1 Introduction
Steroid hormones exert a wide variety of effects on the growth, development, and differentiation, including important regulatory and behavioral functions within the reproductive system, central nervous system, and adrenal axis. These hormones act by binding to specific intracellular receptor proteins that function as signal transducers and transcription factors to modulate the expression of target genes [1,2]. Numerous studies have revealed that the Harderian gland is an androgen target organ. Androgens affect HG morphology and secretory activity [3]. Although a great deal of interest has been focused on the androgen receptor in the hamster's HG, the androgen sensitivity of the desert Meriones libycus was not elucidated so far. HGs are capable of undergoing significant morphological changes from season to season [4]. We strongly believed that there must be a link between steroid hormone action and induction of pheromone production, an idea proposed by Beier et al. [5] based on the localization of androgen receptors within these glands. Indeed, one of the functions assigned to the gland is pheromone production [6].
The Libyan jird, M. libycus, is a seasonal breeding desert rodent that is sexually active during the spring and quiescent in the fall. Based on previous work concerning the HG of desert rodents [4,7,8], we put forward the hypothesis that HG and AR expression changes with the season. We wanted to know whether the HG undergoes structural variations and whether the expression of AR in M. libycus HG undergoes changes during the reproductive cycle.
2 Material and methods
2.1 Animals
For this study, we used 12 males of M. libycus, six in the breeding season and six in the resting season, of mean body weight 92,48 ± 7,31 g, trapped at night in the desert of Béni Abbès (30°07′N 2°10′W). The animals were cared for according to the recommendations of the “Association algérienne des sciences en expérimentation animale” (AASEA) (http://www.aasea.asso.dz/).
2.2 Light microscopy
Harderian glands were removed after anesthesia in light phase. A few glands were fixed in 10% buffered formalin, embedded in paraffin and sectioned at 5 μm for histological study, stained with Van Gieson (Hematoxylin/picrofuchsine). Some HGs were prefixed with glutaraldehyde–paraformaldehyde (pH 7.4) at 4 °C, and subsequently fixed in 1% osmic acid solution for 2 h and embedded in epoxy resin blocks. Semithin sections (1 μm) were cut with ultramicrotome (LKB Bromma, 8800 Ultratome III). Samples were stained with toluidine blue and examined by light microscopy Zeiss and photographed with a high-resolution optics microscope camera (MA88-500: Premiere®, 5.0 Megapixels with a 1280 × 1024 resolution) using TSView 6.2.4.5 software.
2.3 Morphometric study
A morphometric study of semithin sections during breeding and resting periods was made using Axio Vision 4.8 (Carl Zeiss Micro Imaging GmbH, Germany).
The principal cell height was measured using a 100× objective. Results were expressed as means ± standard measurement error (SEM).
2.4 Immunohistochemistry
The Harderian glands were fixed in paraformaldehyde at 4% during 24 h and embedded in paraffin blocks. Five-micrometer-thick sections were cut with a microtome (American Optical Corporation), and mounted on superfrost glass slides. Tissue sections were deparaffined with xylene and rehydrated with graded ethanol. The samples were washed in tap water for 5 min then in PBS-T for 5 min. After incubation in 3% H2O2 for 30 min, the sections were encircled using Dakopen (Dako, Glostup, Denmark) and incubated with a horse serum for 30 min to block nonspecific sites. Afterwards, the slides were incubated with primary antibody AR (ab 74272, Abcamplc, Cambridge, UK). The AR antibody was used at a dilution of 1/50, and incubated overnight at 4 °C in a wet chamber. Next, sections were washed in PBS solution and incubated with secondary biotinylated antibody (Anti-Mouse IgG/Rabbit IgG; BA-1400, Vectastain Universal, Vector Laboratories, Burlingame, CA, USA) for 30 min in a wet chamber. After missing in PBS, samples were incubated with an avidine/peroxydase complex for 30 min. The slides were washed in PBS and stained by Novared chromogen (ImmPACT NovaRED, Peroxidase Substrate, SK-4805. Vector Laboratories, Burlingame, CA, USA) for 3 min, then the reaction was stopped in a PBS solution. Some sections were then counterstained with hematoxylin (QS, H-3404, Vector Laboratories) for 3 min, then dehydrated and preserved with Eukitt. Tissue sections used as negative controls were incubated with PBS solution instead of the primary antibody. The observation of immunostaining was done under a Zeiss light microscope.
