1 Introduction
Organotin halogeno-species have already been extensively studied by both vibrational spectroscopy and X-ray diffraction [1–4]. The crystal structure of (Me2SnCl, terpyridyl)+(Me2SnCl3)− has also been reported by Einstein and Penfold [5], while the dimethyltin trichloride anion with tetraethylammonium as counter cation has been reported by Debye et al. [6]. While studying the interactions between the tetrabutylammonium hydrogenosulphate and dimethyltin dichloride, Bu4NSnMe2Cl3 has been isolated. We report here the X-ray structure of Bu4NSnMe2Cl3 to examine the influence of the cation.
2 Experimental
2.1 Synthesis
Ethanolic solutions containing Bu4NHSO4 (1.54 g; 9 mmol) and SnMe2Cl2 (0.99 g; 9 mmol) have been mixed and stirred at room temperature for more than 1 h.
After removing the precipitate, the filtrate was allowed to evaporate to give colourless crystals of the title compound. Analytical data: % found (% calculated): %C: 43.40 (43.50); %H: 8.37 (8.35); %N: 2.81 (2.79); %Cl: 21.40 (21.60); %Sn: 23.85 (23.90) [171° dec., yield 80%].
The reaction is:
Bu4NHSO4 + 2 SnMe2Cl2 → SnMe2ClHSO4 + Bu4NSnMe2Cl3.
The elemental analyses were performed by the ‘Service central d'analyses’, CNRS, Vernaison, France.
2.2 Infrared and Raman data (cm−1)
The IR and Raman spectra were obtained as described in [7a] and [7b], respectively.
569s (526w) νas SnC2; 515m (516vs) νs SnC2; 316m (320m) νs SnCl3; 240m (262w) νas SnCl3 (the values in parentheses are related to the Raman spectrum).
2.3 Mössbauer data (mm s−1)
The Mössbauer data are obtained as described in [8].
I.S. = 1.39; Q.S. = 3.56; Γ = 0.94.
2.4 NMR data [CDCl3; δ (ppm); J (Hz)]
The NMR spectra were recorded in chloroform at the ‘Centre régional des mesures physiques de l'Ouest’, Rennes, France.
- 1H: 1.02(t) (12H) –CH2–CH3; 1.36(q) (6H) SnMe2; 1.44(m) (8H) N–CH2; 1.65(m) (8H) CH2–CH2–CH2; 3.24(m) (8H) CH2–CH2–CH3; 2JSn–H = 84.5; 88.5
- 13C: 13.72(s) –CH2–CH3; 18.21(q) SnMe2; 19.79(s) N–CH2; 24.09(s) CH2–CH2–CH2; 59.06(s) CH2–CH2–CH3; 1JSnC: 712.10; 681.33
- 119Sn: −100.94.
2.5 Crystallography
X-ray-quality crystals were grown by slow evaporation of a methanol solution. A single crystal of approximate dimensions 0.68 × 0.60 × 0.36 mm was used for data collection. Experimental details relating to the crystal class, method of data collection and data manipulation are given in Table 1. Selected geometric data are given in Table 2.
