C. The supernatant was carefully aspirated with a fine glass tube. The periodide crystals were dissolved in 9 ml of 1,2-dichloroethane. After 2 h, the absorbance was measured at 365 nm using GB as standard.
2.4 Proline metabolizing enzymes
2.4.1 γ-Glutamyl kinase [ATP: L-glutamate 5-phosphotransferases (EC 2.7.2.11)] activity
γ-GK activity was assayed by the method of Hayzer and Leisinger [40]. Plant samples (1 g) were extracted with 50 mM Tris-HCl buffer and centrifuged at 40,000 g for 30 min at 4 °C. 0.1 ml reaction buffer was prepared by adding 0.1 ml 10 × ATP and 1.8 ml of extract and incubated at 37 °C for 30 min, 2 ml of stop buffer was added. γ-GK activity was measured at 535 nm and expressed in units (U mg−1 protein). One unit of enzyme activity is defined as μg of γ-glutamylhydroxamate formed min−1 mg−1 protein.
2.4.2 Proline oxidase [L. Proline: O2 Oxidoreductase (EC 1.4.3.1)] activity
PROX activity was determined according to the method outlined by Huang and Cavalieri [41]. Plant samples (1 g) were extracted with 5 ml of Tris-HCl buffer (pH 8.5) grinding medium, and centrifuged at 10,000 g for 10 min at 4 °C. The supernatant was again centrifuged at 25,000 g at 20 min at 4 °C. A 3-ml assay mixture was prepared by taking 0.1 ml of extract, 1.2 ml of 50 mM Tris HCl buffer (pH 8.5), 1.2 ml of 5 mM MgCl2, 0.1 ml of 0.5 mM NADP, 0.1 ml of 1 mM KCN, 0.1 ml of 1 mM phenazine methosulphate (PMS), 0.1 ml of 0.06 mM 2,6-dichlorophenol indophenol (DCPIP), and 0.1 ml distilled water instead of PRO. The reaction was monitored at 600 nm at 25 °C using PRO to initiate reaction; the OD value's increase was noted at 0, 1, 2, 3, 4, and 5 min. PROX activity was expressed in U mg−1 protein (one U = mM DCPIP reduced min−1 mg−1 protein).
2.5 Antioxidant enzyme extractions and assays
2.5.1 Superoxide dismutase (SOD, EC 1.15.1.1)
The activity of SOD was assayed as described by Beauchamp and Fridovich [42]. The reaction mixture contained riboflavin, 0.1 M methionine, KCN, and nitroblue tetrazolium (NBT) salt dissolved in 3 ml of 0.05 M sodium phosphate buffer (pH 7.8). Three millilitres of the reaction medium were added to 1 ml of enzyme extract. The mixtures were illuminated in glass test tubes by two sets of Philips 40-W fluorescent tubes in a single row. Illumination was started to initiate the reaction at 30 °C for 1 h; identical solutions that were kept under dark served as blanks. The absorbance was read at 560 nm in the spectrophotometer against the blank. SOD activity is expressed in U mg−1 protein (U = change in 0.1 absorbance h−1 mg−1 protein).
2.5.2 Peroxidase (POX, EC 1.11.1.7)
POX was assayed by the method of Kumar and Khan [43]. The assay mixture of POX contained 2 ml of 0.1 M phosphate buffer (pH 6.8), 1 ml of 0.01 M pyrogallol, 1 ml of 0.005 M H2O2 and 0.5 ml of enzyme extract. The solution was incubated for 5 min at 25 °C, after which the reaction was terminated by adding 1 ml of 2.5 N H2SO4. The amount of purpurogallin formed was determined by measuring the absorbance at 420 nm against a blank prepared by adding the extract after the addition of 2.5 N H2SO4 at zero time. The activity was expressed in unit mg−1 protein. One unit is defined as the change in the absorbance by 0.1 min−1 mg−1 protein.
2.5.3 Catalase (CAT, 1.11.1.6)
The activity of CAT was measured according the method of Chandlee and Scandalios [44] with small modifications. The assay mixture contained 2.6 ml of 50 mM potassium phosphate buffer (pH 7.0), 0.4 ml of 15 mM H2O2, and 0.04 ml of enzyme extract. The decomposition of H2O2 was followed by the decline in absorbance at 240 nm. The enzyme activity was expressed in units mg−1 protein (U = 1 mM of H2O2 reduction min−1 mg−1 protein). The enzyme protein was estimated by the method of Bradford [45] for all the enzymes.
