1 Introduction
In colonial birds and mammals, the recognition between parents and their offspring is an essential condition for reproductive success, enabling the exclusivity of parental care to offspring among numerous conspecifics (e.g. [1,2]). High-density colonies especially favor the individual identity by distinctive cues due to the high risk of confusion among individuals [3–7]. In birds, constraints for recognition processes differ with the nesting patterns. In strictly nidicolous species, the parents are usually ensured of feeding their own chicks on coming back to the nest using location cues (e.g., Black-headed gull Larus ridibundus [8]). In this context, the offspring recognition does not appear crucial in terms of fitness gain for the parents [9,10]. Nevertheless, some parents can use the individual distinctive calls to recognize and preferentially feed some nestlings [11–13]. In nidifugous species, parents face the difficulty of discriminating their offspring among many others without relying on location cues (e.g., Slender-billed gull Larus genei [8]). Comparative studies among penguins have shown that, in non-nesting species, this recognition is based on a sophisticated system of vocal coding (King penguin Aptenodytes patagonica and Emperor penguin A. forsteri), while in nesting species, parents have poorer call recognition abilities and first use location cues to find their chicks (e.g., Gentoo penguin Pygoscelis papua, Adelie penguin P. adeliae, Rockhopper penguin Eudyptes chrysocome) (review in [1]). Finally, some species combine both nesting patterns throughout the development of offspring: chicks are first reared in the nest and are still food-dependent of their parents at the post-fledging stage [14,15]. In territorial species, the timing of recognition may coincide with the onset of brood mobility [7,16–18]. In colonial birds, the risk of confusion among conspecifics may lead to greater pressure for offspring recognition than in territorial species. Some studies have shown that this recognition appears earlier in nidifugous species [19] than in nidicolous ones [20] but choice tests should be carried out at different stage of development to clarify these findings. Surprisingly, few studies assessed the period when the emergence of offspring recognition takes place in such colonial species.
Since acoustic cues are efficient over short and long distances, individual vocal recognition seems to be a key component of parent–chicks recognition [9,21–24]. To support the individual recognition process, vocalizations have to show a highly individualized vocal signature. An acoustic parameter encoding individual identity has to show a strong individual stereotypy, i.e. a weak intra-individual variability combined with a high inter-individual variability [25–31].
The present study aimed at investigating the acoustic cues of signaler identity carried in the begging calls of a colonial species experiencing food-dependence after fledging. Zebra finches (Taeniopygia guttata castanotis) are an excellent model to carry out this study. They are gregarious songbirds of subarid regions of Australia which form life-long pair bonds and breed in loose colonies of hundreds of pairs [32,33]. Zebra finches' chicks leave the nest at 17–22 days of age and are still fed by their parents during 13–18 days after fledging [33]. The call-based mate recognition skills of zebra finches have been demonstrated in adults of both sexes [25,34] but little is known about the recognition process of offspring by parents guiding their investment at the post-fledging stage.
Given that chicks still beg after fledging to obtain food, we hypothesized that: (1) parents should be able to discriminate between their offspring and the other chicks in the colony when they fledged; and (2) the offspring recognition was based on the acoustic cues of begging calls. To test these hypotheses, we performed playback tests broadcasting the begging calls of offspring and of familiar to parents and analyzed the acoustic structure of begging calls when chicks left the nest. Then, to determine when the vocal signature takes place in the chick's development, we examined its ontogenesis at the pre-fledging stage and the acoustic parameters which could early support the individual identity coding.
2 Material and methods
2.1 Subjects
To assess the existence of a vocal signature and investigate its ontogenesis, the begging calls of 12 nestling zebra finches (six males and six females) were studied at three ages (at the age of 11 days, 15 days and the day before fledging, see below). Begging calls were recorded daily in the nest until the fledging of the nestlings. Each monitored breeding pair was arbitrarily formed before being placed in separate cages () equipped with an enclosed wicker nest and one main opening. Food and water were provided ad libitum and the temperature room was maintained at between 25 and 28 °C (14L/10D photoperiod). Each nest was checked daily to establish the precise day of hatching and fledging.