Crystal data, data collection, solution and structure refinement for the title compound
| Empirical formula | C18H42Cl3NSn |
| Colour, habit | Colourless, irregular |
| Crystal size (mm) | 0.68 × 0.60 × 0.36 |
| Crystal system | Monoclinic |
| Space group | C2/c |
| Unit cell dimensions | a = 26.633(2) Å |
| b = 9.880(2) Å, β = 114.82(2)° | |
| c = 21.510(2) Å | |
| Volume | 5137.0(7) Å3 |
| Z | 8 |
| Formula weight | 497.6 |
| Density (calc.) | 1.287 Mg/m3 |
| Absorption coefficient | 1.3087 mm−1 |
| F(000) | 2064 |
| Diffractometer used | Siemens P4/PC |
| Radiation | Mo Kα (λ = 0.71073 Å) |
| Temperature (K) | 293 |
| Monochromator | Highly oriented graphite crystal |
| 2θ Range | 3.0°–55.0° |
| Scan type | ω/2θ |
| Scan speed | Variable; 4.00°–511.00° in ω |
| Scan width (ω) | 0.78° |
| Background measurement | Stationary crystal and stationary counter at beginning and end of scan, each for 25.0% of total scan time |
| Standard reflections | 3 Measured every 97 reflections |
| Index ranges | 0 < h < 34, 0 < k < 12, −27 < l < 25 |
| Reflections collected | 6026 |
| Independent reflections | 5900 (Rint = 2.69%) |
| Observed reflections | 3330 [F > 4.0σ(F)] |
| Absorption correction | Semi-empirical [9] |
| Min./max. transmission | 0.2324/0.2982 |
| System used | Siemens SHELXTL PLUS (PC version) [10] |
| Solution | Direct methods |
| Refinement method | Full-matrix least-squares |
| Quantity minimized | ∑w(F0 − Fc)2 |
| Absolute structure | N/A |
| Extinction correction | X = 0.00003(2), where F∗ = F[1 + 0.0008F2/sin(2θ)]−1/4 |
| Hydrogen atoms | Riding model, fixed isotopic U |
| Weighting scheme | w−1 = σ2(F) + 0.0008F2 |
| Number of parameters refined | 209 |
| Final R indices (obs. data) | R = 5.37%, wR = 6.42% |
| R Indices (all data) | R = 9.62%, wR = 7.68% |
| Goodness-of-fit | 1.27 |
| Largest difference peak | 0.93 eÅ−3 |
| Largest difference hole | −0.68 eÅ−3 |
| Deposit number (CCDC) | 140722 |
Selected bond lengths [Å] and angles [°]
| Sn(1)–Cl(1) | 2.563(2) | Sn(1)–Cl(2) | 2.560(2) |
| Sn(1)–Cl(3) | 2.387(3) | Sn(1)–C(1′) | 2.104(10) |
| Sn(1)–C(2′) | 2.110(11) | N(1)–C(1) | 1.520(10) |
| N(1)–C(5) | 1.523(9) | N(1)–C(9) | 1.528(9) |
| N(1)–C(13) | 1.506(7) | C(1)–C(2) | 1.514(9) |
| C(2)–C(3) | 1.509(12) | C(3)–C(4) | 1.496(12) |
| C(5)–C(6) | 1.498(9) | C(6)–C(7) | 1.521(14) |
| C(7)–C(8) | 1.479(13) | C(9)–C(10) | 1.508(11) |
| C(10)–C(11) | 1.540(14) | C(11)–C(12) | 1.496(16) |
| C(14)–C(13) | 1.507(11) | C(14)–C(15) | 1.518(11) |
| C(15)–C(16) | 1.515(13) | ||
| Cl(1)–Sn(1)–Cl(2) | 178.0(1) | Cl(1)–Sn(1)–Cl(3) | 88.9(1) |
| Cl(2)–Sn(1)–Cl(3) | 92.1(1) | Cl(1)–Sn(1)–C(1′) | 91.5(3) |
| Cl(2)–Sn(1)–C(1′) | 89.9(3) | Cl(3)–Sn(1)–C(1′) | 106.1(4) |
| Cl(1)–Sn(1)–C(2′) | 89.3(2) | Cl(2)–Sn(1)–C(2′) | 88.7(2) |
| Cl(3)–Sn(1)–C(2′) | 110.2(3) | C(1′)–Sn(1)–C(2′) | 143.7(5) |
| C(1)–N(1)–C(5) | 111.1(5) | C(1)–N(1)–C(9) | 105.9(6) |
| C(5)–N(1)–C(9) | 110.3(5) | C(1)–N(1)–C(13) | 111.0(4) |
| C(5)–N(1)–C(13) | 107.1(6) | C(9)–N(1)–C(13) | 111.5(5) |
The asymmetric unit used in text and tables is shown in Fig. 1.