2.5 Statistical study
The intensity of immunoreactivity during breeding and resting periods was measured on no-counterstained sections using NIH Image J software (http://www.mirror.imagej.net/docs/examples/stained-sections/index.html).
The intensity of the immunostaining of prismatic cells was measured using a 40× objective. The results were expressed as means ± standard measurement error (SEM). The statistical analysis was conducted by testing the distribution of the variables for normality. One-way Anova was used to test the effect of the reproductive cycle followed by Tuckey comparison test on cell height and androgen receptor intensity. Differences were considered significant at P < 0.05 (Statistica version 6).
3 Results
3.1 Organisation and cytoarchitecture of the Harderian gland in M. libycus
Our cytoarchitectural investigation of the Harderian gland of M. libycus consists in a tubule-alveolar structure with a wide lumen lined by columnar epithelial cells (Fig. 1).
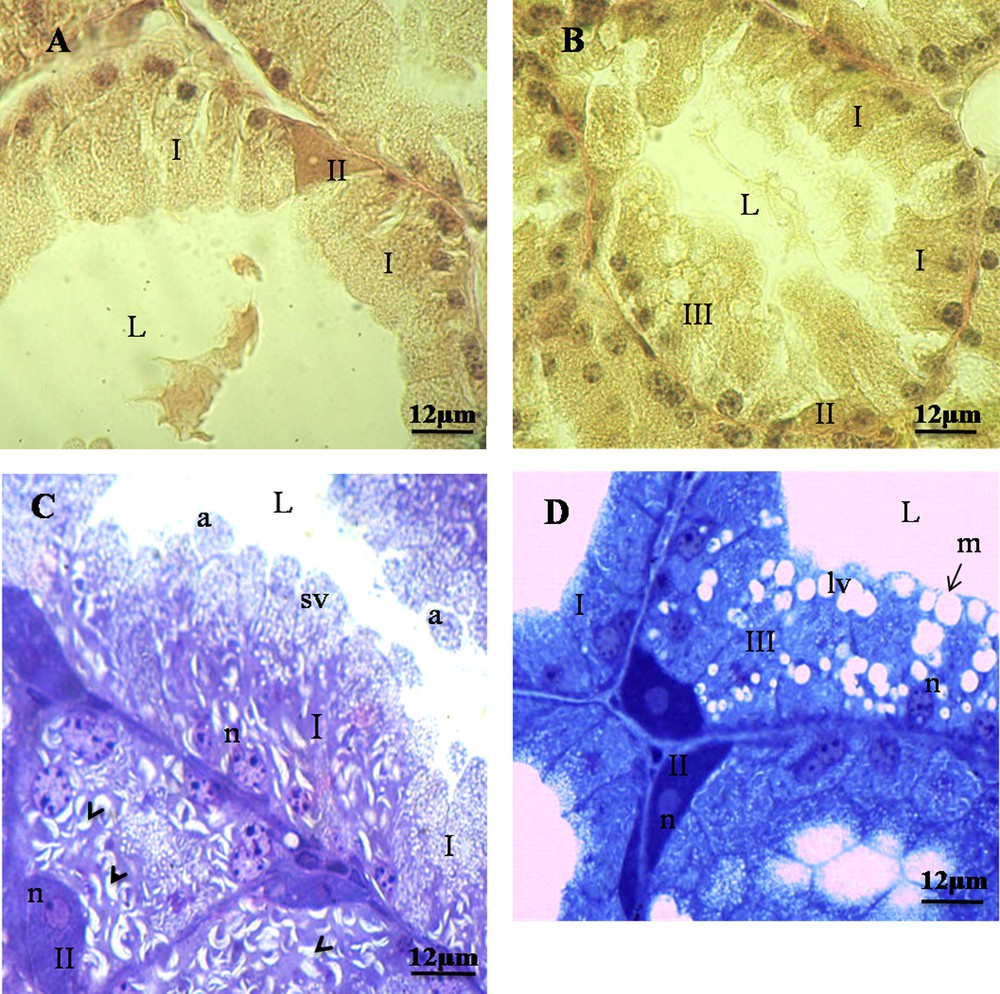
Harderian epithelium structure in Meriones libycus during reproductive cycle. A and C. Harderian epithelium in the resting period. Type-I cells appear prismatic with acidophilic cytoplasm, basal nuclei (n), presence of small lipidic vacuoles (sv), and cytoplasmic slashes (Head Arrow); type-II cells are interposed between type-I cells and are pyramidal in shape with basophilic cytoplasm. B and D. Harderian epithelium in breeding period. Type-III cells resemble type-I cells, with a prismatic and acidophilic cytoplasm, with larger lipidic vacuoles (lv); merocrine (m) and apocrine (a) modes of secretion. L: lumen. Masquer