To assess the vocal signature, we studied the calls' acoustic structure at the age of 11 days, 15 days and the day before fledging. On average, the chicks studied fledged at 19 days old (, ). We favored the monitoring of broods with a single nestling and broods of more than two chicks were not recorded to ensure the reliability of the emitter's identification. In two nests, we had to limit the brood size at two nestlings placing the additional chick in another nest where some eggs were hatching. Previous studies on begging calls indicate that the presence of nestmates does not modify the acoustic structure of begging calls [35]. Between the three ages, the identity of the 12 recorded nestlings was not consistently preserved. At 11 days old, the studied nestlings came from ten different breeding pairs and 11 grew up in a one-chick brood (Table 1). At 15 days old, the studied nestlings came from seven different breeding pairs and six grew up in a one-chick brood. The studied nestlings, which were a day before fledging, came from eight different breeding pairs and eight nestlings grew up in one-chick brood. In two-chick broods, we used video recordings to identify the emitter's begging calls. To discriminate nestlings by video identification, one chick was marked with bright nail polish on claws and, at 8 days old, its plumage was safely coloured with picric acid (yellow coloration).
| Ages | No. of onechick broods | No. of twochick broods | No. of breeding pairs |
| 11 days | 11 | 1 | 10 |
| 15 days | 6 | 6 | 7 |
| dBF | 8 | 4 | 8 |
2.2 Recording procedures
2.2.1 Video recordings
To identify the chick begging in two-chick broods, an IP video LAN camera (IP DLink DSC-900) was focused on each nest opening. Birds were acclimatized to the camera over a four day period prior to potential hatching. Videos were recorded at 5 frames/s on a Bi Xeon Intel Workstation located in a different room. Video files were recorded with IPView Lite Software version 3.88 and read with Media Player Classic.
2.2.2 Audio recordings
The roof of nests was equipped with an omni-directional tie microphone (MKE-2 SENNHEISER, frequency response almost flat between 50 Hz and 10 kHz). The microphone position and its small size did not disturb the behavior of nestlings and parents. Microphones were connected via a shielded cable to the audio card of the Bi Xeon Intel Workstation. Recordings were automated and programmed to begin one hour after the artificial dawn and to last three hours, every day, with the Avisoft-SASLab Pro Recorder software (version 4.39, Avisoft Bioacoustics; sampling frequency: 48 kHz). The time code on video recordings was defined by the computer clock and enabled to match with time on audio files.
2.3 Playback procedures
Zebra finch parents were tested with begging calls of their own chick versus begging calls of a familiar chick ( adults, 4 males and 3 females; each individual was tested once, except 1 male and 1 female that were tested with begging calls of their two chicks). The playback tests were performed between one and five days after fledging, a period where chicks are still dependent on parents for feeding.
Prior to the experiment, all the tested birds were accommodated in the same aviary room, each pair and its nest being in its own cage (cage dimension: ). All birds were thus able to hear the vocalizations of other adults' chicks. On the evening of the day before the playback, the tested parent was separated from its partner and placed with its own chick in an experimental cage () within a soundproof chamber with a 14L/10D photoperiod. The other parent was placed in another room. Four other cages accommodating familiar adult pairs that were not currently breeding chicks were placed around the experimental cage, thus offering a familiar social context for the tested bird [34]. The identity of audience changed at each test and enabled us to assume the independence of parent's response. The next morning, the chick was removed from the experimental cage two hours before starting the playback test and placed with the other parent.
The begging calls used for the playback experiments were recorded the day before the fledging of chicks and the files (.wav) were created using GoldWave software (5.22 version). As begging calls are usually emitted in a cluster (sequence of calls) a playback test consisted in broadcasting six sequences of begging calls of the offspring and six sequences of begging calls of a familiar chick (sequence duration: range 6–9 s; sequences were chosen at random among the ones recorded the day before fledging and the number of calls per sequence was kept intact ( calls) in order to preserve the natural modulation of intensity between the begging calls). The playback tests were performed with a Marantz PMD690/W1B recorder and an amplifier (Yamaha AX-396) connected to two loudspeakers (Triangle Comete 202, sounds emitted at 60 dB at 1 m) located at either end of the cage. A playback test lasted 10 minutes during which begging sequences were alternatively broadcasted every 40 seconds (total of begging sequences per playback test; one loudspeaker broadcasting the six sequences from the offspring of the tested parent and the second one the six sequences from a familiar chick; the role of each loudspeaker was balanced between tested individuals to avoid a potential location preference).
