1. Introduction
An important aspect of the cardiac studies is the neuroanatomy of this organ [1, 2, 3, 4, 5, 6]. Research on nerve structures has given new insights into the pathological aspects of the heart function. The acquired knowledge is already used for further investigations, i.e. clinical trials [2, 5]. The research has been mainly conducted on the human heart [3, 7, 8] as well as in several animal species including the mouse [5], rat [9], guinea pig [10], dog [11], rabbit [12], heifer [6] and pig [1, 2, 13, 14]. A very interesting field of science, which is being intensively developed is xenotransplantology [13, 14, 15]. Domestic pigs are currently thought to be the best candidates for organ donation. This fact encourages wider and more thorough research on this animal species. Current experiments in xenotransplantation most often use pigs as the donor and baboons as human models. Pigs, when it comes to their physiological parameters, are very similar species to humans [13, 14, 15], among others, they have a very similar cardiac output, close blood pressure or urine osmolarity [13, 14, 15]. Despite considerable knowledge of cardiac neuroanatomy [1, 2, 3, 13, 14], little is known about the innervation of the foetal heart in mammals, including the pig. The existing data mainly concern the human foetus heart [3, 16, 17]. These data indicate that the development of cardiac autonomic innervation takes place in several stages and continues to early postnatal life [17] well as autonomic neurons and their precursors display substantial plasticity [17, 18]. Disorders in the normal developmental path, even at early stages of development, may result in cardiovascular disease in childhood or adulthood. An example of this is sinus bradycardia in mice lacking Nrp1 (neuropilin 1) and Sema3a (semaphorin 3A), important axonal growth-cone protein. Their absence is caused a defective trunk of neural crest cells (NCC) migration and results in ectopic sympathetic ganglia and a malformed stellate ganglion as the main centre of neurons whose axons supply the heart tissue [17, 19, 20, 21, 22]. Another example is hypertension and coronary heart disease, which early birth or low birth weight infants are predisposed to because the maturation of the autonomic nervous system has not yet been completed and the heart is under the dominant influence of sympathetic innervation. Due to the fact that the domestic pig is an important animal model in experimental neurocardiology [2, 13, 14] and xenotransplantology [13, 14, 15, 23], it is worth more closely investigating the proper development of heart innervation during prenatal development. Considering the rapid rate and high complexity of the development of the heart innervation, the delayed advancement of its the sympathetic component, as well as the first appearance of some biologically active substances (e.g. CGRP) in the nerve structures not earlier than in mid-pregnancy [17, 19, 20, 21, 22], it was decided to examine the porcine foetus heart nerve supply in the second half of pregnancy. Therefore, the present study aimed at investigating the distribution and chemical coding of nerve structures in the hearts of 10-week-old porcine foetuses (these foetuses, as a unique slaughterhouse research material, constituted the oldest foetal group obtained) and can be considered as a starting point for further investigations dealing with the developmental changes occurring in the earlier and later stages of pregnancy.
2. Materials and methods
Ten-week-old porcine foetuses (n = 5) were obtained from the “Warmia” slaughterhouse (Biskupiec, Poland). According to Polish law and an EU directive (No. 2010/63/EU) the experiments performed in the present study do not require the approval of the Ethics Committee. The age of foetuses was marked according to the crown-rump length (CRL) method. CRL sets the distance from the top of the head of the embryo or foetus entering the lower limit of the buttocks [24]. After removing the foetuses from the uterus, the thorax and abdominal skin were cut for better penetration of the fixative solution in all collected animals. The foetuses were fixed by immersion in 4% buffered paraformaldehyde (pH 7.4; 4 h), rinsed with phosphate buffer (pH 7.4; overnight, in the fridge) and transferred into an 18% buffered sucrose solution (pH 7.4) where they were stored until further processing. The hearts were dissected out, cut on 12 μm-thick cryostat sections and processed for single- or double-labelling immunofluorescence (as described earlier; [25, 26]) using primary antibodies against PGP, DβH, VAChT, CGRP and corresponding secondary antibodies (Table 1). After air-drying at room temperature (RT) for 30 min the sections were pre-incubated with a blocking mixture containing 10% normal horse serum, 1% bovine serum albumin and 0.05% Tween 20 in PBS (1 h, RT). They were then incubated with a mixture of appropriate secondary antibodies (listed in Table 1). After rinsing in PBS (3 × 10 min) the slides were cover-slipped with carbonate-buffered glycerol (pH 8.6).
