1 Introduction
Heteroatom-based dendrimers occupy a relatively marginal place when compared to organic dendrimers [1, 2], but a rich diversity of structures is already available, particularly for silicon- and phosphorus-based dendrimers [3]. We described nine years ago the first method of synthesis of neutral phosphorus dendrimers, and since that time, we have developed the chemistry and the reactivity of such compounds [4]. These dendrimers possess interesting, and in some cases, unprecedented properties, which led to some potential applications in three main fields [5]: catalysis, materials science, and biology. In this paper, we will show in particular how the nanometric size is an important criteria to be considered, and how some properties and applications depend on the generation of the dendrimers.
2 Syntheses of phosphorus dendrimers
The first method of synthesis of phosphorus dendrimers that we have described is also the one we use in most cases. It consists in the repetition of two quantitative steps outlined in Fig. 1, i.e. the reaction of hydroxybenzaldehyde in basic conditions with a core having P–Cl functions, then the condensation of the aldehyde groups with a phosphorhydrazide [6, 7]. The repetition of both steps was carried out until the obtaining of the twelfth generation 1a-G12, which is the highest generation described up to now for dendrimers. This sequence of two reactions can be applied starting from various cores having P–Cl or aldehyde functions. The presence of P(S)Cl2 or aldehyde end-groups at each step allowed us to develop a rich diversity of reactions [8–15], using mainly nucleophilic substitutions of Cl with functionalized amines or phenols, and condensation reactions with functionalized hydrazines. A few of the functional groups grafted on the surface of the dendrimers are displayed in Fig. 2. We have also developed other methods of synthesis [16], in particular methods that afford functional groups available for further reactions within the structure of the dendrimer [17–23], or at the core [24]. The synthesis of dendrimers being time consuming, we also developed improved methods of synthesis: in a first attempt we synthesized each generation in one step [25] (instead of two steps by classical ways); recently we proposed a new improvement for multiplying rapidly the number of functional groups by using AB5 and CD5 monomers (Fig. 3) [26]. These methods give layered dendrimers, i.e. dendrimers having two types of repetitive units. A comparison between the number of end-groups at each step, for the methods shown in Fig. 1 (AB2 + CD type) and the method shown in Fig. 3 (AB5 + CD5 type), starting in both cases from the hexaaldehyde 2b-G0, shows an extraordinary increase: 24 end-groups after three steps in the first case, 750 in the second case (Fig. 4). We have also synthesized hyperbranched polymers in only one step by polycondensation of an AB2 type monomer (Fig. 5) [27]. These polymers can be an interesting alternative to dendrimers, since their degree of branching is relatively high (0.83–0.85) even if their polydispersity is higher (1.16–2.51) than that of dendrimers made of the same repeat unit (1.008 for generation 3).
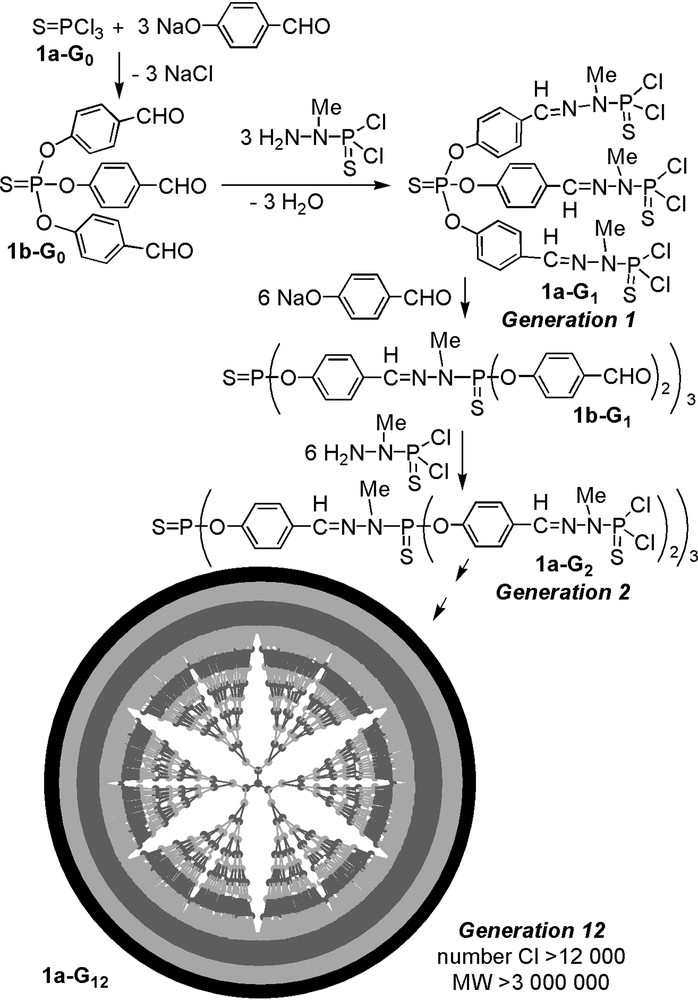
First method of synthesis of neutral phosphorus dendrimers.