The behavioral response to playback was assessed by an experimenter hidden behind a black curtain and blind to conditions. The following four parameters were measured observing the behavior of tested parent and using a stopwatch: (1) latency time before the first vocalization (call or song); (2) latency time before the first locomotion; (3) number of vocalizations emitted during the playback and the following 15 seconds; and (4) the time spent during the playback and the following 15 seconds in each of the three zones in the experimental cage defined in relation to the loudspeaker position (left side zone, central zone, right side zone, each zone being of identical size and equipped with one feeder cup, one water fountain and three roosts). When the parent did not respond to playbacks, the latency time was recorded as the maximum length of the time used to monitor behavior (including the duration of playback and the following 15 seconds).
To compare the responses of parents between the begging calls from its offspring or from a familiar chick, we calculated the median value for each measured parameter and we compared these with Wilcoxon matched pairs tests. The total time spent in the different zones was compared with a Friedman ANOVA test. To keep independent tests we pooled the playback tests of the two individuals (one male and one female) that were tested twice (for each of their two offspring). As a whole, seven independent playback tests were analyzed.
2.4 Begging calls analysis
To study independent calls we analyzed 10 begging calls coming from 10 different sequences for each nestling and for each studied age. We isolated the call in the midst of each sequence. A total of 360 begging calls were analyzed using Avisoft-SASLab Pro version 4.39 and Praat version 4.4.10. To assess the vocal signature in begging calls, one observer measured 19 spectral, temporal and amplitude acoustic cues. Begging calls could have two distinct segments (Fig. 1): the first segment appears amorphous with a blurred-frequency, called the background noisy segment and the second segment, called the structured segment, was modulated in frequency and presented a fundamental frequency associated with several harmonics. With regards to the parameters measured, all durations are given in ms, frequencies in Hz and intensities in Pa.
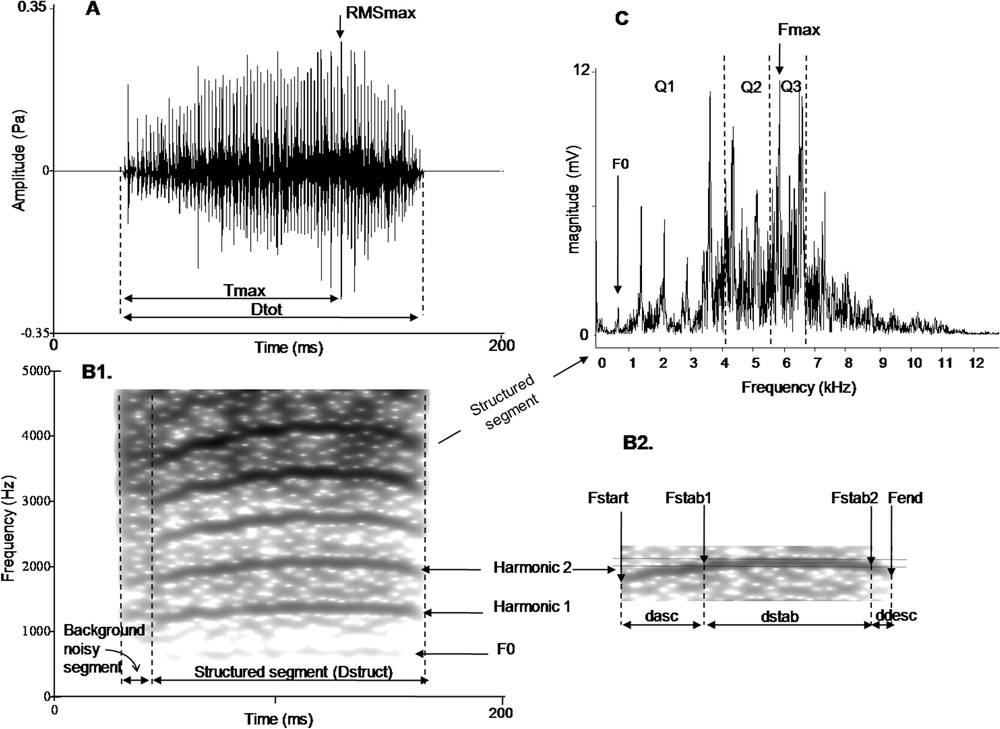
Analysis of the begging calls; see text for details.