List of primary antisera and secondary reagents used in the study
| Antigen | Species | Code | Dilution | Supplier |
|---|---|---|---|---|
| Primary antibodies | ||||
| PGP 9.5 | Mouse | 7863-2004 | 1:400 | Biorad |
| DβH | Mouse | MAB308 | 1:500 | Millipore |
| DβH | Rabbit | BML-DZ1020-0050 | 1:500 | Enzo |
| VAChT | Rabbit | V5387 | 1:200 | Sigma |
| CGRP | Rabbit | C8198 | 1:600 | Sigma |
| Secondary reagents | ||||
| Goat AlexaFluor 488 anti-mouse | A11001 | 1:400 | Invitrogen | |
| Goat AlexaFluor 488 anti-rat | A11006 | 1:400 | Invitrogen | |
| Goat AlexaFluor 555 anti-rabbit | A11010 | 1:400 | Invitrogen | |
The omission of primary antisera and their replacement by normal, non-immune sera (rabbit, mouse or rat) were used to investigate the specificity of immunohistochemical labelling. No fluorescence was observed in any of these control stainings, which confirmed the specificity of the staining. The labelled sections were viewed under a Zeiss Axiophot fluorescence microscope equipped with epifluorescence and an appropriate filter set for AlexaFluor 488 and AlexaFluor 555. The total number of cardiac nerve clusters or neurons in the large cardiac ganglia were counted in every fourth slice considering the size of the whole of cardiac neurons (neurons were not larger then 20 μm). To determine the percentage (as described earlier, [25, 27]) of particular neuronal populations, at least 100 neuronal profiles for each combination of antisera were counted. The sections of sinoatrial and atrioventricular ganglia were collected from different, representative regions of the ganglia (from its upper, middle and lower one-third).
3. Results
3.1. Nerve cell bodies
Immunohistochemical staining for PGP showed that numerous clusters of nerve cells were observed throughout the foetus heart (Table 2). The majority of the clusters were found beneath the epicardium around the root of the aorta, pulmonary trunk and main veins. The sinoatrial ganglion was found to comprise the largest number (262 ± 12) of the PGP-positive nerve cell bodies (Figure 1a). In the right atrial wall, a large PGP-positive nerve cell cluster was also observed – the atrioventricular ganglion (236 ± 13). Neurons forming both of the ganglia were mainly oval with a large nucleus located in the cell centre. The size of these neurons was 16–20 μm. Around the cardiac segment of the ascending part of the aorta, numerous scattered spherical clusters consisting mostly of over a dozen nerve cells were observed. The others, containing from 10 to 30 PGP-positive neurons, were distributed near the pulmonary artery and smaller clusters (5–10 neurons) were observed near the venous vessels. Numerous smaller clusters consisting of 6–10 PGP-positive neurons were observed in the epicardium of both atria, in the vicinity of coronary arteries (Figure 1g). The lowest number of neurons was observed in the epicardium of the heart ventricles. They were single nerve cells or gathered into small clusters. The number of small nerve cell clusters in the whole heart area was 117 ± 21. The size of neurons in small cell clusters was slightly smaller (11–17 μm) than those in the large ganglia.
Longitudinal-section of sinoatrial ganglion in the heart of 10-week-old foetus pig (a–c) labelled for PGP (a) and VAChT (b). The large number of PGP-positive neurons contained immunoreactivity to VAChT. Merged images 1a and 1b (c). Nerve cell cluster located around the cardiac segment of the ascending part of the aorta (d–f). Some neurons contained immunoreactivity to DβH (d) but were VAChT-negative (e, arrows). In the small nerve body cluster (g–i) which was observed in the epicardium of left atria, in the vicinity of coronary arteries, single PGP-positive neurons (g) contained CGRP (h). Merged images 1g and 1h (i).
Total number of PGP-positive neurons counted in different areas of the heart and percentages of particular neuronal subpopulations containing substances investigated in cardiac PGP-positive neurons
| Ganglia | Total number of neurons | Percentage of particular neuronal subpopulations | ||
|---|---|---|---|---|
| PGP | PGP/VAChT | PGP/CGRP | PGP/DβH | |
| Sinoatrial | 262 ± 12 | 53,8% | 13,2% | 1,6% |
| Atrioventricular | 236 ± 13 | 51,7% | 13,9% | 1,2% |
| Smaller clusters of neurons | 117 ± 21 | 41,22% | Single | 9,42% |
Double immunohistochemical stainings revealed that the majority of the PGP-positive neurons contained immunoreactivity to VAChT (Figure 1a–c), CGRP (Figure 1g–i) and the single nerve cells were DβH-positive (Figure 1d–f). In the sinoatrial and atrioventricular ganglion in the heart of 10-week-old foetus pigs, a large number of PGP-positive neurons contained immunoreactivity to VAChT (53,8% and 51,7% respectively), some to CGRP (13,2% and 13,9% respectively) and a few were DβH-positive (1,6% and 1,2% respectively). Single neurons were DBH/VAChT-positive. In the smaller nerve body clusters, neurons mainly contained VAChT (41,22%), a few DβH (9,42%) and single nerve body cells were CGRP-positive (Figure 1g–i).
