1 Introduction
The pathogens responsible for the infectious diseases that affect human populations are seldom strictly confined to our species. Among the about 1415 currently known human infectious diseases, c.a. 58 to 61% have an established animal origin [1,2]. Ruminants, carnivores, rodents, birds and primates constitute, in the order, the five major animal categories that have transmitted pathogens to human populations [3]. This percentage increases to 73–75% if only the “emerging” or “re-emerging” diseases, which have appeared in the last 30 to 40 years, are considered [2,3]. The work by Woolhouse et al. [4] has also shown that these pathogens have an important range of host reservoir species as about 60% of them have 3 or more host species (in addition to humans), thus highlighting the notion that pathogen dynamics driven by a multiplicity of hosts is a more realistic picture for emerging infectious diseases [5].
Without doubt, the origin of the most important pathogens and parasites that affect human populations is zoonotic (in this work, we will use the term “parasite” to refer to agents of infectious diseases). Therefore, it is easy to recognize the importance of a better understanding of animal species as potential hosts and sources of novel contaminations in humans [6]. The recent appearance and propagation of the highly pathogenic H5N1 avian influenza (bird flu), West Nile fever, Lyme disease, rabies as well as of Severe Acute Respiratory Syndrome (SARS), all of animal origin, have truly highlighted the role of animal species as major reservoirs for (new) human infections. Understanding how they are transmitted in animal species communities and identifying the underlying ecological and evolutionary mechanisms is essential to lessen the transmission risk to humans [7]. Anthropogenic changes in landscapes, particularly habitat destruction and fragmentation [4] and their subsequent effects on biological diversity have led to disease emergence in a number of situations [8]. Here, we discuss new insights gained from studies that explicitly acknowledge the contribution of multi-host species systems to the dynamics of emerging pathogens. Throughout this review, we also argue on the necessity to better cross-fertilize the two separate research fields of epidemiology and ecology of infectious diseases if we want to anticipate future epidemics. Finally, we recommend an increased attention to the role of the many epizootic host species and the weakly competent vectors in disease transmission not only among themselves, but also in driving the infection towards human communities.
2 “In-a-nutshell” basic principles of community ecology
Many definitions can be used to describe what an “ecological community” is. This profusion of definitions does not simplify communication within this research field. In this article, we have chosen to use the relatively neutral, but also vague, definition by Putman [9]. An ecological community is thus defined as “a group of species that interact in a given geographical area and whose functions and dynamics are somehow interdependent”.
Classically, a community structure is described using a species abundance/richness curve that associates a number of species for each abundance “rank” (e.g., 20 species involving between 2 and 4 individuals each). These relations can take different forms according to the animal communities under study. For instance, Fisher et al. [10] showed statistically that this relation follows a geometrical law (Fig. 1) for butterfly communities. A few years later, Preston showed that this relation takes more frequently the shape of a log-normal distribution in mammal communities [11,12] (Fig. 1). These two explanations have been unified only recently following Hubbell's demonstration that both relations correspond to two specific situations of another more general distribution called zero-sum multinomial [13].