2.6 Indole alkaloid accumulation
Total alkaloid extraction and determination was carried out by following the standard extraction method of Uniyal et al. [46], modified by Misra and Gupta [21]. Freshly harvested leaf, stem, and root samples of treated and untreated plants were oven-dried at 60 °C for 48 h and powdered. A known amount of each plant material was extracted in 90% ethanol (three times), filtered, and concentrated to dryness. Dried residue was re-dissolved in ethanol, diluted with an equal volume of water, and acidified with 3% hydrochloric acid. The mixture was extracted with hexane (three times), hexane fraction discarded, and aqueous extract cooled to 10 °C and basified with 3% ammonium hydroxide to pH 8.5. This portion was further extracted with chloroform (three times). The combined chloroform extract was washed with distilled water, evaporated to dryness, and weighed.
2.7 Statistical analysis
Statistical analysis was performed using the one-way analysis of variance (ANOVA) followed by Duncan's Multiple Range Test (DMRT). The values are mean ± SD for six samples in each group. P values ⩽ 0.05 were considered as significant.
3 Results
3.1 H2O2 content
H2O2 content (Fig. 1a) increased with salinity treatment in shoot and root, the highest content being 90 DAS in root. Under CaCl2 treatment in combination with NaCl slightly reduced the H2O2 content, but higher than in unstressed plants. CaCl2 alone also increased the H2O2 content.

Effect of NaCl (80 mM), CaCl2 (5 mM), and their combination on (a) H2O2 accumulation and (b) lipid peroxidation (LPO) in Catharanthus roseus on 90 DAS. Values are given as a mean ± SD of six samples in each group. Bar values that are not sharing a common superscript (a, b, c, d) differ significantly at p⩽0.05 (DMRT).
3.2 Lipid peroxidation (TBARS content)
Oxidative damage to tissue lipid was estimated by the content of total TBARS. The plants under treatments of NaCl showed a trend of increasing content of TBARS (Fig. 1b). The CaCl2 treatment reduced the TBARS content in both root and shoot.
3.3 Proline content
Another compatible solute, which accumulates under salt stress in plants, is PRO. In the present study, an increase in PRO accumulation in C. roseus seedlings under salinity, with a concomitant increase in γ-GK (PRO synthesizing enzyme), and a decrease in PROX (PRO degrading enzyme) activities (Fig. 2a) was observed. PRO content was diminished upon the addition of CaCl2; anyhow, CaCl2 alone increased PRO when compared to unstressed control.
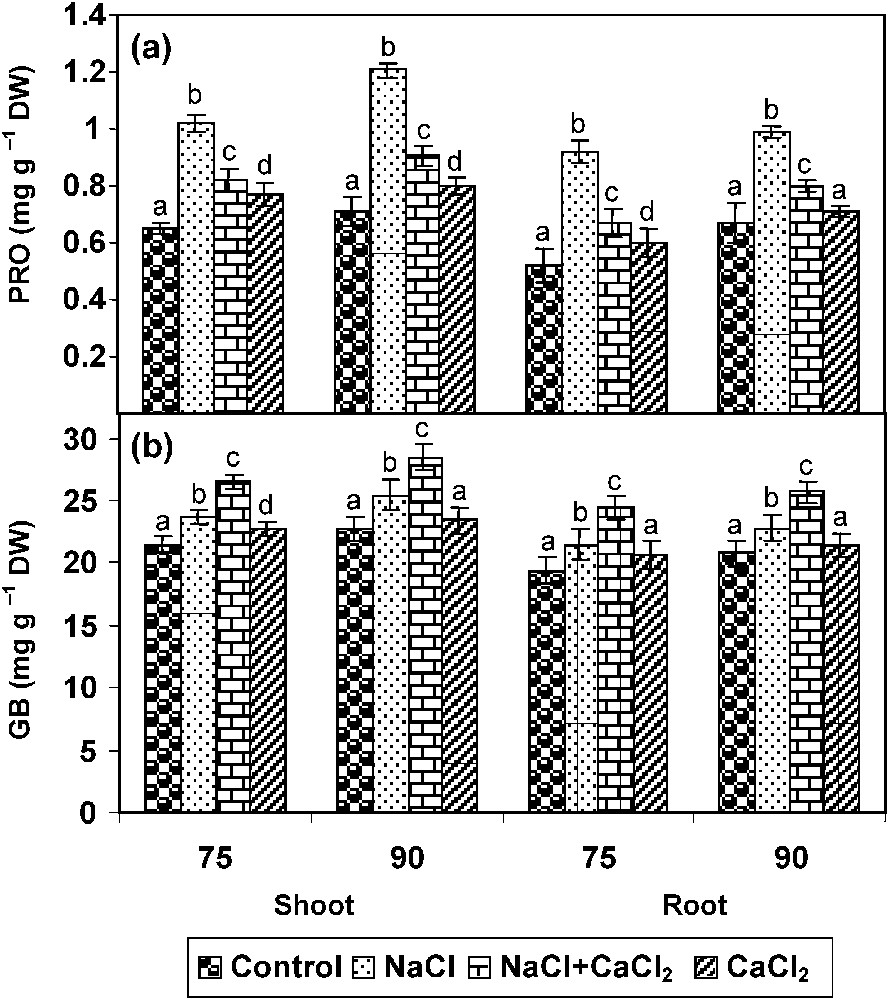
Effect of NaCl (80 mM), CaCl2 (5 mM), and their combination on (a) proline (PRO) and (b) gycinebetaine (GB) accumulations in Catharanthus roseus on 90 DAS. Values are given as a mean ± SD of six samples in each group. Bar values that are not sharing a common superscript (a, b, c, d) differ significantly at p⩽0.05 (DMRT).