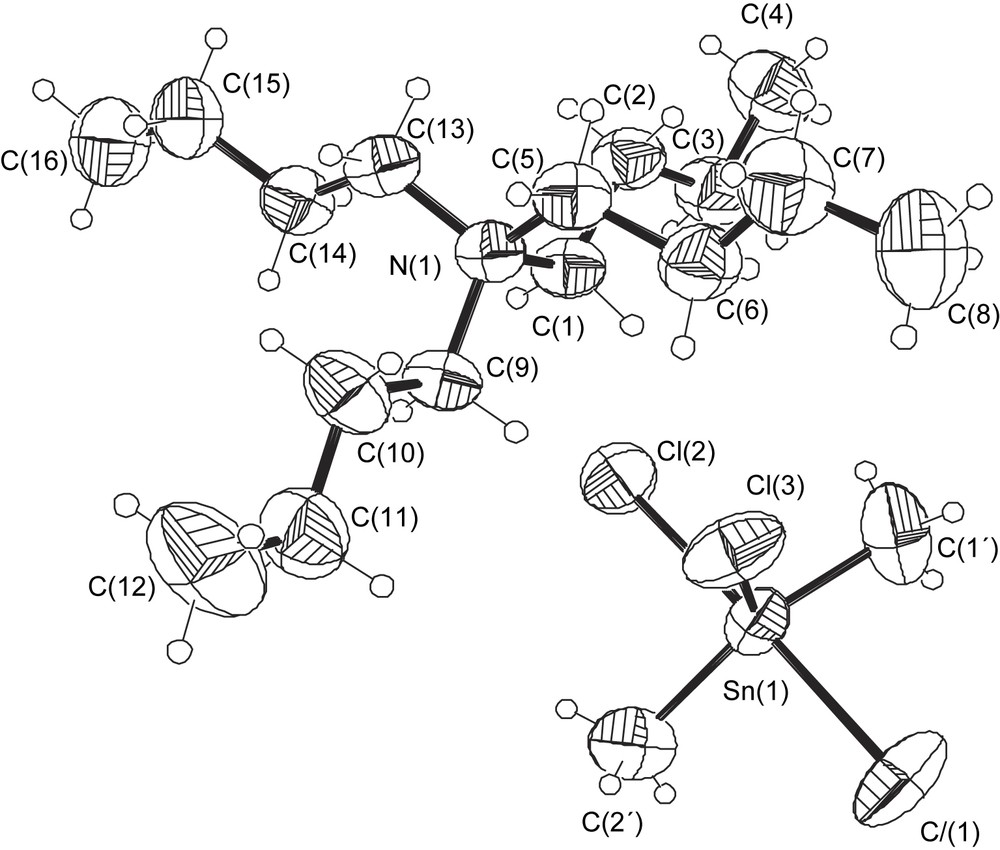
ORTEP drawing of the title compound with the atomic numbering scheme.
3 Results and discussion
The presence of a band at 515 cm−1 due to νs SnC2 is indicative of a non-linear SnC2 group [11]. The Q.S. value [Q.S. = 3.56 mm s−1] is in the range of pentacoordinated tin centers [12].
The 119Sn NMR exhibits one resonance at −100.94 ppm (in SnMe2Cl2 δ 119Sn = 141 ppm [13], the values of the coupling constants are 1JSnC = 712.1; 681.3 Hz and 2JSnH = 84.5; 88.5 Hz). The 2J value larger than 83 Hz is indicative of an SnC2 angle above 135° [14].
Fig. 1 shows an ORTEP drawing of the asymmetric unit with the labelling scheme of the structure; Fig. 2 shows the packing arrangement in the crystal. It consists of an assemblage of Bu4N+ and SnMe2Cl3− entities, in which the tin atom adopts a trigonal bipyramidal environment with Cl(1) and Cl(2) in the axial positions and Cl(3), C(1′) and C(2′) in the equatorial positions. The axial SnCl bonds are almost collinear [Cl(1)–Sn–Cl(2) = 178.0°]. The only important angular distortions from a regular trigonal bipyramidal configuration are within the equatorial plane. The angle between the methyl groups is 143.7°, while the two Me–Sn–Cl angles are 106.1° and 110.2° (the sum of the angles around the tin atom in the equatorial plane is 360°).