Harderian epithelium structure in Meriones libycus during reproductive cycle. A and C. Harderian epithelium in the resting period. Type-I cells appear prismatic with acidophilic cytoplasm, basal nuclei (n), presence of small lipidic vacuoles (sv), and cytoplasmic slashes (Head Arrow); type-II ... Lire la suite
We observed, in histological and semithin sections, two types of cells during the resting season (Fig. 1A and C), while we observed the presence of three types of cells in HG during the breeding season (Fig. 1B and D).
We have shown type-I cells, frequently observed in epithelial tissue. They appear prismatic and some cells are binucleated, with acidophilic cytoplasm and basal nuclei (Fig. 1). They possessed also small lipidic vacuoles and cytoplasmic slashes (Fig. 1C).
Our results show type-II cells. They are located between the prismatic cells. They are reduced in number and have pyramidal shape with basophilic cytoplasm and often with two nuclei (Fig. 1).
We have observed in the Harderian gland of M. libycus that type-III cells resemble type-I cells, with a prismatic shape, an acidophilic cytoplasm, and larger lipidic vacuoles. These cells appear only in the breeding period (Fig. 1B and D).
We have found different modes of secretion in the HG of the male of M. libycus, including merocrine, apocrine (Fig. 1C and D), and holocrine (Fig. 2).
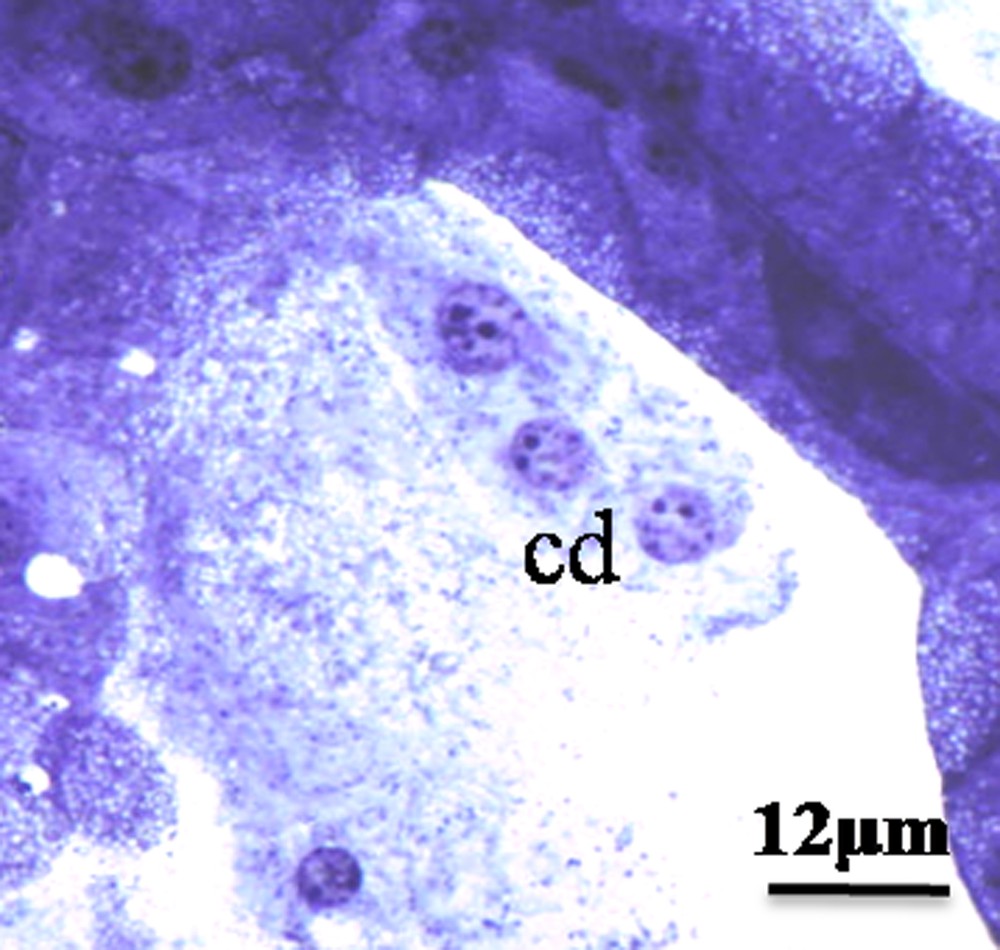
Holocrine, mode of secretion. Note the presence of nuclei and cytoplasmic rest in the large lumen; cd: cells debris; L: lumen.