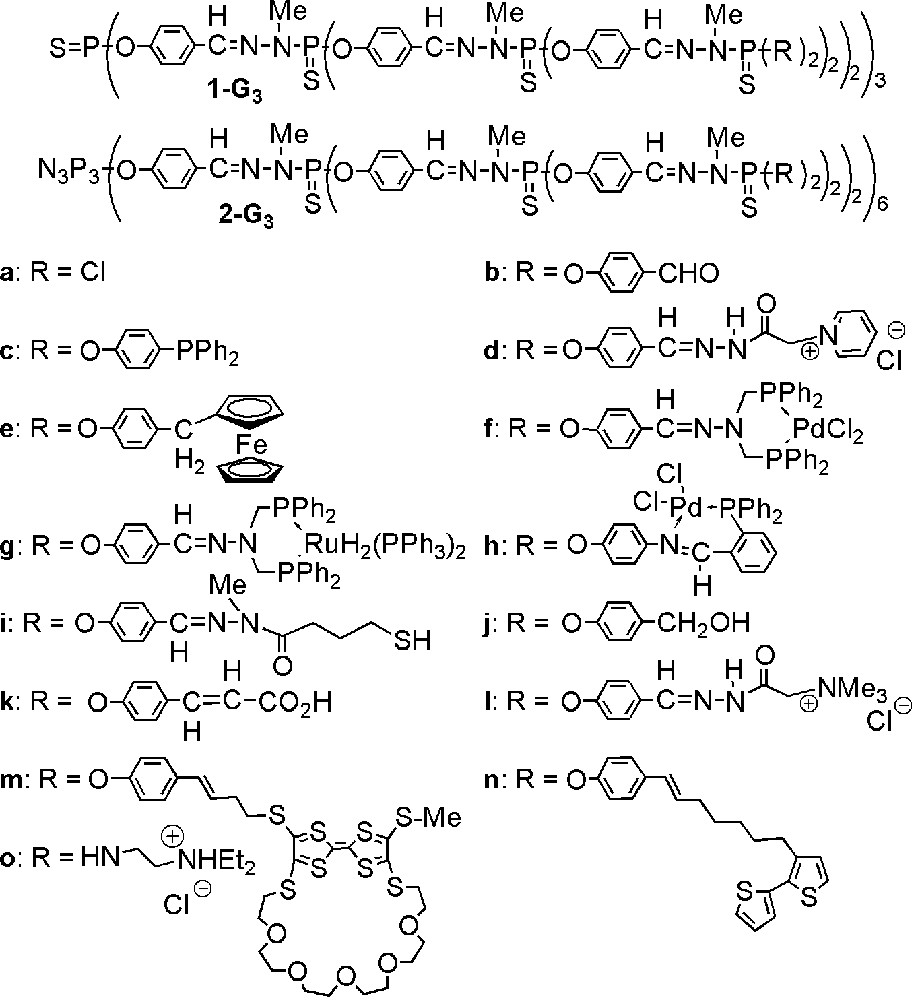
Some end-groups grafted on the surface of phosphorus dendrimers built from two types of cores.