To describe the amplitude change over time, we measured from the oscillogram: the total length of the call (), the highest amplitude in the call (RMSmax), the duration between the beginning of the call and the time at which the highest amplitude in the call occurs () and the mean intensity of the entire call represented by the root-meansquare signal level (RMSaver) according to Beeman [36]. We then calculated the variable . To describe the frequency modulation, we focused on the structured segment (Fig. 1B2). The structured segment is divided in three parts: an initial part defined by a loud ascending frequency modulation, then a long and loud second part with no frequency modulation (the stable part), and finally a third part defined by a rapid descending frequency modulation. We measured from the spectrogram four temporal parameters on the fundamental frequency or on one harmonic (Fig. 1B2): its total length (), the duration of the ascending frequency modulation (), the duration of the stable part (), and the duration of the descending frequency modulation (). Then we calculated the variable and . Five spectral parameters were also measured: the start frequency (), the frequency of the beginning of the stable part (), the frequency of the end of the stable part ( which was very similar to ), and the end frequency of the call (). These parameters enabled us to calculate: , the slope of the ascending frequency modulation (Hz ms−1) [calculated as ], and , the slope of the descending frequency modulation (Hz ms−1) [calculated as ].
To describe the energy spectrum, we separately measured the energy distribution of signals on the background noisy and structured segments for each quartile (Q1: 25% of the energy of the signal; Q2: 50%, Q3: 75%). Finally, we measured the maximal frequency () for each segment of the signal and the fundamental frequency () on the structured segment.
For the statistical analysis, we performed a non-parametric analysis of variance (Kruskall–Wallis ANOVA, ) to determine which variables would contribute to individual identity information. To describe the intra and inter-individual variations of each variable, we used the coefficient of variation (CV) [37]. For each variable we calculated CVi (within individual CV) and CVb (between individual CV) according to the formula for small sample size:
All statistical tests were performed using Statistica version 8.
3 Results
3.1 Differential response of parents
Playback tests showed a differential vocal response in parents in favor of offspring begging calls (Table 2). They replied both more quickly (latency vocal response, , ) and more often (number of emitted calls/songs, , ) to their own offspring begging calls than to familiar ones. No differences were found in the latency of locomotive response (, ) or in the time spent in each zone of the experimental cage (Friedman ANOVA, , df =2, Chi Sqr. =0.857, ). Globally, parents tended to come and go in the cage during broadcastings as if they were searching for their young or as a means to locate them.
| Number of vocalizations in response to own chick calls | Number of vocalizations in response to familiar chick calls | Response latency to own chick calls (in seconds) | Response latency to familiar chick calls (in seconds) | |
| female 1 | 7.5 | 2.5 | 18 | 19.5 |
| female 2 | 5 | 1 | 5.5 | 9.5 |
| female 3 | 3 | 1 | 7 | 11 |
| male 1 | 2.5 | 0 | 13.5 | 30 |
| male 2 | 1 | 1 | 20 | 24 |
| male 3 | 3 | 1 | 10.5 | 21 |
| male 4 | 6 | 2 | 3 | 14.5 |
3.2 Vocal signature
The day before fledging, 30% of chicks' begging calls (; 10 calls/individual) included a background noisy segment (Fig. 1). The structured segment started by an initial ascending frequency modulation in 92% of calls and ended by a descending frequency modulation in 75%. Whatever the calls, these frequency modulations were always separated by a stable frequency part. Only one individual systematically started its call by a background noisy segment while all other chicks had never more than five begging calls (out of 10) including a such segment (range: 0–5). The structured segment in this individual started by an ascending frequency modulation in only one call out of 10. This chick was the earliest to fledge in the studied population (16 days old). The presence of a descending frequency modulation was more equitably distributed among individuals (presence in calls/individual), except with one individual (3/10 calls; fledging at 17 days). All the following statistical analyses were performed on 15 variables (exclusion of the four ones related to the background noisy segment for which numerous values were not available).