3.2. Nerve fibres
Immunohistochemical staining for PGP showed that many bundles of nerve fibres have been observed throughout all cardiac chambers of the porcine foetal heart (Table 3). The richest network of PGP-positive nerve fibres was observed in the base of the heart in the vicinity of the main cardiac blood vessel openings (Figure 2a). The nerve bundles were sometimes associated with single neurons or small clusters of nerve cells. Most of the nerve bundles were observed in the epicardium, with less in the myocardium and endocardium. Generally, many of them were found in the left and right atrial epicardium and were less numerous observed in the left and right ventricular epicardium. Many PGP-positive nerve fibres were distributed in the endocardium, in the interatrial septum and some in the interventricular septum. Thinner bundles or single nerve fibres were observed in the myocardium of the atria and ventricles (Figure 2g) of the heart.
The richest network of nerve fibres was observed in the base of the heart in the vicinity of the main cardiac blood vessel openings (a–f). The majority of PGP-positive nerve fibres (a) were DβH-positive (b). DβH-positive nerve fibres (d) were VAChT-negative (e). PGP-positive nerve fibres (g, arrow) were observed in right ventricle myocardium. Some of them contained immunoreactivity to CGRP (h). Merged images 2g and 2h (i).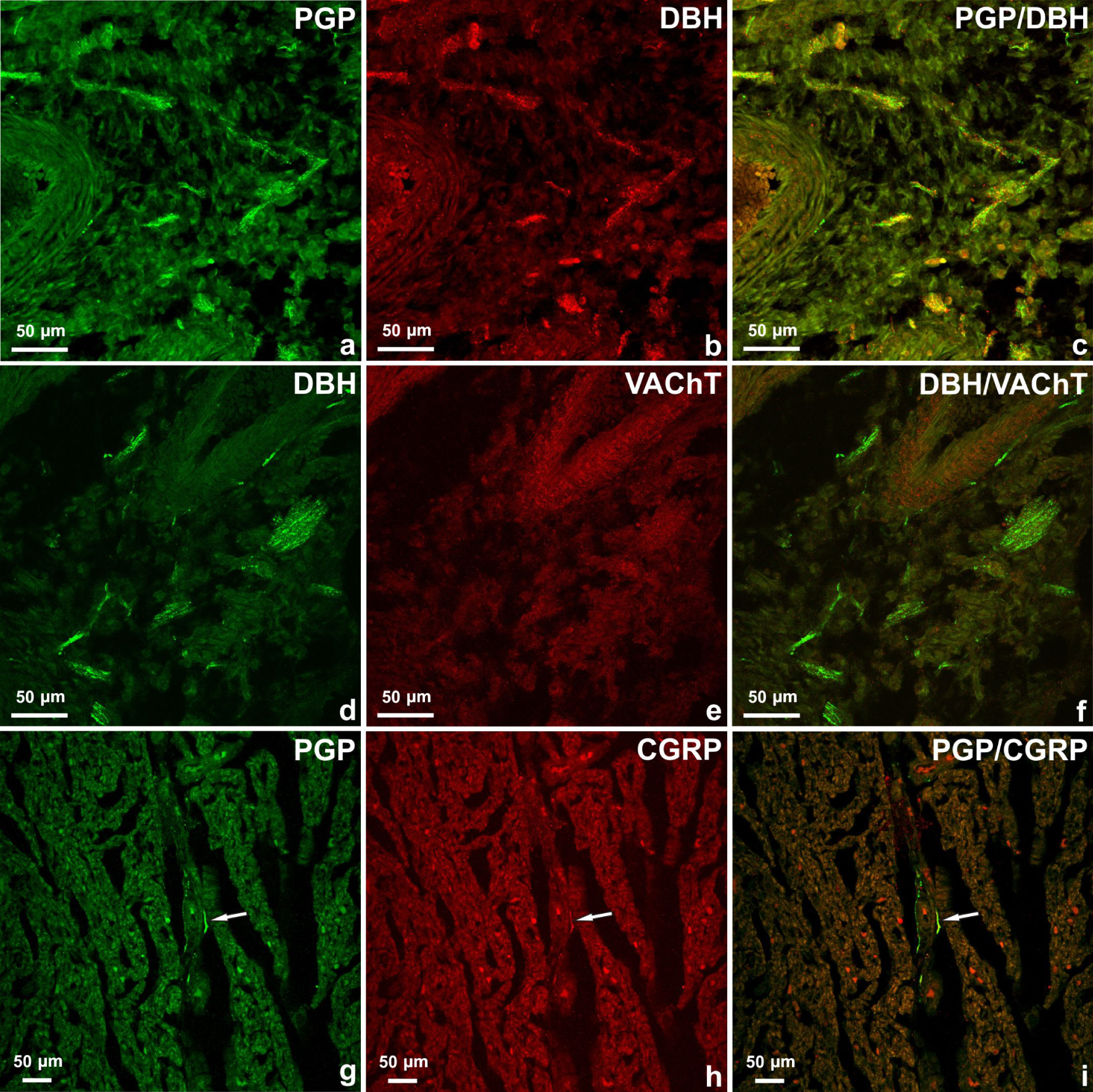
Relative frequency of PGP-IR, DβH-IR, VAChT-IR and CGRP-IR nerve fibres in the 10-week-old porcine foetus heart
| Nerve fibres | Right atrium | Right ventricle | Left atrium | Left ventricle | |
|---|---|---|---|---|---|
| Epicardium | PGP | + + + ++ | + + + + | + + + ++ | + + + + |
| PGP/DβH | + + + + | + ++ | + + + + | + ++ | |
| PGP/VAChT | + + | + | + + | + | |
| PGP/CGRP | + + | + | + + | + | |
| Myocardium | PGP | + ++ | + ++ | + ++ | + ++ |
| PGP/DβH | + + | + + | + + | + + | |
| PGP/VAChT | + | + | + | + | |
| PGP/CGRP | + + | + + | + + | + + | |
| Endocardium | PGP | + ++ | + ++ | + ++ | + ++ |
| PGP/DβH | + + | + + | + + | + + | |
| PGP/VAChT | + | + | + | + | |
| PGP/CGRP | + + | + + | + + | + + | |
Many fibres + + + ++, moderate number of fibres + + + +, low number of fibres + ++, few fibres + +, single fibres +.
The majority of epicardial, myocardial and endocardial nerve fibres were DβH-positive (Table 2), (Figure 2a–c). A low number of fibres were VAChT-positive. DBH/VAChT-positive nerves were not observed (Figure 2d–f). Few axons observed in the epicardium or myocardium contained immunoreactivity to CGRP and single nerves were found in the endocardium of four heart chambers (Figure 2g–i). There was no co-localization of CGRP with other studied substances in the nerve fibres supplying the foetus heart.