Use of AB5 and CD5 monomers for the rapid synthesis of phosphorus dendrimers.
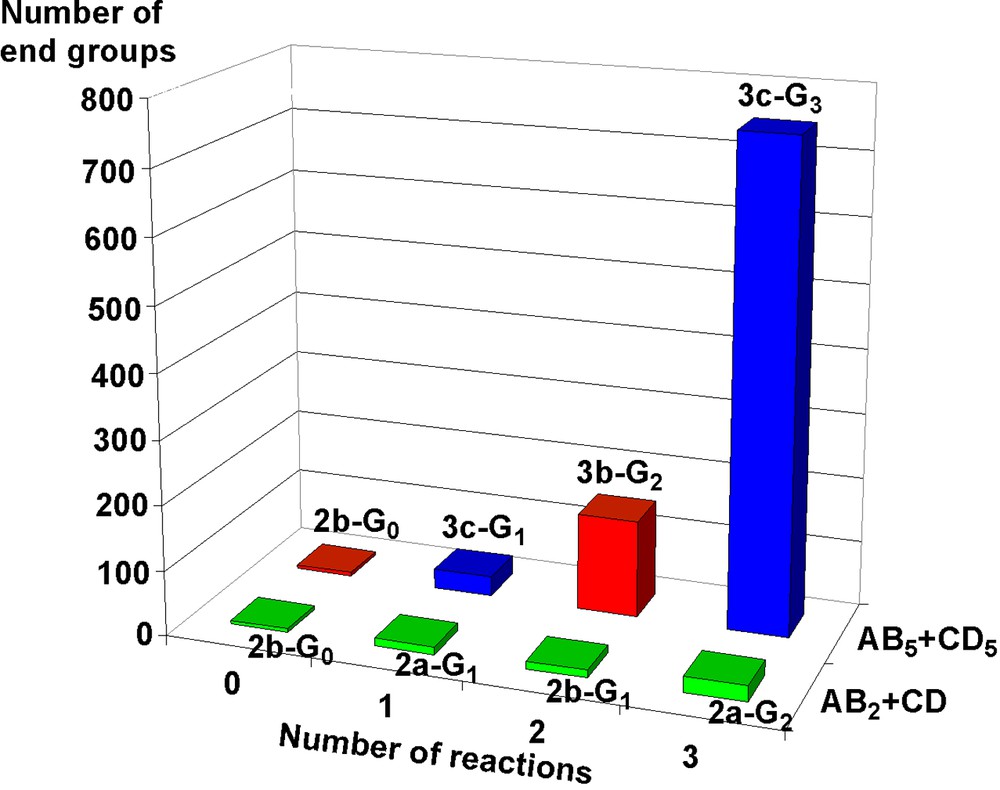
Comparison of the number of end-groups at each step for the methods of synthesis shown in Fig. 1 (AB2 + CD) and Fig. 3 (AB5 + CD5).

One-step synthesis of a hyperbranched polymer.
The various methods of synthesis and reactivity at the surface, within the branches or at the core that we have described allowed us to obtain compounds having the diversified topologies illustrated in Fig. 6.