All the studied variables significantly differed between the individuals (Kruskall–Wallis ANOVA, , ; Table 3). However, among different individual pairs differences occurred according to the variable considered (multiple comparisons of mean ranks for each individual pair). Therefore, each variable contributed to the chick's vocal signature but, separately considered, did not enable all 12 chicks to be discriminated. For all these variables, the coefficients of variation within individuals were smaller than those among individuals (PIC values >1; Table 3). Thus all the variables were potential cues for individual identity coding. The PIC values of two frequency parameters, the fundamental frequency () and the frequency of the end (), were greater than two, and one spectral parameter (Q1) was equal to two.
| Mean ± Standard Deviation | Mean CVi | CVb | PIC CVb/mean CVi | Kruskall–Wallis ANOVA p-value | |||||||||||
| Ages variables | 11 d | 15 d | dBF | 11 d | 15 d | dBF | 11 d | 15 d | dBF | 11 d | 15 d | dBF | 11 d | 15 d | dBF |
| (ms) | 114.6 ± 24.9 | 112.2 ± 21.4 | 112.5 ± 28.3 | 13.90 | 14.33 | 16.49 | 21.80 | 19.12 | 25.19 | 1.57 | 1.33 | 1.53 | ⁎⁎⁎ | ⁎⁎⁎ | ⁎⁎⁎ |
| RMSaver (Pa) | 0.064 ± 0.027 | 0.073 ± 0.04 | 0.053 ± 0.029 | 26.13 | 30.53 | 29.32 | 42.09 | 56.84 | 54.76 | 1.61 | 1.86 | 1.87 | ⁎⁎⁎ | ⁎⁎⁎ | ⁎⁎⁎ |
| RMSmax (Pa) | 0.294 ± 0.124 | 0.35 ± 0.20 | 0.253 ± 0.163 | 25.14 | 31.45 | 40.68 | 42.16 | 59 | 64.66 | 1.68 | 1.88 | 1.59 | ⁎⁎⁎ | ⁎⁎⁎ | ⁎⁎⁎ |
| 0.564 ± 0.144 | 0.62 ± 0.14 | 0.601 ± 0.207 | 24.33 | 22.19 | 32.73 | 25.58 | 23.18 | 34.47 | 1.05 | 1.04 | 1.05 | ⁎⁎ | ⁎ | ⁎⁎⁎ | |
| 0.739 ± 0.134 | 0.916 ± 0.11 | 0.960 ± 0.071 | 12.65 | 7.15 | 4.29 | 18.16 | 12.05 | 7.36 | 1.44 | 1.68 | 1.72 | ⁎⁎⁎ | ⁎⁎⁎ | ⁎⁎⁎ | |
| 0.767 ± 0.240 | 0.815 ± 0.18 | 0.746 ± 0.136 | 17.61 | 10.85 | 13.33 | 31.31 | 14.42 | 18.31 | 1.78 | 1.33 | 1.37 | ⁎⁎⁎ | ⁎⁎⁎ | ⁎⁎⁎ | |
| (Hz) | – | 532.9 ± 102.65 | 659 ± 158.7 | – | – | 13.24 | – | – | 24.12 | – | – | – | – | ⁎⁎⁎ | |
| (Hz) | – | 21.97 ± 8.08 | 10.86 ± 6.30 | – | – | 44.16 | – | – | 58.19 | – | – | 1.32 | – | – | ⁎⁎⁎ |
| (Hz) | – | 648.16 ± 97.6 | 761 ± 179.6 | – | 10.67 | – | 23.45 | – | 2.20 | – | ⁎⁎⁎ | ⁎⁎⁎ | |||
| (Hz) | – | −25.13 ± 21.82 | −12.39 ± 9.79 | – | 65.52 | – | −79.20 | – | 1.21 | – | ⁎⁎ | ⁎⁎⁎ | |||
| (Hz) | 3867 ± 875 | 3449 ± 857 | 13.56 | – | – | 22.68 | – | – | 1.67 | – | – | ⁎⁎⁎ | – | – | |
| (Hz) | 5651 ± 986 | 5914 ± 817 | 10.45 | – | – | 17.48 | – | – | 1.67 | – | – | ⁎⁎⁎ | – | – | |
| (Hz) | 7873 ± 1520 | 7555± | 10.41 | – | – | 19.35 | – | – | 1.86 | – | – | ⁎⁎⁎ | – | – | |
| (Hz) | 4153 ± 2475 | 3597 ± 2358 | 61.44 | – | – | 59.72 | – | – | 0.97 | – | – | ⁎⁎ | – | – | |
| (Hz) | 945 ± 187 | 870.3 ± 107 | 863 ± 126 | 4.85 | 4.63 | 5.84 | 19.87 | 12.28 | 14.58 | 4.09 | 2.66 | 2.50 | ⁎⁎⁎ | ⁎⁎⁎ | ⁎⁎⁎ |
| (Hz) | 4362 ± 600 | 3997 ± 585 | 4418 ± 712 | 8.09 | 10.62 | 8.09 | 13.78 | 14.68 | 16.14 | 1.70 | 1.38 | 2 | ⁎⁎⁎ | ⁎⁎⁎ | ⁎⁎⁎ |
| (Hz) | 5884 ± 864 | 5488 ± 685 | 5914 ± 812 | 8.73 | 10.25 | 7.68 | 14.72 | 12.51 | 13.77 | 1.68 | 1.22 | 1.79 | ⁎⁎⁎ | ⁎⁎⁎ | ⁎⁎⁎ |
| (Hz) | 7536 ± 1358 | 5097 ± 771 | 7272 ± 1157 | 11.31 | 9.74 | 10.46 | 18.06 | 11.00 | 15.95 | 1.60 | 1.13 | 1.52 | ⁎⁎⁎ | ⁎⁎ | ⁎⁎⁎ |