3.4 Glycine betaine content
One of the most important mechanisms exerted by higher plants under salt-stress conditions is the accumulation of compatible solutes such as GB. In the present study, the amount of GB content increased with individual and combined treatments of NaCl and CaCl2 in C. roseus plants. The GB accumulation was higher in CaCl2 treatment when compared to unstressed plants (Fig. 2b).
3.5 γ-Glutamyl kinase activity
The γ-GK activity has been increased largely in shoot and root in the NaCl-stressed C. roseus plants when compared with control (Fig. 3a). NaCl-with-CaCl2-treated plants showed decreased γ-GK activity when compared to NaCl-stressed and control plants. CaCl2 alone also increased the level of γ-GK when compared to control, but less than in NaCl treatment.

Effect of NaCl (80 mM), CaCl2 (5 mM), and their combination on (a) γ-glutamyl kinase (γ-GK) and (b) proline oxidase (PROX) activities in Catharanthus roseus on 90 DAS. Values are given as a mean ± SD of six samples in each group. Bar values that are not sharing a common superscript (a, b, c, d) differ significantly at p⩽0.05 (DMRT).
3.6 Proline oxidase activity
PROX activity has been inhibited largely by NaCl, CaCl2 and their combination in all parts of C. roseus when compared with control. The lowest value was recorded in NaCl treatments. Addition of CaCl2 to NaCl-treated plants increased the PROX activity when compared to NaCl-stressed plants, but less than in unstressed plants (Fig. 3b).
3.7 Superoxide dismutase
The SOD activity has been increased in all parts of the NaCl- and CaCl2-stressed plants when compared to control. Addition of CaCl2 to NaCl-treated plant increased SOD activity when compared to NaCl-stressed plants (Fig. 4a).
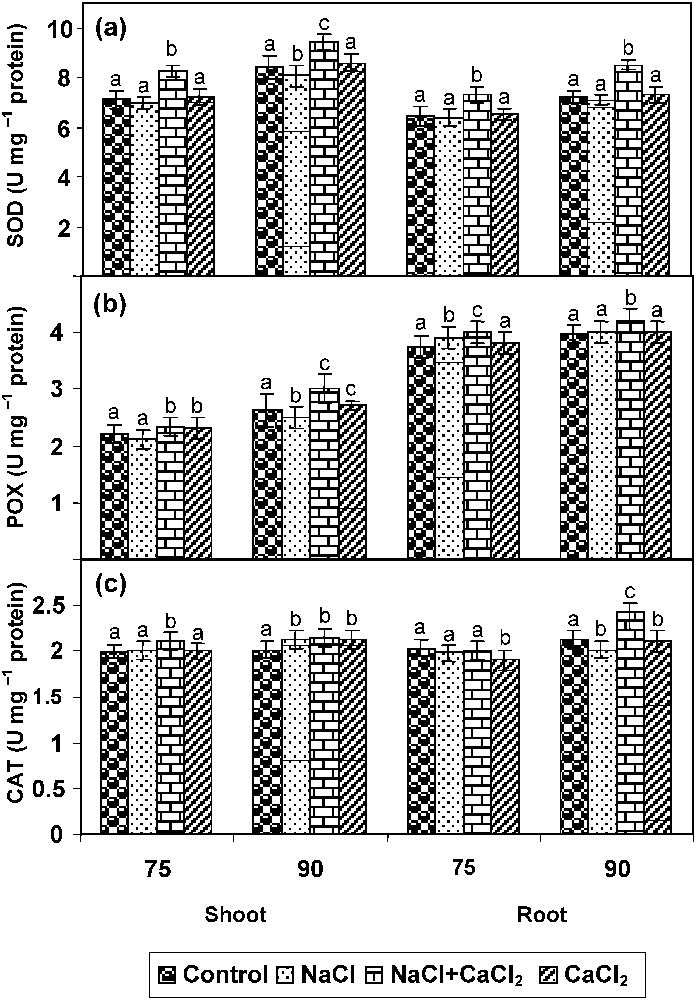
Effect of NaCl (80 mM), CaCl2 (5 mM), and their combination on (a) superoxide dismutase (SOD), (b) peroxidase (POX), and (c) catalase (CAT) activities in Catharanthus roseus on 90 DAS. Values are given as a mean ± SD of six samples in each group. Bar values that are not sharing a common superscript (a, b, c, d) differ significantly at p⩽0.05 (DMRT).