Our morphometrical study shows that the reproductive cycle affects the size of the prismatic cells (P < 0.001, one-way ANOVA). The height of the prismatic cells during the breeding season was found significantly higher than in the resting season (36.66 ± 0.86 μm vs. 15.5 ± 1.01 μm, respectively; P < 0.001) (Fig. 3A). However, the height of the pyramidal cells does not change between the two periods of sexual activity (2.6 ± 0.21 μm vs. 2.96 ± 0.17 μm; P > 0.05) (Fig. 3B).
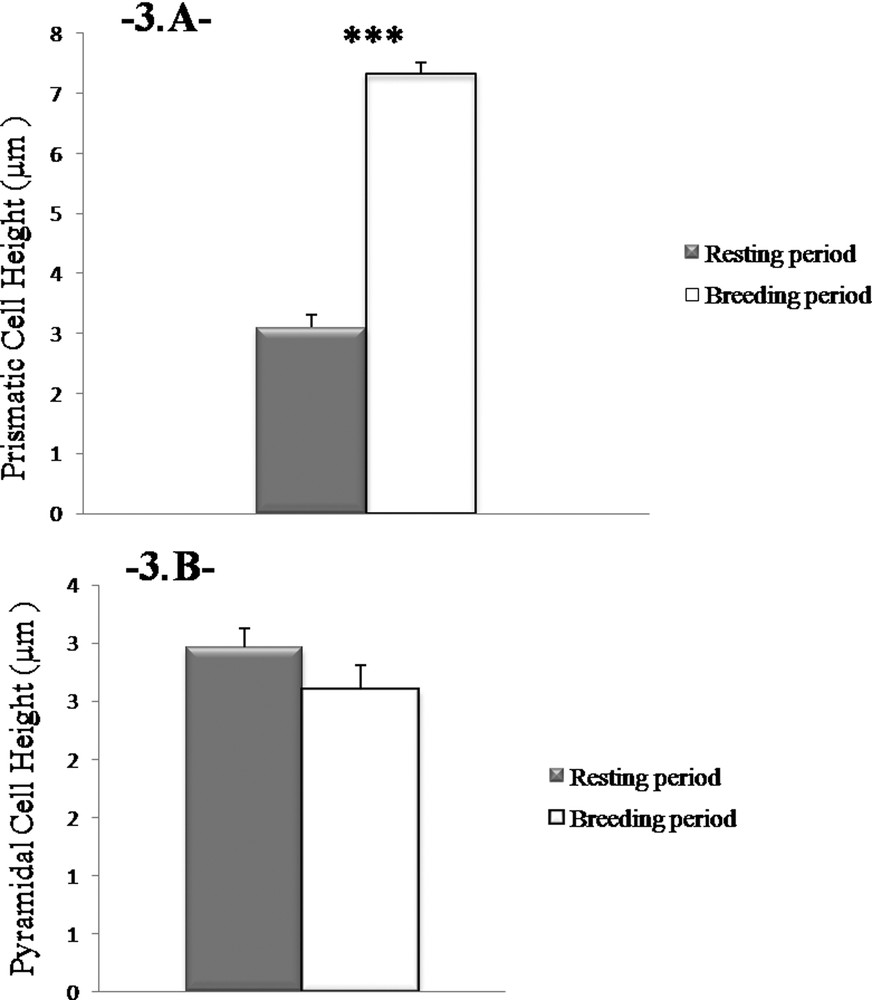
Height changes in prismatic cells. The height of the prismatic cells in the Harderian gland in males is more important during the reproductive period. Values are mean ± SEM, ANOVA, ***P < 0.001.
3.2 Immunoexpression of the androgen receptors during the reproductive cycle
We have observed that the immunoexpression of the androgen receptors are clearly in the cytoplasm of the prismatic cells in both periods of sexual activity and that it is granular in appearance. In the same sections, pyramidal cells appear negative for androgen receptors (Fig. 4A–D; E is a negative control).
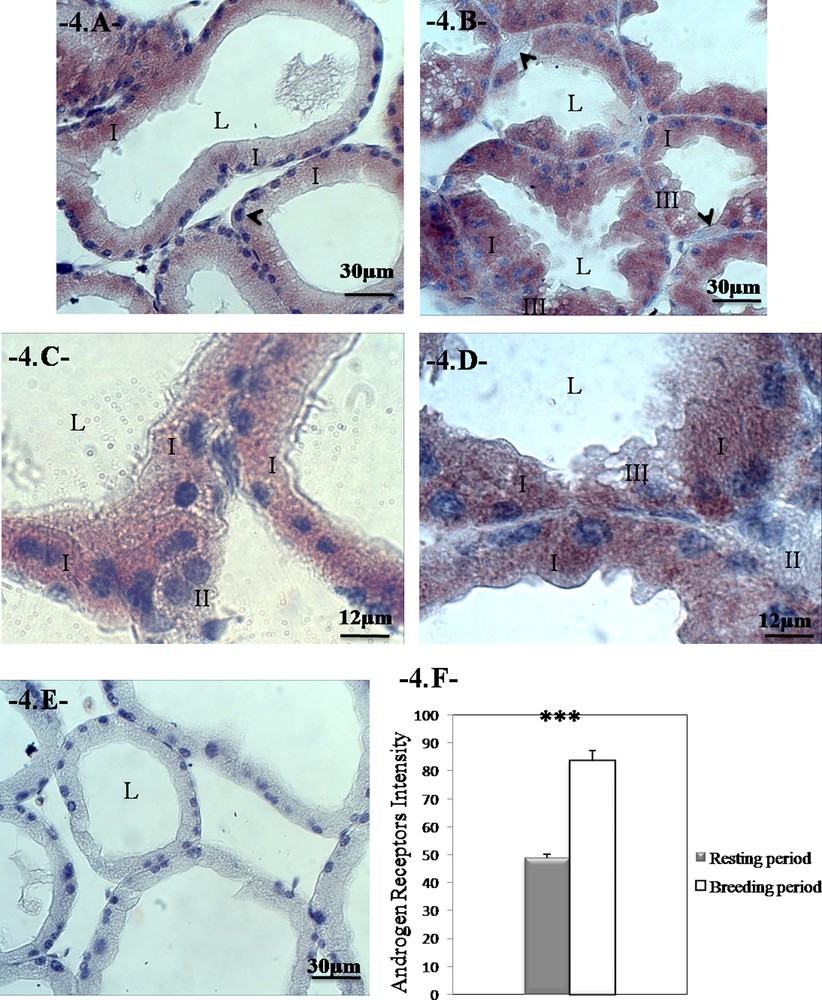
Changes in the distribution of the androgen receptors during the reproductive cycle in the Harderian gland of Meriones libycus. The immunoexpression of AR is localized in the cytoplasm of prismatic cells in the breeding and resting periods. Note the absence of receptors in the pyramidal cells (head arrow); breeding period (A and C); resting period (B and D); negative control (E). The sections were counterstained