| (Hz) | 4951 ± 1465 | 4864 ± 1583 | 862 ± 1695 | 24.80 | 27.91 | 24.20 | 29.65 | 32.61 | 31.58 | 1.20 | 1.17 | 1.30 | ⁎⁎⁎ | ⁎⁎⁎ | ⁎⁎⁎ |
⁎ .
⁎⁎ .
⁎⁎⁎ .
The multivariate approach was performed on 13 variables (exclusion of and for which one individual displayed in only one call an initial ascending frequency modulation; see above). The discriminant analysis successfully discriminated 97.8% of the calls of the 12 chicks (cross-validated DFA: 75.6% of correct assignment), and confirmed the individual signature in the begging call of chick zebra finches at the fledging stage.
3.3 Ontogenesis of vocal signature
The background noisy segment was present in 38% of calls at the age of 15 days () and in 92.5% at the age of 11 days (). At 11 days old, all individual calls included this segment in at least seven calls out of 10 while at 15 days old, only four individuals had this segment in more than half of their calls. Three individuals were devoid of background noisy segment. The initial ascending frequency modulation was present in 81% of calls in individuals aged of 15 days (present in at least eight calls in 10 individuals) but only in 57.5% in individuals aged of 11 days (present in less than five calls in five individuals). The descending frequency modulation was present in 52% of calls in individuals aged at 15 days and only in 32.5% in those aged of 11 days old. According to these results, for the uni- and multivariate approaches and the calculation of PIC, there were 13 variables for which there was sufficient data at the age of 15 days (exclusion of the four variables related to the background noisy segment, and ) and 15 variables at the age 11 days (exclusion of , , and ).
Whatever the stages of development, all the variables studied significantly differed among the individuals (Kruskall–Wallis ANOVA, ; Table 3); however, none of these variables, separately considered, enabled all 12 individuals to be discriminated. Different individual pairs differed according to the variable considered (multiple comparisons of mean ranks for each individual pair). The fundamental frequency () displayed the greatest potential of individual coding (PIC15 days =2.65 and PIC11 days =4.09; Table 3). For all the studied variables at 15 days, the coefficients of variation within individuals were smaller than those among individuals (PIC >1). At 11 days, same results were found except for the maximal frequency () reached in the background noisy segment. The coefficient of variation within individuals of was similar with this among individuals (PIC =0.97). The background noisy segment participated to the identity signal through the variation in energy distribution among individuals (range PICQ1, Q2, Q3: 1.67–1.86).
The multivariate approach revealed that at these early ages, the individual signature in begging calls is already reliable (at 15 days old: 100% of the calls rightly classified, cross-validated DFA: 63.3% of correct assignment, variables, 10 individuals; at 11 days old: 100% of the calls rightly classified, cross-validated DFA: 83.9% of correct assignment, variables, 12 individuals).