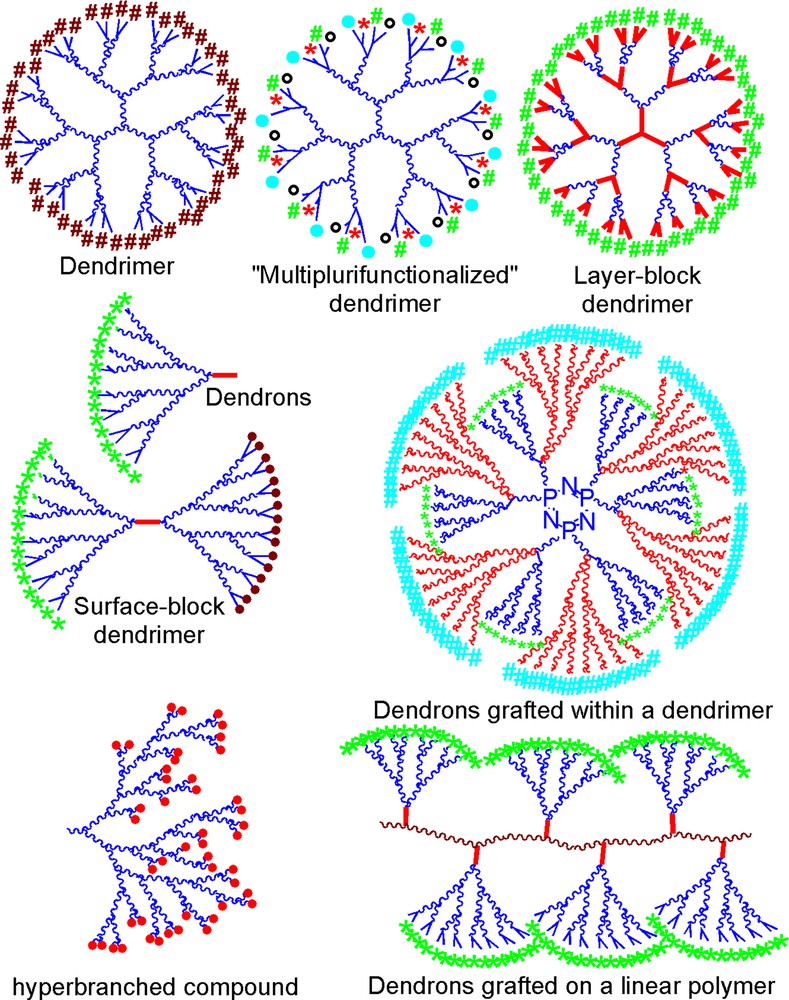
Schematic drawing of the various dendrimeric structures having phosphorus at each branching point.
3 Properties of phosphorus dendrimers
One of the most important properties to be studied in view of using a compound for applicative purposes concerns its thermal stability. We have carried out thermogravimetric analyses for generations 1 to 6 of dendrimers having aldehyde end-groups (3 to 96 end-groups). There is practically no influence of the generation on the thermal stability, as shown in Fig. 7. On the contrary, we have shown a dramatic influence of the type of end-groups on the stability, which varies from 225 °C for pyridinium end-groups (1d-G5), to 376 °C for ferrocene end-groups (1e-G5) [28]. This last value indicates that the internal structure is stable at least up to this temperature.

Thermogravimetric analysis of dendrimers 1b-G1–1b-G6.
We also studied the influence of the generation on various physicochemical properties, in particular on the dipole moment values. Indeed, compounds possessing a P=S group in their structure have generally a relatively important dipole moment value ranging from 2 to 5 D. The presence of such groups in our dendrimers incited us to measure this value versus generation for dendrimers having P(S)Cl2 (1a-Gn) or aldehyde (1b-Gn) end-groups, from generation 1 to generation 11. An exponential increase of the dipole moment value is observed (Fig. 8) [29]. Such behaviour could appear surprising in view of the spherical structure of these dendrimers, particularly for high generations. In fact, there is an enormous compensation of the elemental vectors that compose the global dipole moment value; this compensation is as high as 99% for the eleventh generation.

Variation of the dipole moment value versus generation for dendrimers 1a-Gn and 1b-Gn (n = 1–11).
Physical studies were also carried out with dendrimers having aldehyde end-groups, using in particular Temperature Modulated Calorimetry and Standard Differential Scanning Calorimetry. Both techniques show the metastability of the structure of these dendrimers. The molecular mobility in the solid phase gives rise to a physical aging that decreases upon increasing generations (from generation 1 to 3), and the higher generations (generations 3 to 5) have a higher rigidity [30, 31].