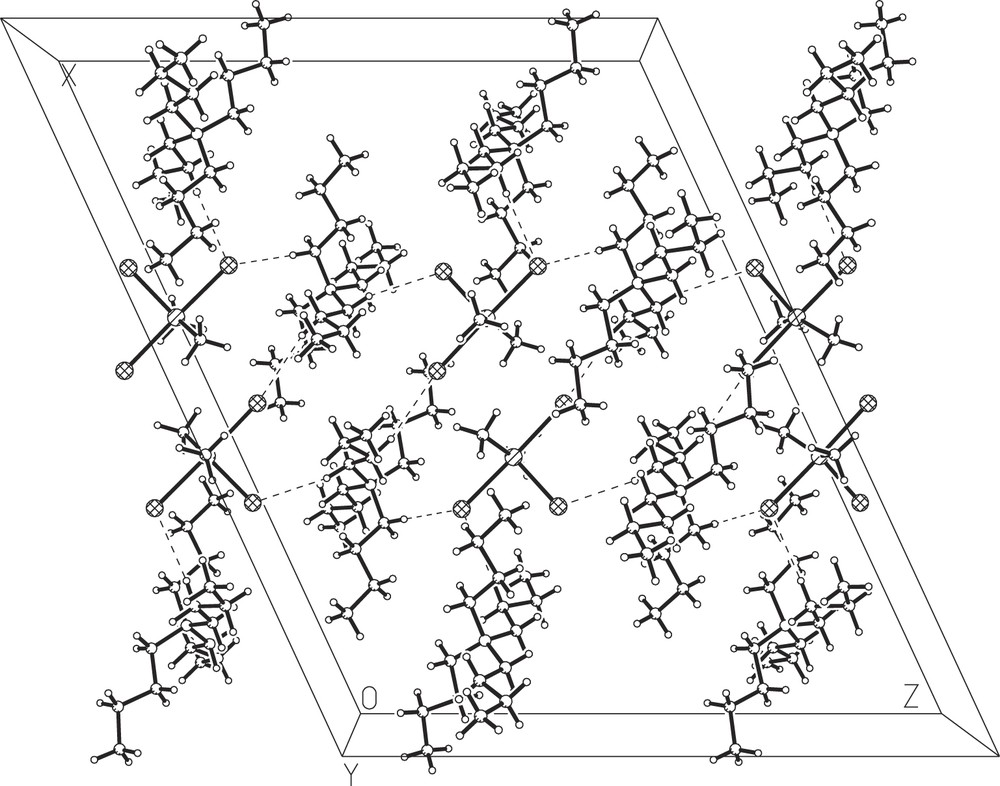
The packing arrangement in the crystal.
These results are in good agreement with those obtained in (Me2SnCl, terpyridyl)+(Me2SnCl3)− [5]. In the latter case, the three equatorial planar angles are 140°, 107° and 113°, while the two axial Sn–Cl bonds are perfectly colinear [Cl–Sn–Cl = 180°].
As it has been observed in Me2SnCl3−-containing compounds, the axial Sn–Cl bonds [2.53(2) Å] are significantly greater than the equatorial one [2.38(3) Å]; in (Me2SnCl, terpyridyl)+(Me2SnCl3)− the distances are 2.54 and 2.32 Å, respectively. The Sn–C distances in the two compounds are also very similar: 2.10 Å in the present case and 2.11 Å in (Me2SnCl, terpyridyl)+(Me2SnCl3)−.
In the cation, the N–C and C–C distances are in the expected ranges [N–C: 1.506(7)–1.528(9) Å; C–C: 1.479(13)–1.540(14) Å], while the C–N–C angles deviate slightly from those in a regular tetrahedron [range: 105.9(6)°–111.1(5)°].
4 Conclusion
The structure of the title compound is discrete; the tin center adopts a trigonal bipyramidal environment, as in the previously published compounds containing SnMe2Cl3− units: it appears that there is no cation effect on the environment of SnMe2Cl3−, the interactions being essentially electrostatic.
Acknowledgements
The Dakar group thanks the Third World Academy of Sciences (TWAS) (Trieste – Italy) for financial support (Grant Number 93318 RG/AF/AC).


