1 Introduction
Allium is the largest and the most important representative genus of the Liliaceae family that comprises 700 species, widely distributed in the northern hemisphere [1,2]. Besides the well-known garlic and onion, several other species are widely grown for culinary use, such as leek (Allium porrum L.), scallion (Allium fistulosum L.), shallot (Allium ascalonicum Hort.) and wild garlic (Allium ursinum L.)[3]. Allium has an important economical value for vegetable and ornamental uses in Europe, Asia and America [4].
For many centuries, several species from the Liliaceae family have been used as vegetables and spices, and as folk medicines for curing various diseases [5]. The first citation of these plants is found in the Codex Ebers (1550 BC), an Egyptian medical papyrus reporting several therapeutic formulas based on garlic and onion as useful remedies for a variety of diseases such as heart problems, headache, bites, worms and tumours [3]. Scientific research on these plants started in the second half of the 19th century with Pasteur (1858), who evidenced the antibacterial properties of garlic.
Several Allium species such as Allium sativum and Allium cepa have been shown in previous studies to exhibit various activities. For example, a wide array of therapeutic effects of garlic has attracted particular attention of modern medicines because of its widespread use as antiatherosclerotic, antidiabetic, antihypertensive, antimicrobial, anticancerous, antioxidant, antifungal and antiviral [6–10].
Allium species, namely, onion, garlic, leek, and chive contain a variety of secondary sulphur compounds [11]. Sulphur-carrying flavour compounds are responsible for the characteristic smell and taste, the source of major active compounds which are the best known properties in Allium plants [1,5–7]. The antibacterial bioactive principal of garlic was identified in 1944 by Cavallito as diallylthiosulfinate and was given the trivial name allicin [12].
As a very polymorphous species, Allium roseum is represented in North Africa by 12 different taxa: 4 varieties, 4 subvarieties and 4 forms [13,14]. In Tunisia, the same authors mentioned the presence of only three varieties: var. grandiflorum, var. perrotii and var. odoratissimum. Considered as an endemic taxon in North Africa [13], the odoratissimum variety is a perennial spontaneous weed [15]. Its oblong bulb grows about 30–60 cm tall [16] and its flowers are wide, rosy or white coloured and with eyelet odor [17].
A. roseum var. odoratissimum prefers poor and sandy soils. It was also found in grassy and bushy places, cultivated fields and fallows, and roadsides. It was used since ancient times as a vegetable, spice or herbal remedy [14]. According to the same author, A. roseum is used to treat head and rheumatisms.
With the increase of bacterial resistance to antibiotics, there is considerable interest to investigate the antimicrobial effects of different extracts against a range of bacteria, to develop other classes of antimicrobials useful for the infection control or for the preservation of food [9].
As far as this literature review could ascertain, few Allium species had been taken into account, except for A. sativum (garlic) and A. cepa (onion), for the assessment of their possible biological activities [18,19].
Since no information is available on the antibacterial activities of the A. roseum var. odoratissimum, in the present study the chemical composition of the essential oil and its antibacterial activity were investigated for the first time. The antibacterial effects of the aqueous and the organic extracts and the total oligomer flavonoids (TOF) of this species were also studied.
2 Materials and methods
2.1 Plant material
A. roseum var. odoratissimum (Desf.) Coss (syn. A. roseum) was collected from the south-east of Tunisia (Bengardane, latitude 33° 86′ 46″ N, longitude 10° 52′ 48″ E, with an arid climate characterized by a mean rainfall of 150 mm/year) at two periods in 2006, in relation to both different stages of the plant growing cycle: the vegetative stage (January) and the flowering stage (February). Botanical identification was carried out in the Range Ecology Laboratory of the “Institut des régions arides”, Tunisia (I.R.A.), according to the “Flora of Tunisia” [13]. Voucher specimens were deposited at the herbarium of the I.R.A. Aerial parts of A. roseum var. odoratissimum samples were air-dried (temperature: 25 °C, humidity: 60%) for 15 days and then used for antibacterial assay. The fresh flowers were separated from the lignified part and were used for hydrodistillation.
2.2 Essential oil extraction
The fresh flowers (100 g) were subjected to hydrodistillation for approximately 4 h, in a Clevenger-type apparatus. The obtained essential oil was dried over anhydrous sodium sulphate and 0.2 μl was used for GC and GC–MS analyses.