4. Discussion
As mentioned earlier, the development of the autonomic innervation of the heart is a multi-stage process [17, 18]. During the fifth week of human prenatal development, the NCC starts to migrate to the dorsal aorta and then differentiate into neurons. After this process, the neurons migrate to form a paravertebral sympathetic chain or parasympathetic cardiac ganglia and finally the axons of nerve body cells elongate and penetrate the cardiac tissue. This process continues until the first weeks after birth [17, 18, 28, 29]. According to the current study, in the heart of the 10-weeks-old porcine foetus, there are typical cardiac nerve structures. However, analysing the obtained results, it is clear that the process of maturation, migration and proliferation of the nervous system structures in the heart area has not yet been completed. This can be concluded by the number of neurons in the sino-atrial, atrio-ventricular ganglion, as well as by the number and distribution of smaller nerve cell clusters compared to juvenile pigs [1, 2, 13, 14]. Previous studies have shown that in the hearts of few- week-old piglets there were an average of 359 ± 178 neurons in the large ganglia [2] while in hearts of 10-week-old pig foetuses there were fewer (249 ± 10) nerve cell bodies observed. The number of small ganglia in piglets [2] were more numerous than in the heart of porcine foetus (361 ± 52 and 117 ± 21, respectively) and the most of them were observed in the left atrial and in the left and right ventricle area of the piglet hearts [2]. The small cardiac ganglia in the foetus were mainly observed on the base of the heart and in the vicinity of coronary arteries while the least were observed in the epicardium of the heart ventricles. There were single nerve cells or gathered into very small clusters. In comparing the results, the differences in the distribution of a small clusters of neurons in the hearts of piglets [2, 13, 14] and 10-week-old foetuses are visible, which strongly suggests that they are still in the process of migration. Moreover, it may seem that the innervation of the porcine heart matures more slowly than in humans. It has been observed that in 24 week-old human foetuses (roughly, proportionately similar pregnancy time in a pig) the distribution pattern of heart innervation has been observed in adults [16]. This may be associated with a more extensive cardiac intrinsic nervous system in the pig than in humans, which is a characteristic feature of the porcine heart but also makes a large difference in the morphology of the heart of both species and may today pose difficulties for the function of the heart donor by the transgenic pig [13, 14, 15].