3.8 Peroxidase
POX activity has been increased in all parts of the NaCl- and CaCl2-stressed plants when compared to control. The NaCl-with-CaCl2-stressed plants increased POX activity when compared to NaCl- and CaCl2-stressed plants (Fig. 4b).
3.9 Catalase
POX activity has been increased in all parts of the NaCl- and CaCl2-stressed plants when compared to control. The NaCl-with-CaCl2 stressed plants increased POX activity when compared to NaCl- and CaCl2-stressed plants (Fig. 4c).
3.10 Indole alkaloid accumulation
The indole alkaloid content increased in individual and combined treatments of NaCl and CaCl2 when compared to unstressed control plants. The highest alkaloid accumulation was recorded in NaCl-treated plants (Fig. 5).

Effect of NaCl (80 mM), CaCl2 (5 mM), and their combination on total alkaloid accumulation in Catharanthus roseus on 90 DAS. Values are given as a mean ± SD of six samples in each group. Bar values that are not sharing a common superscript (a, b, c, d) differ significantly at p⩽0.05 (DMRT).
4 Discussion
The individual treatment salts increased the H2O2 content in all parts of C. roseus plants when compared to control. Nevertheless, the combination of salts decreased the increased H2O2 content. The CaCl2 treatment enhanced different H2O2 scavenging enzymes, like SOD, POX and CAT, and also non-enzymatic antioxidants. This enhancement would have helped in scavenging of ROS in C. roseus.
LPO has been associated with damages provoked by a variety of environmental stresses. Poly-unsaturated fatty acids (PUFA) are the main membrane lipid components susceptible to peroxidation and degradation [23]. The increase in LPO can be correlated with the accumulation of ions and active oxygen species (AOS) production under salt stress [47]. The level of LPO, in case, indicates the extent of salt tolerance, as reported by Bor et al. [48] in sugar beet and wild beet under NaCl treatment.
In a majority of plants, salt stress leads to changes in gene expression, leading to an increased synthesis of osmoprotectors and osmoregulators [49]. Osmotic adjustment is the main component of physiological machinery by which plants respond to soil-salt stress. Free PRO and soluble sugar accumulation in the leaves under stress conditions is of utmost importance for plant adaptation during stress [24]. In most plants, there is an increased accumulation of amino acids and amines (e.g., PRO, B-alanine, GB) in their tissues in response to salt stress. The way these compounds are accumulated differs between species and ranges from only one to several different compounds being accumulated. Generally, plant species that accumulate PRO usually have low amounts of this amino acid when grown in well-watered and non-saline soils, increasing its contents upon imposition of drought or salt stresses [50]. The GB accumulation that resulted from the NaCl-induced oxidative stress is helpful in the stimulation of salt-tolerance mechanisms [51,52].
The induction of PRO accumulation may be due to an activation of PRO synthesis through glutamate pathway involving γ-GK, glutamyl phosphate reductase, and P5CR activities. The PRO metabolizing enzyme, γ-GK, increased under the NaCl salinity in C. roseus seedlings. This enzyme plays an important role in the synthesis of PRO. The γ-GK activity can be inversely correlated with PROX activity and protein content in salt-treated plants [51]. PRO accumulation in NaCl-stressed seedlings can be attributed in part to the increased level of γ-GK activity [53].