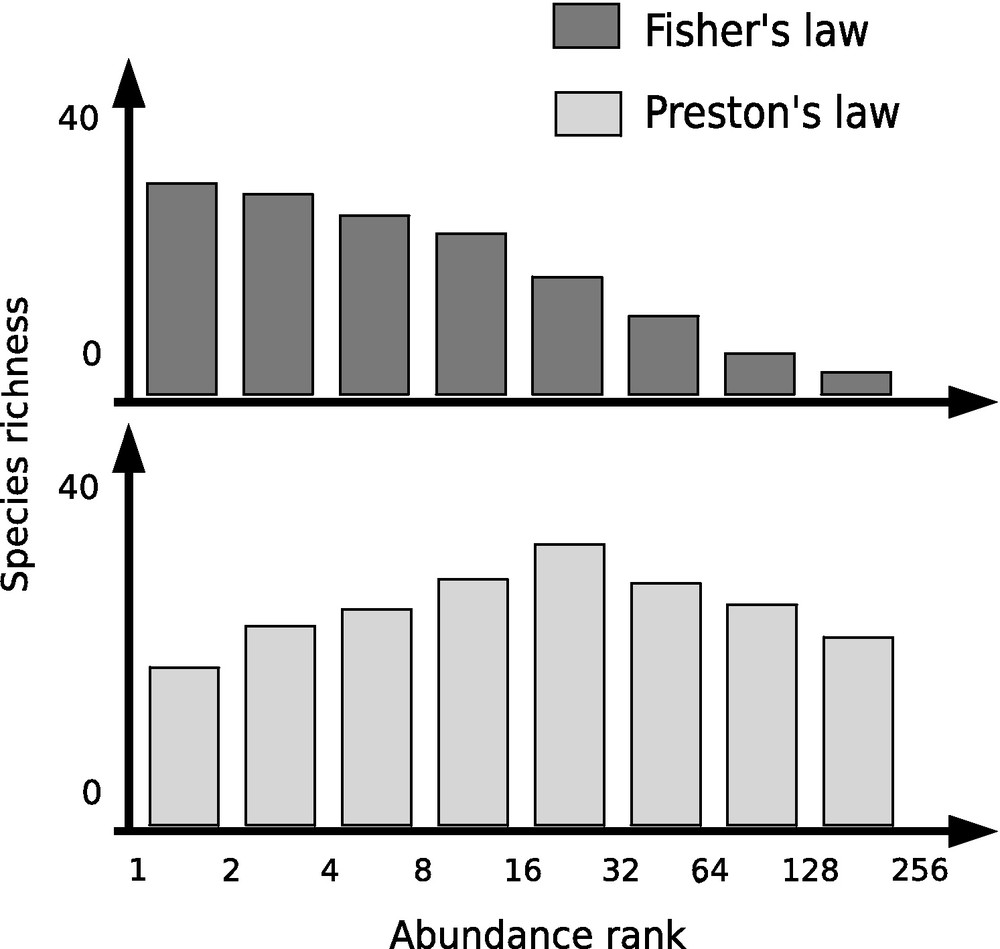
Examples of the species abundance-richness distribution in different animal communities. The Fisher's law is representative of invertebrate species communities, such as insect communities, whereas the Preston's law is typical of vertebrate species communities, such as mammalian communities.
These species abundance/richness relationships are the results of all the interactions that occur within local communities (e.g., preys/predators, competition…) and as such they may constitute a good integrator of all these relations. Since it is not sufficient to account for the observed community structure, trophic networks should not be dismissed as they might be another important contributing mechanism to the dynamics of ecological communities [14]. However, within these networks, the importance of parasitic organisms (e.g., viruses, bacteria, protozoans, helminth worms.) has been frequently ignored due to sampling issues. Parasites are surprisingly important in food webs and their role in food web stability is increasingly recognized [15–17]. First, by inducing a differential mortality among the host species, parasites can positively or negatively influence the abundance and the spatial distribution of the host species they use as resources. Second, and this aspect has been less studied, parasites can affect food webs and interacting host species especially by altering trophic cascades.
3 How can parasites influence the dynamics of host communities?
The first “visible” effect exerted by parasites is the more or less important regulation that virulent infections can have on the size of host populations in wildlife [15]. The literature in this domain is teeming with scientific evidences that describe the parasitic pressures on natural host populations. The case of the accidental introduction of bovine rinderpest in the wild ungulate communities of the Serengeti National Park in 1892 is here emblematic [15]. The disease decimated different animal species. This, in turn, caused the decrease in the predation pressure on the vegetation, leading, at the same time, to a modification of the vegetation cover of the savannah that made it less favourable to fire development.