Analysing the distribution of nerve fibres in the heart area of the porcine foetus and the piglet, differences in the distribution and quantity of fibres are also noticeable. As described by Crick et al. [14], a gradient of immunoreactivity for PGP was observed from dense plexus of nerve fibres in the epicardium and endocardium, to a lesser extent in the myocardial tissue. Considering the distribution of innervation in different areas of the piglet heart, the epicardial tissues displayed a ventricular-to-atrial gradient in density, the myocardial tissues displayed an atrial-to-ventricular gradient and, for the endocardial tissues, a distinct right to left chamber gradient was observed, with the right ventricle possessing the highest and the left atrium possessing the lowest density of nerves [14]. Generally, significantly fewer nerve fibres were observed in the endocardium and myocardium in the pig foetus. Nerve plexuses and fibres were mostly arranged in the epicardium of both atria and were much less observed in the ventricles area, which is a clear difference between the foetal heart and this organ in the animal after birth. It is most probable that in the studied stage of prenatal development, the axons are in the process of elongation and penetrate the cardiac tissue.
Referring to the immunohistochemical characterization of nerve structures in the foetal heart, it was observed that the occurrence of VAChT, DBH or CGRP in nerve fibres is also slightly different than in juvenile pig in the studied stage of development.
It is known that the ontogenesis of parasympathetic and intrinsic cardiac innervation precedes sympathetic innervation [14, 16, 17, 30] and cholinergic nerves were presented in 12 weeks of gestation and adrenergic nerves were not identified in this organ up to 18 weeks of gestation in the human foetus heart [16, 31, 32, 33] but there is no data in relation to the porcine foetus heart. Analysing the obtained results, it was observed that in the ten-week-old porcine foetus, the majority of cardiac neurons contain VAChT while the only few were DβH-positive compared to the piglet heart [14]. This observation may suggest a still-developing sympathetic part of the cardiac intrinsic nervous system. Another observation concerns the nerve fibres supplying the heart of the porcine foetus. Many of the fibres, especially those present in the epicardium and containing DBH, are mainly efferent post-ganglionic sympathetic nerves, while the intrinsic fibres are just forming. VACHT-positive nerve fibres were visible in the epicardium and single fibres were visible in the myocardium and endocardium. These nerves probably originated from the cardiac ganglia and were at the stage of penetration and elongation. CGRP-positive neurons and nerve fibres were the most abundant subpopulation in the piglet heart [14]. Most of these nerve structures originate from the intrinsic cardiac nervous system and simultaneously contain immunoreactivity to AChE [14]. CGRP in these nerve fibres has probably been involved in coronary vasodilatation [34] and may also play a role in the neutrally-mediated negative inotropic effect on myocardial function observed during cholinergic coronary vasoconstriction in the pig [14, 35]. Other CGRP-positive nerve fibres are sensory and originate from vagal or dorsal root ganglia neurons [14, 36]. In the ten-week-old porcine foetus, CGRP-positive neurons were mainly observed in large cardiac intrinsic ganglia (13.55%) and nerves were weakly visible in the entire heart area. These observations confirm the view that the autonomic innervation of the heart is still developing. In human CGRP/SP-positive nerve fibres (sensory) were not detected until 24 weeks of gestation [16]. It is known that sensory innervation appears later than autonomic innervation [13, 14, 16], which is also confirmed in the current study.
In summary, this study has described nerve structures of the heart and their immunohistochemical characterization in ten-week-old porcine foetuses. The distribution of nerve structures in ten-week-old foetuses represent a relatively high level of advancement, however, it is different from that observed in the heart of several-week-old pigs [2, 13, 14]. The neurochemical coding of the heart nerve structures at this stage of development is slightly different to that observed in the hearts of several-week-old pigs [13] and it is associated with a still developing the cardiac nervous system. The present results will constitute the basis for further observations of the developing heart in the porcine foetus and it is worth highlighting these studies, which provide details on the development and functioning of the heart in a pig as a potential donor of this organ for humans.
Conflicts of interest
The authors have no conflict of interest to declare.
Acknowledgements
The authors would like to thank Anna Przyborowska and Aleksander Penkowski for their contributions in preparing this paper.
This publication was supported by RID (Project financially co-supported by Minister of Science and Higher Education in the range of the program entitled “Regional Initiative of Excellence” for the years 2019-2022). Project No. 010/RID/2018/19, amount of funding 12.000.000 PLN.



 CC-BY 4.0
CC-BY 4.0