Such behaviour is coherent with experiments carried out in solution for studying the effect of the ‘burial’ of particular functions inside dendrimers. For instance, electrochemical studies were carried out for dendrimers having ferrocene functions linked to the surface, then progressively ‘buried’, such as for 4-G3 and 4-G3+2 (Fig. 9) or 4-G9 and 4-G9+2 [32]. Such study showed that the ferrocenes linked to the surface of the high generation are less available, as indicated by the percentage of electrolysis, which is 99% for 4-G3 and 85% for 4-G9. Furthermore, ferrocenes linked inside dendrimer 4-G9+2 are less accessible than inside 4-G3+2 (50% electrolysis versus 81%), showing a higher rigidity of the high generations.
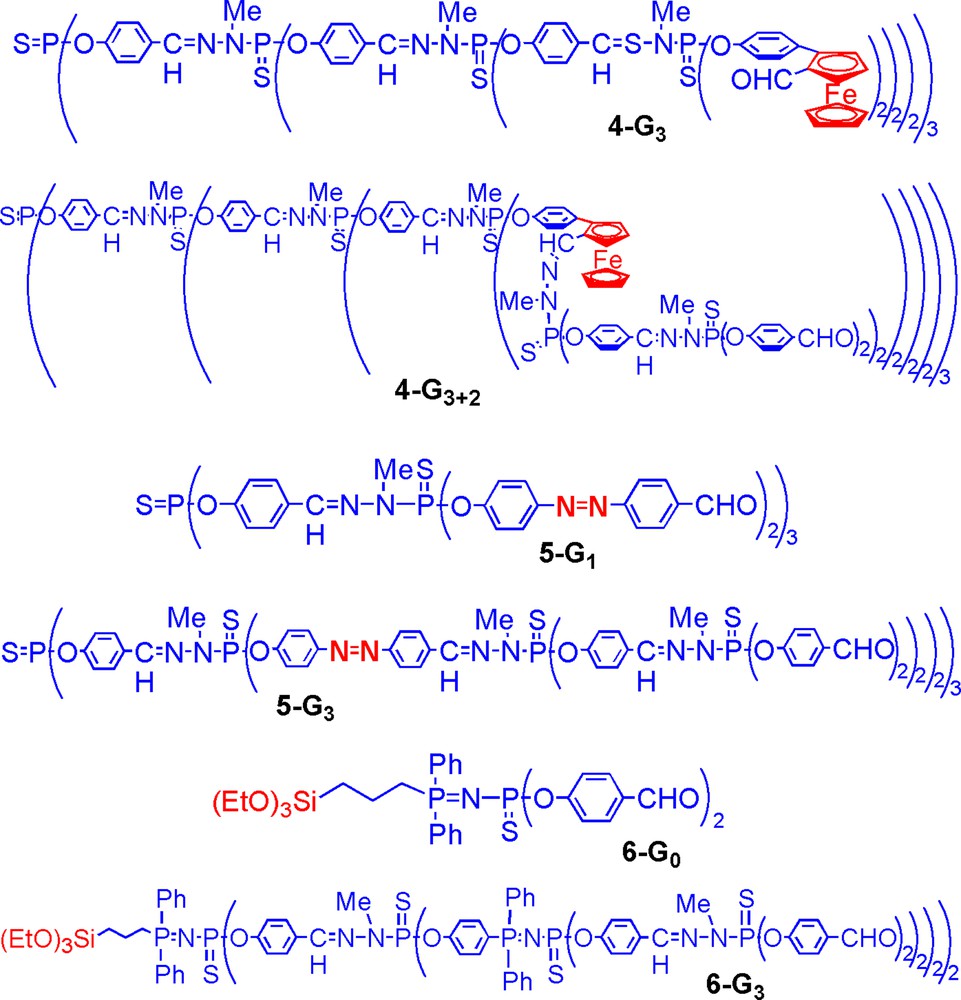
Various types of phosphorus dendrimers having functional groups within the structure or at the core.