Although this example describes well the primordial role exercised by parasites in ecosystems, it is however necessary to distinguish the effects of generalist (nonspecific) and host-specialized parasites on host species communities. In the case of a generalist parasite that can thus infect a significant range of host species, the consequences are generally destabilizing for the host communities. Indeed, if a parasite species can simultaneously attack many host species, the ones that are more tolerant to the parasite (i.e., the host species that can support the reproduction of the parasite while sustaining little damage) will engender an “apparent” competition with the less tolerant host species that will pay for the cost of the parasite virulence. In Scotland, the parasitic nematode Heterakis gallinarum infects both pheasants and partridges [18]. This pathogen multiplies very well in pheasants without decreasing the size of their population that, as a result, shows a great tolerance towards this parasite. For partridges, the situation is the opposite because a large proportion of partridges infected by this nematode will rapidly die. The degree of abundance of pheasants in the community has thus the consequence of increasing locally the nematode incidence and this will directly affect the abundance of partridges, which are more susceptible to the parasite (Fig. 2). Although we cannot propose other examples as representative as this one, in nature such situations must be frequently encountered.

Example of indirect competition induced by a parasite within a local community of hosts. The introduction of individuals of a host species (here symbolized by a pheasant), which is tolerant to the parasite (here a Heterakis nematode worm), leads to an increase of the parasite prevalence within the community. If this tolerant host species interacts with another host species, which is less or nontolerant (here partridges), the increase in the disease prevalence will then cause extra mortality in the non- or less tolerant host species, independently from the type of interactions between tolerant and nontolerant host species.
On the other hand, the effects of host-specialized parasites on host species communities are the opposite of the ones previously described, because generally an important diversity in host species is maintained locally. Indeed, by limiting the abundance of the species they infect, these host-specialized parasites also decrease the local competition pressure among host species and allow the rarest species to freely coexist in the environment [19].
To sum up, parasites can influence the structure of species communities and the consequences of this on the ecosystems can be contrasting due to the leverage action they exert on the coexistence of host species within local communities. Moreover, within natural ecosystems, parasites are undeniably key elements that contribute to the shaping of the observed variety of local species communities. In return, this diversity in species richness and composition can also influence the transmission mode of pathogens.
4 How can animal host communities influence the dynamics of parasites?
Asking about the role played by a community of hosts on the dissemination of a parasite boils down to consider the influence that a local change in the host species composition can have on its transmission. One or more host species can either disappear or appear locally, and this in turn can have important repercussions on the dynamics of parasite transmission.
The consequences of greater host species richness may strongly depend on the transmission mode of the pathogen. For pathogens with “density-dependent transmission”, in general any local addition of host species is likely to enhance disease transmission due to the increase in contact rates among individual hosts within the community [20]. This situation is often observed in the case of directly-transmitted infectious pathogens, like rodent-borne hantaviruses or avian influenza viruses [5,20]. A second situation is that of pathogens with “frequency-dependent transmission” in which contacts among host individuals occur at a constant frequency and do not depend on the host population density. This type of transmission is assumed for sexually transmitted pathogens, like HIV, in which the number of sexual contacts does not increase with the size of the population. Another example of frequency-dependent transmission concerns vector-borne diseases, e.g. malaria, in which the transmission is from host to host via a mosquito vector. This type of transmission is also encountered with pathogens transmitted by ticks, such as Lyme disease for instance. Here, the frequency of blood meals by the arthropod vectors generally does not increase with the abundance of host individuals [21].
For the sake of simplicity, we consider here only the case of vector-borne transmission. During a blood meal from an infected reservoir host, the vector can be contaminated and transmit the parasite to healthy host individuals. In this context, the individuals challenged by the pathogen can be from a single host species (for a host-specialized parasite) or can belong to different host species (for a generalist, nonspecialized parasite). In the case of a nonspecialized, multi-species system, the local conditions of species richness and composition in reservoir hosts will have important consequences on the disease transmission, as it has been shown by Richard Ostfeld et al. [22,23]. These authors have analysed the circulation of the bacterium Borrelia burgdorferi, responsible of Lyme disease in North America. This biological model, which involves numerous reservoir host species and only one vector species (the tick species Ixodes scapularis), represents an interesting first step for the study of pathogen transmission in multi-species systems. Each of these potential reservoir species has naturally a different competence to multiply the pathogen and to transmit it again during a blood meal by noninfected ticks. Here, we will define as susceptibility the probability that a healthy host individual might become infected following a contact with an infected vector [24]. Hence, in animal species communities, each reservoir and vector species has a different susceptibility that is characteristic of that species. In reality, within a host (reservoir or vector) species, important differences in susceptibility can be observed, but it is difficult to integrate this intraspecies variability due to the simplicity of the epidemiological models that are currently used. By focusing on the interspecific variability of susceptibilities, Ostfeld et al. have carried out a series of experimental and field studies that have demonstrated the importance of the local composition in reservoir host species on the transmission of the bacterium responsible of Lyme disease [24].