with hematoxylin. Androgen receptors intensity during the reproductive cycle (F). We can notice higher AR intensity during breeding period. Values are mean ± SEM, ANOVA, ***P < 0.001. (I) Type-I cells; (II) type-II cells; (III) type-III cells. Masquer
Changes in the distribution of the androgen receptors during the reproductive cycle in the Harderian gland of Meriones libycus. The immunoexpression of AR is localized in the cytoplasm of prismatic cells in the breeding and resting periods. Note the ... Lire la suite
We have found in the HG of the male of M. libycus that the reproductive cycle affects the immunoexpression of the androgen receptors (P < 0.001, one-way ANOVA). The intensity of the androgen receptors is significantly greater during the breeding season compared with the resting season (83.167 ± 3.482 vs. 48.897 ± 1.388; P < 0.001; Fig. 4F).
4 Discussion
Lipid production is a common feature of the Harderian glands of rodents and lagomorphs, and their epithelial cells have lipid vacuoles of different sizes. The cell types forming the Harderian epithelium vary from one species to another. We noted throughout our study that the cell types composing the Harderian epithelium vary upon gonadal activity.
Our results show that the glandular epithelium during the resting period is composed of two cell types, type I and type II.
We have observed type-I prismatic cells characterized by small apical lipidic vacuoles (sv). This cell type was found in the HG of Meriones meridianus and Meriones unguiculatus [9,10], of the hamster Mesocricetus auratus [11,12], and in the white lobe of the (Angora) rabbit's HG [13]. In our study model, M. lybicus, these cells have an acidophilic cytoplasm loaded with small lipidic vacuoles. The origin of the lipidic vacuoles is still not known, and according to some authors [14–16], these lipidic vacuoles could be formed from the SER (Smooth Endoplasmic Reticulum).
The main characteristic of type-I cells in our species is the presence of cytoplasmic slashes at the basal level, which is consistent with the observations in Gerbillus and Meriones crassus [17], in Psammomys obesus and Gerbillus tarabuli [18] and in Gerbillus cheesmani [19]; the cited studies suggest a functional relationship in Kuwait's gerbil between cytoplasmic slashes and lipidic vacuoles. However, these cytoplasmic slashes seem to be cholesterol ester [8].
We have found that the second type in the Harderian epithelium of M. libycus corresponds to type II, whose number is reduced with respect to the one measured with type I. These pyramidal cells are displaced at the base; they contain a parabasal nucleus and a basophilic cytoplasm. This cell type has been reported in our team's earlier work on Gerbillus gerbillus and M. crassus [17,20] and G. tarabuli [18]. We have shown that these cells are characterized by a rich heterochromatin nucleus, numerous mitochondria with a dense matrix, and a highly-developed GER organized in concentric lamellae. The role of type-II cells has not been elucidated yet, but because they are present in our desert species, they could be involved in an adaptation mechanism to arid environments.