2.3 Analysis of essential oil
2.3.1 Gas chromatography
Agilent 6890N Network GC system is equipped with: flame ionization detectors (FID), HP-Innowax (polyethyleneglycol, 30 m × 0.25 mm ID, 0.25 μm film thickness fused capillary column). The carrier gas was nitrogen (1.2 ml/min). The oven temperature was programmed at 50 °C for 1 min, then 7 °C/min to 250 °C for 5 min. The injection port temperature was 240 °C, detector heaters 250 °C. Percentages of the constituents were calculated by electronic integration of FID peak areas without response factor correction.
2.3.2 Gas chromatography and mass spectrometry
The volatile constituent's analyses were achieved on a Hewlett-Packard gas chromatograph GC: 5890 series II. The fused HP-Innowax capillary column (polyethyleneglycol, 30 m, 0.25 mm ID, 0.25 μm film thickness) was directly coupled to the mass spectrometer. The carrier gas was nitrogen, with a flow rate of 1.2 ml/min. The oven temperature was programmed from 50 °C (1 min) to 250 °C (5 min) at 7 °C/min. The temperature of the injector port was held at 250 °C, the temperature of the detector was set at 280 °C. The mass spectrometer was operating (full scan-mode) in the EI-mode at 70 eV.
2.3.3 Component identification
The compounds of the essential oil were identified by comparison of their mass spectra with those of a computer library (Wiley 275 library). Further confirmation was done by referring to retention index data generated from a series of known standards of alkanes (C9–C28) [20].
2.4 Preparation of the other extracts
2.4.1 Aqueous extract
The powdered aerial parts of A. roseum (20 g) were extracted with boiling water (250 ml) for 20 min. After this step, the decoction was filtered and then freeze-dried (aqueous extract)[21].
2.4.2 Organic extracts
Petroleum ether, chloroform, ethyl acetate and methanol extracts were obtained by Soxhlet extraction of 20 g of aerial parts for 6 h in about 250 ml of each solvent used separately. These four types of organic extracts, with different polarities, were concentrated to dryness and the residue was kept at 4 °C [21].
2.4.3 Total oligomer flavonoids (TOF)
In order to obtain an extract enriched with oligomeric flavonoids (TOF), 100 g of the powder from aerial parts of A. roseum were macerated in 500 ml acetone/water mixture (1:4 v/v), during 24 h with continuous stirring at room temperature. The extract was filtered and acetone was evaporated under low pressure in order to obtain the aqueous phase. The tannins were removed by precipitation with an excess of NaCl during 24 h at 5 °C, and the supernatant was recovered. The latter was extracted with ethyl acetate, concentrated, and finally precipitated with an excess of chloroform. The precipitate was separated by centrifugation at 10 000 rpm for 15 min [21].
2.5 Antibacterial activity
2.5.1 Bacterial strains
For the determination of antibacterial activity, standard and isolated strains of the following six bacteria, including the Gram positive bacteria, Staphylococcus aureus ATCC 25923, Staphylococcus epidermidis CIP 106510, Micrococcus luteus NCIMB 8166 and the Gram-negative bacteria Salmonella typhimurium NRRLB 4420, Escherichia coli ATCC 25922 and Pseudomonas aeruginosa ATCC 27853, were used. The microorganisms were storied on Mueller–Hinton Agar (Bio-Rad) at 4 °C. Microorganisms were obtained from the culture collection of the “Laboratoire d'analyses, traitement et valorisation des polluants de l'environnement et des produits”, Faculty of Pharmacy of Monastir, Tunisia.
The nutrient broth (Bio-Rad) and the Mueller–Hinton agar were used, respectively, for growing and diluting the microorganism suspensions for the antimicrobial assays.
2.5.2 Extract dissolution
The aqueous extract and the organic extracts as well as the TOF and the essential oil were dissolved, respectively, in water, DMSO (5%) and Tween 80, in order to have an initial concentration of 5 mg/ml.
2.5.3 Agar diffusion method
Antibacterial activities of different extracts from aerial part of the plant were assessed using the paper disk agar diffusion method according to Rios et al. [22] and Freney et al. [23]; inoculates grown in the nutrient broth at 37 °C for 24 h were diluted to approximately 2 × 106 CFU/ml in molten nutrient agar. The concentration of the suspension used for inoculation was standardised by adjusting the optical density to 0.5 at 570 nm wavelength (spectrophotometer UV/visible, Jenway 6405). Absorbent disks (Whatman disk No. 3 of 6-mm diameter) were impregnated with 20 μl of different extracts and then placed on the surface of inoculated plates (90 mm) and incubated at 37 °C for 24 h. Negative controls were prepared using a disk impregnated with the same solvent as that used to dissolve the plant extracts. Antimicrobial activity was assessed by measuring the inhibition zone. This was the diameter of the zone visibly showing the absence of growth, including the 6-mm disk. Where there was no inhibition, the value 0 mm was assigned to the tested sample. All the tests were performed in triplicate.