These authors observed a decrease of pathogen prevalence in Ixodes ticks and a lower human prevalence with greater reservoir host species richness [22]. Further studies on the West Nile virus, which is transmitted by different mosquito species and many bird species in the USA, have also highlighted a tendency to lower pathogen circulation in the presence of greater host bird richness [25]. The results of the few empirical studies, which have been carried out, converge and indicate that local communities with low richness in reservoir host species tend to present higher levels of disease prevalence (Fig. 3), whereas local communities that involve a larger number of reservoir hosts species see this level decreasing (Fig. 3). Ostfeld et al. [22–24,26] have called this phenomenon the “dilution effect” in reference to the process of deviation to which is subjected the pathogen in communities which are rich in reservoir species and a significant proportion of which are less or not susceptible to infection.

Schematic representation of the dilution effect proposed by Richard Ostfeld and his collaborators. For the sake of simplicity, it is considered that one vector individual can bite only twice during its lifespan. In the left panel, an infected vector transmits the pathogen to two individuals from a susceptible reservoir species. These two highly susceptible reservoir individuals will infect in turn two new vector individuals. On the right, the same initial conditions, but with the introduction of an individual from a nonsusceptible reservoir species. If the vector is generalist, there is a nonnegligible probability that the infected vector will bite this nonsusceptible reservoir individual. Hence, only one reservoir individual will be infected and transmit the disease to one vector individual. Overall this results in a decline of the pathogen prevalence in the vector populations.
To the best of our knowledge, only two theoretical studies tried to formally explain the dilution effect. First, using an epidemiological model of the Susceptible-Infected-Resistant (SIR) type that relies on allometric laws to quantify the different parameters, Dobson [21] analysed the epidemiological consequences of host reservoir species inclusion (up to six, and progressively less susceptible) in a virtual host community. Among other outputs, Dobson [21] studied the basic reproduction number (R0) that quantifies the mean number of new infections caused by a single infected host individual. He underlined that, in the case of frequency-dependent transmission, the value of R0 decreases when the richness in host species increases, which represents a direct application of the previously described “dilution effect” phenomenon. This first theoretical formalism had the merit of introducing a theoretical framework to explain the dilution effect observed empirically. Nevertheless the low reservoir species richness involved (6 species) and the fact that Dobson's work considered only directly transmitted infections did not allow the generalization of these results to vector-borne diseases, especially when multiple vector species are involved.
In a more recent theoretical work, Begon [5] has discussed the effects of the addition of a second, less competent host species on transmission pathways, pathogen persistence and abundance. In the case of Louping ill virus, which is transmitted in red grouse by the vector tick species Ixodes ricinus, Begon [5] concluded that in the presence of a second, less competent host species, a transmission event linked infectious individuals with incompetent susceptible host individuals that generated many fewer new infections than a competent host would have. The second host species therefore served only to dilute the transmission process, but in general, it is difficult to separate a dilution effect due to greater host species richness or composition.
To continue the works by Dobson [21] and Begon [5], a recent study by Roche et al. [27] has proposed a more general theoretical formalism by borrowing empirical observations derived from community ecology to mimic the relationships observed in nature between local species richness and relative abundance in species (see section 2, i.e., species richness-abundance curves). This study concentrated on the intensity of transmission of a pathogen in a local community of host species by studying the observed maximum prevalence of locally infected cases that represents a close estimate of the R0 [27].