A similar behaviour is observed when azobenzene groups are precisely placed inside dendrimers. A progressive burying of these functions induces a progressive reluctance of the –N=N– bond to isomerise upon irradiation (E → Z), as shown by the percentage of Z isomer at the photostationary equilibrium, that is 63% for 5-G1 and 42% for 5-G3 [33].
4 Uses and applications of phosphorus dendrimers
We develop applications in many fields, ranging from materials to biology, and including many aspects of chemistry. One of the first applications we studied is catalysis. Indeed, the possibility to recover and reuse the dendrimeric catalyst, due to its large size, can be interesting particularly if expensive metals or ligands have to be used [34]. We have carried out catalytic experiments with the dendrimeric complexes of palladium 1f-G3 and 2h-G1 for Stille coupling reactions, and with the dendrimeric complexes of ruthenium 1g-Gn (n = 1, 3) for Knoevenagel condensations and Michael additions [35, 36]. In all the cases tested, the third generation of the dendrimeric catalyst can be recovered and reused at least two times with only a slight decrease of the catalytic efficiency, whereas analogous monomeric catalysts cannot be recovered. Furthermore, we have shown in some cases an increased efficiency of the catalysis when the size of the catalyst increases, at least up to generation 3, when compared to the corresponding monomeric catalyst, as shown in Fig. 10 for the Knoevenagel condensation of malonitrile with cyclohexanone [35].
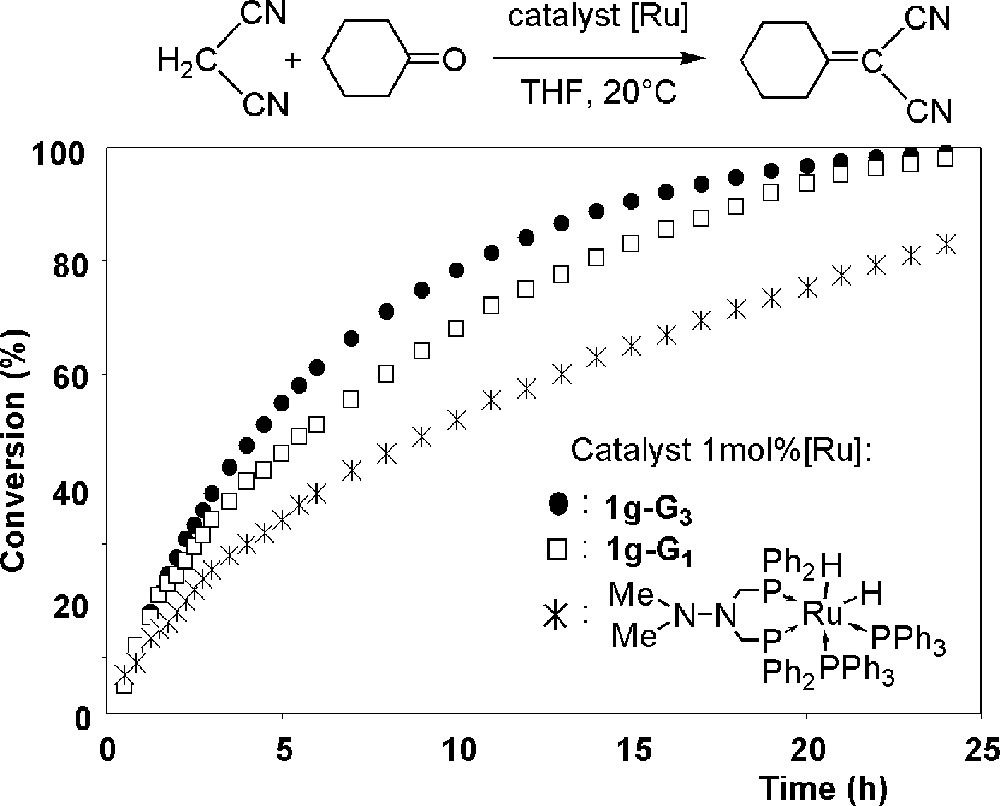
Conversion versus time monitored by 1H NMR for the Knoevenagel condensation of malonitrile with cyclohexanone catalysed by various ruthenium derivatives.