Our investigation shows, that compared to the resting period, the HG exhibits structural variations during the breeding season. Indeed, during the breeding season, in addition to the two cell types (types I and II) observed in the Harderian epithelium, we have evidenced the appearance of a third type. The last type recalls type I by its acidophilic cytoplasm and the presence of lipidic vacuoles. However, type-III cells are characterized by large lipidic vacuoles and by the absence of cytoplasmic slashes. This cell variety was found in the male of the hamster (type II) [21,22], in the rat (type A) [23,24], and in the Angora rabbit [13]. This cell type has also been demonstrated in desert rodents [17,18].
The appearance of type-III cells in the HG of M. libycus is supposed to be related to gonadal activity. Indeed, steroid receptors have been identified in the Harderian gland in many species: rats, hamsters, frogs, mice, and rabbits [25–28]. The type-III cells that appear in our species during the breeding season could be responsible for the production of pheromones during the reproduction phase, when the gonads synthesize steroids that stimulate the Harderian cells to produce in turn pheromones, like it has been shown in P. obesus and G. tarabuli [18].
Our results show that the glandular lumen contains many products, released by different modes of secretion. We found that some substances were dumped by merocrine, others by apocrine, and we observed whole cells in tubular lumens also assuming the holocrine mode. These different modes have been described in P. obessus, G. tarabuli [17,18], in G. gerbillus, M. crassus, Ctynodactylus vali [20], and in the rat [24].
Our morphometric study shows that the Harderian epithelium increases in height during the reproductive period; this would be related to the secretory activity of the cells. The same observations were made in rabbit during the autumn [29]; in the green frog Rana esculenta, the cell height and secretory activity changed with the season and might be controlled by hormonal and environmental stimuli [30].
Further, we have examined the distribution of the androgen receptors in the gland cells. The androgen receptors are cytoplasmic in prismatic cells (types I and III) in both periods (breeding and resting), similar observations have been made in the HG of the male rabbit [29]. Other authors observed in the HG of rats that most type-I and II glandular cells displayed strong nuclear AR immunoreactivity, and that the intensity of the receptors’ reactivity was similar in type-I and II glandular cells [31]. Indeed, the mRNA for the androgen receptor is present in both type-I and II cells, suggesting that type-I and type-II cells are two forms of the same cell [32], probably denoting different activity or secretory statuses [33].
As expected, we have observed, in the HG of the male of M. libycus, an increase in the intensity of the androgen receptors during the breeding period (February to July) compared with the resting period (September to January). The immunohistochemical expression with the androgen receptors in the autumn revealed high immunoexpression, while in winter there was a moderate immunoreaction; but during both spring and summer, a negative immune expression was evidenced [29]. This simulates the observation in the male rat [25], in both sexes of the golden hamster [26], and in guinea-pigs, mice, ducks, chickens, marine turtles, and lizards [28].
Taken together, our data may indicate a close association between androgen receptors and the functional changes observed in this gland.
5 Conclusion
Our findings demonstrate that AR expression is related to histophysiological changes in the Harderian gland during the reproductive cycle in the male of M. lybicus. It seems that androgen receptors contribute to reproduction-associated mechanisms by modulating the pheromone synthesis rate during the seasonal reproduction cycle.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Contributors
N. Khennaf-Hanniche conducted the experimental part, the collection, the interpretation of data, and completed the first draft of the manuscript. Ouanassa Saadi-Brenkia contributed to the creation of the research design, supervised the experimental part of the work, participated in fata analysis and the correction of the paper. All authors have approved the final article.
Disclosure of interest
The authors declare that they have no competing interest.
Acknowledgments
The authors thank the team members, especially H. Touati, R. Hammi, I. Chaabane, as well as the personnel of the Research Station of Dry Land of Béni Abbès (Algeria).