By taking into account that, in the case of vector-borne zoonotic diseases, two categories of animal communities interact, on one side, the reservoir species and, on the other side, the insect species, this study allowed highlighting the following findings [27]. For two local communities with the same reservoir species richness and abundance but with different species compositions, an increase in the variation of the reservoir species susceptibility corresponded to a greater number of reservoir species with lower level of susceptibility. An immediate consequence is that an important number of vectors, all other proportions kept identical, will feed on reservoir individuals which are less able to transmit the pathogen. Thus, the interspecific variation in susceptibility of the host reservoirs is an important criterion that on its own can explain the decrease in prevalence of a pathogen in vector populations. In practice, these pathogen dynamics are usually the result of a balance mainly between efficient transmission by competent hosts and abortive transmission by poorly competent hosts that can likely lead to a dilution effect (Fig. 4). Indeed, it is not, strictly speaking, the local richness in reservoir species that acts on the level of prevalence of the pathogen in the vectors, but the proportion of reservoir species with low susceptibility, which tends to augment in species-rich communities, that decreases the intensity of vector-borne transmission.
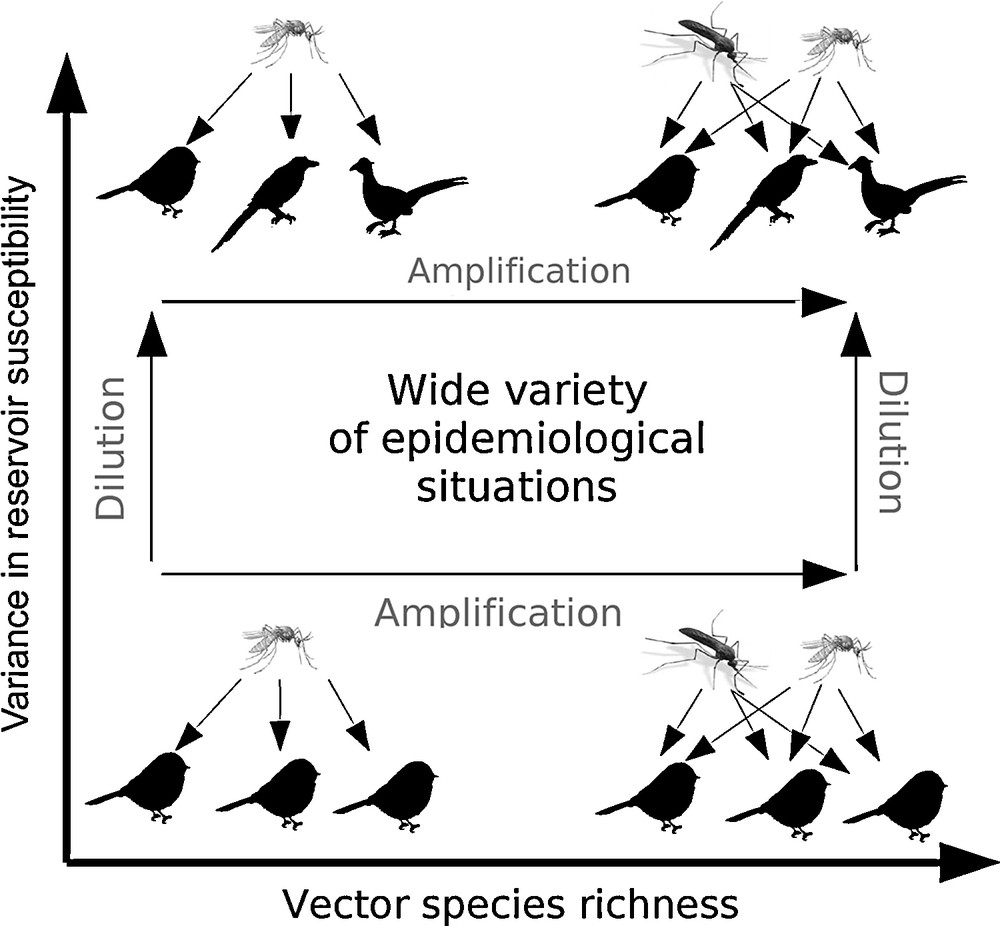
Dual effects of the richness in host reservoir and host vector species on the transmission of vector-borne pathogens of zoonotic origin. In species-rich reservoir communities, generally a decrease in the prevalence of disease pathogens in the vectors is observed. This is illustrated by the case of Borrelia burgdorferi that is transmitted by the Ixodes scapularis ticks in many vertebrate hosts in the USA. The diversity of reservoir hosts dilutes disease transmission (dilution effect) and the pathogen dynamics drops. On the other hand, in species-rich communities of vectors, a larger proportion of vectors, even if poorly competent, can acquire the pathogen from infectious hosts and then amplify the transmission of the disease to susceptible hosts (amplification effect). In natural conditions, these two antagonistic effects can lead to very contrasting epidemiological situations depending on the characteristics of the local ecosystems and their biological diversity, as observed for the emerging disease caused by West Nile virus (see text for further explanations).
So far, we have focused on the community of host reservoir species and its role in the transmission of pathogens in multi-host systems. Nevertheless, the vector species community also plays an important part in disease transmission. The dilution effect has been most thoroughly studied in vector-borne diseases, especially Lyme disease, which, in the USA, is transmitted by a single tick species, I. scapularis [22,23,26]. This, therefore, might not be representative of the many indirectly transmitted zoonotic pathogens for which several vector species may be more or less competent in disease transmission. For zoonotic vector-borne infections, there are two types of contacts: (i) between infectious vectors and more or less susceptible reservoir hosts and (ii) between infectious host reservoirs and more or less susceptible vectors. Indeed, the