In the field of materials science, various examples show the tremendous importance of the size of the dendrimers on the properties. For instance, dendrimers 1i-G2 and 2i-G4 having thiol end-groups are able to deprotect the gold clusters Au55(PPh3)12Cl6, and to induce for the first time the formation of crystals of naked Au55 gold clusters [37], potentially useful in nanoelectronics. The size and the shape of the crystals of Au55 are highly dependent of the size and the density of thiol end-groups of the dendrimer, as shown in Fig. 11.

Crystals of naked Au55 obtained from Au55(PPh3)12Cl6 and 1i–G2 (left) or 2i–G4 (right).
Most of the uses of dendrimers in materials science pertain to two categories: either the dendrimers are incorporated inside the materials during their creation, or the dendrimers are used to modify the surface of an existing material. Phosphorus dendrimers were found useful in both fields. Indeed, hybrid materials were obtained by connecting first generation dendrimers having alcohol (1j-G1) or acid (1k-G1) end-groups with the titanium oxo cluster Ti16O16(OEt)32. New mesostructured hybrid materials were obtained, in which the dendrimers act like spacers in the arrays of clusters [38]. Dendrons 6-G0–6-G3, built from a triethoxysilyl core (Fig. 9) were also used to create new functionalized silica by reaction with tetraethoxysilane (TEOS) and water. The possibility to obtain silica is highly dependant on the amount of TEOS and on the generation of the dendron: 10 equiv of TEOS are needed to observe the gelation using dendron 6-G0, whereas 140 equiv of TEOS are needed for dendron 6-G3 [39]. The dendrons are covalently linked within the silica through their core; the end-groups of the dendrons should be available for further uses since these silica are mesoporous in several cases, with a narrow pore size distribution (around 50 Å).
Another type of gel was obtained using the water-soluble dendrimers 1d-Gn and 1l-Gn (n = 1–4). They are able to form hydrogels when heated for a few days at 65 °C in water at low concentrations (around 1% in weight), whereas the analogous monomeric compounds never gave a gel. These hydrogels are translucent and rigid, they do not flow, and they can even be crushed into pieces [40]. Freeze fracture electron microscopy indicates the presence of small chains made of dendrimers associated by supramolecular interactions. At the lowest concentration of 1d-G4, each molecule of dendrimer is able to gel 500 000 molecules of water! These hydrogels are able to encapsulate various types of water-soluble substances during the gelation process (Fig. 12), thus they are potentially useful for the controlled release of active substances. Other types of supramolecular assemblies of water-soluble phosphorus dendrimers afforded bilayered vesicles [41].
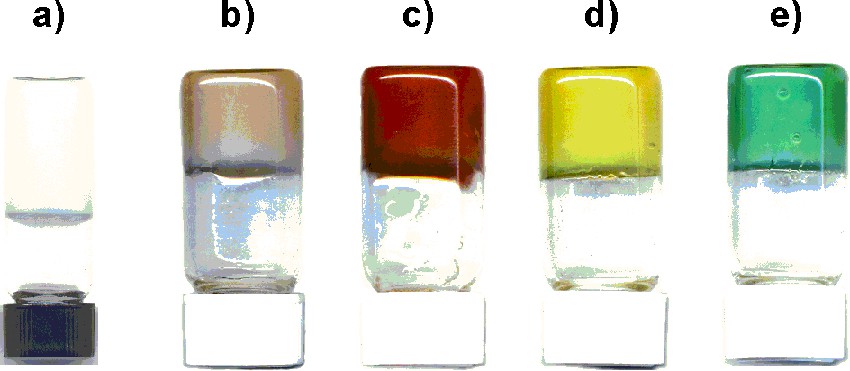
Gels in reversed flasks obtained from 1.5% of dendrimers 1d-G4 or 1l-G4 in water (a) alone, (b) with Er(OAc)3, (c) with ascorbic acid, (d) with (HOCH2)3CNH2, (e) with Ni(OAc)2.