4 Discussion
4.1 Offspring recognition by vocal cues
Although obtained from a small number of individuals, our results show unambiguously that zebra finch parents are able to discriminate between the begging calls of their offspring and of familiar chicks recorded the day before fledging. During the experimental tests, adults vocally replied both more quickly and intensely to the begging calls of their offspring than to the others. Parents have thus a mean using vocal cues to selectively feed their offspring during the food-dependence period following fledging. No differences in the locomotive response of parents were found between the begging calls of their own offspring and those of familiar chicks. Although we cannot exclude that these results were influenced by the experimental conditions (parent tested in a cage), these findings point to the first channel of communication, which at this stage is an acoustic channel. It does not however exclude that other sensory channels can be secondarily used at short distances such as visual cues. Locomotive responses could be only displayed when parents are able to see their offspring.
4.2 Individual vocal signature
The analysis of acoustic structure of begging calls revealed that the offspring recognition by parents could rely on acoustic cues. Indeed, the begging calls of chicks showed a highly individualized vocal signature since the age of 11 days. Thus, as soon as nestlings could produce acoustically structured begging calls, they emitted individually distinctive calls. The individual identity coding was multi-parametric and encoded in spectral, temporal and amplitude domains. Among frequency parameters in particular, the fundamental frequency of calls was the highest individualized acoustic cue whatever the studied stage of development. The day before fledging, the frequency of the end of the descending frequency modulation and, among spectral parameters, the energy spectrum of the structured segment of the call were also greatly modified by the identity of the signaler. Previous studies in both birds and mammals have shown that frequency modulation is often a major cue for support acoustic coding of identity in both adult and young (e.g. [2,13,25,26,39–42]). Our study indicated that all the considered parameters participated in the identity signal and only their combination enabled the discrimination between all the chicks. Consequently, parents are likely to use information about individual identity encoded in a combination of these acoustic cues. Additional experiments using modified signals could be performed to focus on the acoustic cues and the combinations mostly used in offspring recognition. It would be also interesting to assess how often zebra finch parents make mistake feeding other chicks once offspring left the nest. Some playback experiments to test the offspring recognition by parents after fledging are now running (H. Mulard, pers. comm.). Another way to assess how often parents feed other chicks could be to observe ringed birds in aviary and count the number of feeding of offspring versus familiar. In budgerigars, adults display high discriminatory abilities among many individuals of various aged and their discrimination of chick calls increased as the brood aged [43].
The selection pressure to signal its individual identity is stronger and occurs earlier in non-nesting species. In these species, the possibility of costly confusion between the signaler and other individuals is high as soon as hatching has taken place [6]. This selection pressure should favor the production of highly distinctive identity signals, relying on few specific acoustic parameters, to facilitate the accurate recognition of offspring in non-nesting species living in colony. In zebra finches, although the vocal signature of individuals was reliable at the fledging period, no single acoustic parameters were distinctive enough to be used for individual identity coding (this study). Consequently, the successful recognition process of offspring might be more dependent on the receiver's abilities to use multi-parametric acoustic cues than on the signaler who efficiently signals its identity. The recent works of Vignal et al. [25,34] in adult zebra finches have suggested that they have the skills required to use multiple acoustic cues to discriminate calls in background noise. To further our understanding of the parent-offspring recognition at the post-fledging stage, the role of the vocal recognition of parents by offspring in this species is currently investigated (H. Mulard, pers. comm.).