dilution effect requires that vector contacts must be wasted on poorly competent reservoirs, but also that vector species richness, by increasing their abundance, cannot compensate for these wasted contacts to overcome the dilution effect. In the case of vector-borne pathogens, the analysis of the species richness-abundance curves of the local vector communities in the different models allows the assessment of the effect of the number of vector species in a given community on the pathogen persistence and prevalence. This raises two important questions concerning: (i) the effects on local disease transmission of the accidental introduction or biological invasion by exotic vectors, even when they show a low competence to transmit the infection; and (ii) the role of low to very low competent resident vector species on the general circulation of pathogens. Moreover, it highlights what is perhaps a key issue in the understanding of vector-borne emerging pathogens. Although medium to very high competent vector species are usually credited with the maintenance and transmission of a disease, the role of the many poorly to very poorly competent vectors should not be underestimated as recent evidences have shown for bovine catarrhal fever (also known as Blue Tongue disease) in Northern Europe [28], Chikungunya in Italy [29] and Reunion Island [30], or malaria in Corsica [31].
It is interesting to note from the multi-host dynamic model by Roche et al. [27] that, while an increase in the number of reservoir host species generally dilutes the transmission of a pathogen, a local increase in vector species richness amplifies transmission (Fig. 4). To understand why, it is necessary to consider the number of new additional bites that occur when local vector species richness increases, even though some vector species might be poorly to very poorly competent for the pathogen locally.
These opposite effects of dilution and amplification of the pathogen circulation must be taken into consideration when analysing vector-borne disease transmission. Indeed, depending on the local species richness and composition in host reservoirs and vectors, we may observe different disease trends (Fig. 4; and see also Begon [5]). This model clearly illustrates the mechanics and the potential role played by the biological diversity of host reservoirs and vectors in vector-borne infections. A possible example of this is represented by the transmission of West Nile virus. West Nile virus is a Flavivirus commonly found in Africa, West Asia, Middle East, Eastern Europe, Northern and Central America and West Indies that causes encephalitis (inflammation of brain and spinal chord) in humans and horses. West Nile disease is transmitted by different mosquito vectors, which bite and infect birds. Many mosquito species have been tested positive for this pathogen [32], but in experimental studies the most susceptible one seems to be the Culex species complex [33]. Infections have been identified mostly in wild birds (and even domestic ones), which are the primary reservoirs for the virus. Although most wild bird species are not affected and rather act as more or less amplifying reservoirs, members of the Corvid family, including crows, blue jays, magpies and ravens, are very susceptible to the effects of West Nile virus. The virus can also infect many different dead-end hosts in terms of transmission, including humans and other mammals.