PROX activity decreased under NaCl stress in C. roseus seedlings when compared to control. This enzyme converts free PRO into glutamate. Reduction in PROX activity and simultaneous increase in PRO level were reported in low-temperature-stressed wheat [54]. PROX oxidizes the PRO and converts it back to glutamate. This enzyme also influences the level of free PRO. The activities of PRO degrading enzymes, PROX and proline dehydrogenase (PDH), were significantly inhibited in the salt-stressed green gram seedlings [55]. PRO may act as a non-toxic osmotic solute preferentially located in the cytoplasm, or as an enzyme protectant, stabilizing the structure of macromolecules and organelles. Accumulated PRO may supply energy to increase salinity tolerance [5,56]. PRO, as an osmoprotectant compound, plays a major role in osomoregulation and osmotolerance [57]. However, its definite role in exerting salinity resistance continues to be a debate [52].
Several plants, including halophytes, accumulate high PRO levels in response to osmotic stress as a tolerance mechanism to high salinity and water deficit [58]. In plants, PRO is synthesized from either glutamate or ornithine. However, the glutamate pathway is primary route used under osmotic stress or nitrogen limitation conditions, whereas the ornithine pathway is prominent under high nitrogen input [52]. However, recent data suggest that glutamate is the major amino acid involved in PRO synthesis, since transgenic tobacco plants with reduced expression of cytosolic glutamine synthetase accumulated less PRO than non-transformed plants in response to salt stress. Accumulation of PRO in plants under stress is a result of the reciprocal regulation of two pathways: [(-pyrroline-5-carboxylate synthetase (P5CS) and P5CR] and repressed activity of PRO degradation [58]. PRO catabolism is catalyzed by pyrroline-5-carboxylate dehydrogenase and PDH, a mitochondrial enzyme, whose activity had been shown to reduce during salt stress. The first two steps of PRO biosynthesis are catalyzed by P5CS by means of its γ-GK and glutamic-γ-semialdehyde dehydrogenase activities. Subsequently, the P5C formed is reduced by P5CR to PRO [59].
Although the precise role of PRO accumulation is still debated, PRO is often considered as a compatible solute involved in osmotic adjustment [60]. Accumulation of PRO may occur through an increase in its synthesis constantly with inhibition of its catabolism [61], and may be a mechanism for stress tolerance. However, its role in imparting stress resistance under saline conditions is controversial. Anyway, understanding the biosynthesis, degradation, transport, and role of PRO during stress and the signalling events that regulate stress-induced accumulation is vital in developing plants for stress tolerance [62].
SOD activity directly modulates the amount of ROS. Similar results were seen in the case of CAT activity also. The unique importance of Ca2+ for stabilization of members is well known [63]. SOD and CAT activities have been reported to be negatively correlated with the degree of damage of plasmalemma, chloroplast, and mitochondrial membrane systems, and positively related to the indices of stress resistance [64]. CaCl2-treated seedlings maintain higher levels of SOD and CAT activities and lower levels of LPO and POX activity [65]. ROS scavenging depends on the detoxification mechanism provided by an integrated system of non-enzymatic reduced molecules, like AA and glutathione and enzymatic antioxidants [66]. As part of this, antioxidant enzymes play important roles in the defence mechanism against oxidative stress. Antioxidant mechanisms may provide a strategy to enhance salt tolerance in plants, though the detailed mechanisms are not yet clear [21].
The total alkaloid accumulation in both shoot and root of C. roseus was found increased significantly under the oxidative stress resulting from both NaCl and CaCl2, as well as from their combination. The content of alkaloids in C. roseus has been found influenced by individual factor, such as stage of plant growth, salinity stress, and nitrogen fertilization [21], and treatment with triadimefon, a plant-growth regulator [29]. There are reports on the improvement of indole alkaloid production in cell cultures of C. roseus treated by GA3 and Pseudomonas elicitors [31,35]. Recently, it has also been reported that indole alkaloid production in C. roseus can be increased by abiotic stresses, like drought [32–34].
5 Conclusion
From these results, it can be concluded that the addition of CaCl2 to NaCl-stressed C. roseus plants have a significant role in partial alleviation of salinity stress. Thus, it is clear that plants under salt stress are highly regulated by components of the antioxidative system and secondary metabolite contents. Our results indicated that the cultivation of medicinal plants like C. roseus in saline areas would increase its PRO metabolism, defence mechanisms, and the level of active principles. However, the data presented here reflect the importance of a physiological analysis of plant response, which must accompany field experiments and evaluation. Further investigations are required to ascertain this conclusion.