4.3 Ontogenesis of individual vocal signature
The vocal signature formation in zebra finch chicks took place early in their ontogenesis (at least 8 days before fledging) while it was not yet crucial to signal their identity in the nest. The begging calls gained in structure when chicks became older: the background noisy segment progressively reduced and the frequency modulation became established. Similar results in the vocal development were found in budgerigars [43]. Brittan-Powell et al. [43] specially showed that calls were easier to discriminate as the age of brood increased. However, we found that the reliability of begging calls did not increase with the age of zebra finch chick. The precocity of the individual acoustic coding of begging calls could enable zebra finch parents to familiarize with the call features of their offspring during the nesting period. In the framework of the brood division studies, Leedman and Magrath [11] suggested that behavioral interactions between a parent and its offspring could become more efficient with learning. Moreover, in adult zebra finches, there is an increase in the neuronal recruitment in their brain when offspring fledged and were still in need of parental care [44]. These studies suggest that there is a learning process in parents who need to memorize vocalizations of nestlings before they fledge and could explain the precocity in the identity signal in chicks. To assess the potential role of learning in the development of parent-fledgling interactions, additional playback tests should be realized broadcasting the begging calls of their offspring and of familiar chicks at different ages before fledging. To confirm the learning hypothesis, we expect that the rate of the successful discrimination of parents between their chicks and familiar ones increases with the age of their youngsters. However, parents may be able to discriminate their chicks before the age of 11 days, whereas their begging calls do not show a clear harmonic structure. Indeed, we found that the energy distribution in the background noisy segment was well individualized in post-11 days calls. It is possible that calls of younger individuals carry an individual signature as well. It would therefore be worthy to investigate the acoustic basis of this early recognition by recording and assessing the vocal signature in begging calls from younger individuals. Offspring that produce early distinctive calls may have been favored by selection if they garner a greater portion of their parents' parental care. The conflict between siblings to benefit of parental care particularly stimulates the identity signal (e.g. [45,46]). Indeed, feeding-preference for different chicks based on vocal recognition has been demonstrated in different species [13,47] but must still be investigated in zebra finches whose mean brood size is four nestlings [33]. A next step is thus to experimentally test the existence of parental vocal discrimination between offspring within the nest.
Identity signals may be a developmentally fixed and a genetically determined character [48,49] while signals that indicate quality are expected to express high degrees of phenotypic variation (e.g. [49–52]). For instance in the cliff swallow, some cross-fostering experiments suggested that variance in their begging calls is genetically determined [5]. It could be similar in zebra finches. Parents may have the opportunity to familiarize with the call features of their offspring as soon as they are audible. The period just before fledging could therefore be less crucial than expected in the recognition process. Globally, the ontogenesis of the vocal signature might play a secondary role in the recognition process. However, through the genetic signature hypothesis, we cannot exclude that they use a signature-matching process (i.e. no learning process) for kin recognition in the post-fledging stage [48]. It means that parents would determine kinship directly by matching with their own signature or those of previous offspring. A multi-locus genetic structure mechanism that would permit discrimination of kin from non-kin was early described [48] but contrary to visual signatures for which a strong genetic component was demonstrated [53,54], the potential genetic acoustic signature received less attention.
In conclusion, this study has pointed out the multi-parametric coding of identity signal in begging calls and underlined the major role of the recognition abilities of the receiver to discriminate its offspring among many potential candidates. It raises new issues, notably on the vocal recognition and memory processes. Many studies investigate song learning and song memorization processes in juveniles (e.g. [55–58]), but few have assessed the recognition memory of parents. For instance, King penguins Aptenodytes patagonicus can find their offspring among a crèche of thousands of chicks after months of separation [59] or mother northern fur seals Callorhinus ursinus respond to their pup's call even after a four-year separation [60]. Considering that vocal recognition is based on learning process, species like the zebra finch which produce more than one clutch per breeding season have to go through this learning process more frequently than single clutch breeders. Some previous works in other bird species suggest that parental hormonal profile is maintained by the food begging calls. The parental stage could be prolonged hearing the food-begging calls of nestlings and an interruption of this auditory feedback is soon followed by the onset of a new breeding cycle [61]. Following our findings, additional experiments are proposed in this paper in order to increase our understanding of the complex parent-offspring interactions in birds living in colony, food-dependent after the fledging stage and breeding several consecutive broods in one reproductive season.
Acknowledgements
We are grateful to Nicolas Ogier for bird marking and developing the video recordings and to Colette Bouchut for taking care of the birds. This study has been funded by the Agence Nationale de la Recherche (A.N.R., project “BIRDS'VOICE”) and Saint-Etienne Métropole. C.V. is supported by a Young Investigator Sabbatical of the Université de Saint-Etienne. N.M. is supported by the Institut universitaire de France and by the Miller Institute for Basic Research in Science, University of California, Berkeley (Visiting Miller Professorship).