In the case of West Nile virus, the “take home” message from the multi-host/multi-vector models is that the local richness and composition in host reservoirs and vectors may lead to different combinatorial effects on disease transmission. In Southern Europe, like in the Camargue area, where the bird reservoir species are poorly to moderately susceptible to infection and only two vector species, one being moderately and the other one poorly susceptible, are present, the prevalence of West Nile virus is highly sporadic [34]. Conversely, in the USA, where there are a multitude of bird reservoir species (of which a large part appears to be very susceptible) and an important diversity in vector species (some vectors being highly competent), huge epidemics that have propagated to the entire country, from east to west, in only a few years have been reported [32]. The absence of previous immunity in bird populations to this virus (West Nile Fever appeared in North America in 1999) cannot explain this pattern due to the fast demographic turnover within avian communities.
In the simplified context of Fig. 4, the combination(s) of local reservoir and vector species richness and composition that can either amplify or dilute the infection may help to explain many other (new) emerging disease spillovers [8]. Bearing in mind that, generally, pathogen dynamics are driven by the dynamics of the overall biological diversity of the community and not of one single reservoir or vector host species, then future research should concentrate on revisiting the idea of disease transmission using a broader community-scale perspective than the one generally applied.
5 Conclusions and future research perspectives
Our understanding of the influence of reservoir host and vector diversity on pathogen epidemiology and disease dynamics is still rudimentary. The possibility of strong effects due to a local multiplicity of hosts and vectors is however high for a large number of emerging pathogens [1]. For some pathogens, like for instance West Nile virus, different species of reservoirs (birds) and vectors can be involved locally, and changes in both reservoir and vector diversity from place to place can result in functional changes in transmission that have strong effects on the disease dynamics [32]. Indeed, animal viruses with multiple hosts are more likely to be designated as emerging threats than the ones with single hosts, a finding that emphasizes the importance of better examining the role played by host and vector diversity [3,7]. Acknowledging a multiplicity of hosts in many zoonotic diseases [4] may also complicate the way these diseases should be studied and analyzed when compared to simple one host-one vector systems.
Our examination of emerging infectious diseases with a zoonotic origin indicates that an explicit assessment of the influence of multiple host reservoirs and vectors must be taken into account. Undoubtedly, it is realistic to acknowledge that the local and regional heterogeneity in vector and reservoir diversity can more or less affect disease behaviour and spread in nonspecialized parasites. Overall, the evidence that the local host diversity influences the outcomes of disease dynamics seems clear for both Lyme disease and West Nile virus. As a consequence, not considering a priori the potential role played by poorly to very poorly competent hosts – the immersed part of the iceberg –, and particularly of vectors, under the pretext of a low or very low supposed or proven participation in the transmission, can have important ecological and epidemiological consequences. In many situations, the onset of emerging infectious diseases might be due to the multiplicity of hosts and vectors and its local variability and the role of the many epizootic reservoirs and poorly competent vectors should not be underestimated, not only in driving the disease dynamics in the system, but also in increasing the risk of infection to humans.