The modification of surfaces can be carried out by chemical or electrochemical processes. Dendrimers having electroactive end-groups such as ferrocenes 1e-Gn [42], TTF derivatives 1m-Gn [43], or bithiophene derivatives 1n-Gn [44] (Fig. 2) deposit onto the electrode during the oxidation step as a conducting film. The deposit is reversible for 1e-Gn and 1m-Gn during the reduction step, whereas it is irreversible for 1n-Gn due to the polymerization of the bithiophene end-groups. It was shown that increasing the generation number from 1n-G0 to 1n-G4 increases the length of the polybithiophene segments, thus enhancing the effective conjugation. The modified electrodes can be used as electrochemical sensors, as shown for instance by the ability of electrodes reversibly modified by 1m-Gn to detect and quantify the presence of barium [43].
Quartz or glass surfaces modified by aminopropyl triethoxysilane react with dendrimers 1b-Gn or 2b-Gn to afford surfaces equipped with aldehyde groups (Fig. 13), whose wettability is lowered compared to the bare quartz plate. These modified surfaces are suitable for the immobilization of proteins [45]. These first researches recently induced a new development in the field of biology, for the creation of biochips. Indeed, dendrimers are strongly bound to a surface by several of their end-groups, but a lot of other end-groups remain available for further reactions, such as the grafting of oligonucleotides; the dendrimers can be considered as linkers which move away the oligonucleotides from the solid surface. When complementary oligonucleotides having a fluorescent label are used, the hybridization is detectable by fluorescence with a high sensitivity (up to 1 pm). Due to the strong bonding of the dendrimers to the solid surface, deshybridization processes can be applied to the biochip to reuse it (Fig. 14). Ten hybridization/deshybridization sequences were carried out, without any significant loss of the sensitivity of the biochip. Various sizes of dendrimers were used; the best compromise between efficiency and the time necessary for synthesizing the dendrimer was for generations 3 and 4 [46].

AFM image of a quartz surface modified by dendrimers 1b-G5.

Principle of preparation of biochips using dendrimers.
The size of the dendrimer is also an important criterion in other parts of biology, for instance for transfection experiments. Indeed, generations 1 to 5 of dendrimers having tertiary ammonium end-groups 2o-Gn were tested for transfecting the luciferase plasmid into mammalian cells. A dramatic increase of the efficiency is observed from generation 1 to 3, then a plateau is reached for generations 3 to 5 (Fig. 15) [47]. It is interesting to note in these cases that the transfection efficiency of these dendrimers is comparable with that of PolyEthyleneImine, one of the chemical standards used in transfection experiments. Very recently, a study concerning the potential use of phosphorus dendrimers as anti-prion agents also pointed out the importance of the generation on the property, the best results being obtained with the fourth generation [48].

Efficiency of various generations of dendrimers 2o-Gn (n = 1–5) for the transfection of plasmid pCMVLuc in NIH3T3 cells (murine fibroblast) in the presence of serum, with five or ten equivalents of ammoniums/phosphate. Comparison with polyethyleneimine.
5 Conclusion
We have shown that phosphorus dendrimers possess a lot of promising properties, usable for applications in various fields, from catalysis to materials sciences and biology. In most cases, the size of the dendrimer (the generation) is an important parameter, and the dendrimeric structure often offers dramatic improvements compared to monomeric compounds. Work is in progress to develop the chemistry and study the properties of these new macromolecules offered to the imagination of chemists.
Acknowledgements
The authors wish to express their gratitude to their past co-workers and students and to the numerous collaborative researchers whose names appear in the references.