This highlights possible research key issues for understanding pathogens in multi-species systems. Given the recent emergence of important infectious diseases in several complex communities [8], more attention should be given to generalist pathogens and to recording the number and identity of all potential vector and reservoir species locally, instead of focusing only on the more competent, well-accepted hosts and vectors. It is clear from this review that to better understand multi-species dynamics of diseases, we should rather collect qualitative information on the largest possible number of actors in disease dynamics than to obtain quantitative and substantial data but only for one or two reservoir hosts or vectors that are a priori suspected to be involved in the transmission. Moreover, since we can observe both spatial and temporal variations in local species diversity, such analysis should be repeated in several different communities and for different periods of time in order to infer on disease regional dynamics and on its effects on host assemblages, and vice versa. In addition, the potential for an accidental introduction in an originally untouched geographical area of some vector specimens, even if they are not very competent in transmitting the pathogen, should not be underestimated especially because they might originate or exacerbate the onset of an epidemic. The same could be said also about resident vectors and reservoir hosts that are accepted as being putatively low to very low competent hosts, but might play a key starting role in the epidemiology of a disease.
Besides, it comes back also to better understand the links that exist between the relative competence of reservoir and vector species and their life-history strategies, particularly the fact that competent hosts and vectors are often ubiquitous and generalist organisms. Other life-history characteristics of reservoir and vector species should be further explored, such as reproductive strategies, feeding habits or body features, for instance. From an evolutionary point of view, pathogens should select for the best host vessels that facilitate their own survival and dissemination. A multiplicity of hosts might thus complicate the evolutionary dynamics of pathogens, if it introduces conflicting selective pressure between being embarked into the main reservoir host(s)/vector(s) or into a more occasional host/vector [5].
On the other hand, there is no clear evidence that the pathogen prevalence for vector-borne infections is lower in richer reservoir communities. Indeed, by studying the transmission of a generalist plant virus in experimentally manipulated plant communities, Power and Mitchell [35] showed that the pathogen prevalence was entirely driven by the single, most competent plant species. In this case, therefore, the link between pathogen prevalence and species richness was a reflection of the community composition rather than of the community richness. To what extent, this observation might be applied to other multi-species systems is still a matter of debate. Nevertheless, this suggests again that it would be more fruitful to explore the links between biological diversity and emerging infectious diseases by focusing on community composition and species life-history strategies.
The community epidemiology perspective we advocate here may present also some practical aspects. Indeed, it could be envisaged to exploit the natural complexity of ecological systems by introducing locally animal baits in the form of reservoir species with low susceptibility that might thus divert vector species from their human targets. By choosing preferentially these baits, vectors will consequently reduce disease transmission to humans [36]. This type of control is called zooprophylaxy. The development of livestock rearing in Western countries, by increasing the herd of animals with low susceptibility, has been suggested to be responsible, according to Bruce-Chwatt [37], to the disappearance of malaria in Europe. This method, which has been theoretically studied for malaria [38], is still considered for application in some regions of Asia [39]. This calls into question the importance, in practice, of the community function in the epidemiology of infections. Of course, more complete theoretical and experimental studies must be carried out because the introduction of these animal baits could have other indirect and even more dramatic consequences.
Community epidemiology is still in its early days. Limited so far by a too simplistic perspective (the triad “one agent-one vector-one reservoir”) on the complexity of natural communities, its development in the next years should stem from a partnership between specialists of complex systems, especially those working on multi-agent systems, ecologists, evolutionary biologists and epidemiologists. We conclude this review with an idea that has been pervasive throughout this essay: different epidemiological and ecological outcomes for a same disease can occur in different places, and one important and probably missing ingredient in the understanding of emerging infectious disease is the role of biological diversity and its heterogeneity in space and time.
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.
Acknowledgments
This review was supported by the Institut de Recherche pour le Développement (BR, JFG), the French School of Public Health (JFG), the Centre National de la Recherche Scientifique (JFG), the University Pierre et Marie Curie (BR) and the EDEN project, EU grant GOCE2003010284 EDEN (BR, JFG). This publication is catalogued by the EDEN Steering Committee as EDEN0245 (http://www.edenfp6project.net/). The contents of this publication are the sole responsibility of the authors and do not necessarily reflect the views of the European Commission. Additional note: We recommend the reading of a recent review paper by Keesing et al. published in Nature (2010), vol. 468: 647–652, and that appeared during the editing process of the present work.