3 Results and discussion
3.1 Extraction yield of aerial part
The aerial part extraction with different solvents showed the highest yields for water, and then for methanol. The TOF fractions were present at low concentration (Table 1). The high yield of extraction in polar solvents exhibited rich polar constituents of the aerial part of the A. roseum. The essential oil yield obtained was 0.05% (v/w). The family of Alliums is widespread and well known for its flavours caused by sulphurous compounds. This property was recently studied by Jones et al. [1] who provided information by measuring sulphur compounds to suggest a biosynthetic pathway of the flavour precursors of Alliums.
Yield of extracts from aerial parts of Allium roseum var. odoratissimum collected from Bengardane, south-east of Tunisia (February, 2006)
| Extracted fractions | Yield (%) |
| Aqueous extract | 32.360 |
| Methanolic extract | 29.650 |
| Petroleum ether extract | 3.300 |
| Ethyl acetate extract | 3.100 |
| Chloroform extract | 0.650 |
| Essential oil | 0.050 |
| TOF extract | 0.028 |
3.2 Chemical composition of the essential oil
The essential oil isolated by hydrodistillation from the fresh flowers of the A. roseum var. odoratissimum had a light yellow colour and a pungent odour at room temperature. The global chromatographic analysis of this essential oil showed twenty compounds, representing 91.4% of the whole oil constituents (Table 2).
Composition in percentage of the essential oil from fresh flowers of Allium roseum (Bengardane, Tunisia)
| No | Compound | RIa | Percentage |
| 1 | 2,4-Dimethylthiophene | 1250 | 2.3 |
| 2 | 2-Propenyl methyl disulfide | 1215 | 1.0 |
| 3 | 1-Propenyl methyl disulfide | 1285 | 0.9 |
| 4 | Dimethyl trisulfide | 1382 | 2.3 |
| 5 | Tetradecane | 1400 | 9.6 |
| 6 | Decanal | 1467 | 0.6 |
| 7 | Methional | 1480 | 17.1 |
| 8 | Camphor | 1517 | 13.4 |
| 9 | Pulegone | 1662 | 2.9 |
| 10 | Methyl benzoate | 1632 | 7.2 |
| 11 | Heptacosane | 2700 | 1.3 |
| 12 | Methyl eugenol | 1985 | 1.4 |
| 13 | Eugenol | 2151 | 12.7 |
| 14 | Carvacrol | 2246 | 0.5 |
| 15 | Eugenol acetate | 2263 | 1.0 |
| 16 | Tricosane | 2300 | 11.1 |
| 17 | Octadecanal | 2329 | 3.9 |
| 18 | Benzyl benzoate | 2570 | 0.7 |
| 19 | Hexadecanoic acid | 2891 | 1.4 |
| Sulphurous compounds | 23.6 | ||
| Non-sulphurous compounds | |||
| Hydrocarbons | 22.0 | ||
| Phenolic derivatives | 14.6 | ||
| Aldehydes | 4.5 | ||
| Cyclic ketones | 16.4 | ||
| Aromatic esters | 8.9 | ||
| Fatty acid | 1.4 |
a Retention Index: calculated against C9–C28 n-alkanes on HP-Innowax column.
3.2.1 Organo sulphurous compounds
Table 2 shows a relatively high content of organic sulphurous compounds (about 23.6% of the total eluted compounds). The volatile compounds in the Allium species are mainly represented by thiosulfinates, which are very unstable compounds and give rise to further rearrangements leading to a wide variety of derived organo sulphurous compounds (Table 2). Their structure, biogenesis and variability are well studied [3].
While in garlic compounds, alliins (S-alk(en)yl-l-cysteine sulfoxide) are the major compounds (85%), in onion isoalliin (1-propenyl residue) is the main metabolite [7,24] and in A. roseum the dimethylated compounds are predominant (Table 2). The abundance of the compounds was also mentioned in A. sativum and A. fistulosum L. essential oil [25–27], where five compounds were mainly identified (2,4-dimethylthiophene, 2-propenyl methyl disulfide, 1-propenyl methyl disulfide and dimethyl trisulfide).
The essential oil composition of the studied plant has not been reported before and these results are first evidenced on the composition of this unique and endemic species. Methional presented the highest percentage (17.1%) among all the compounds of the essential oil of A. roseum var. odoratissimum (Fig. 1). This compound is going to be mentioned for the first time in the essential oil of Allium genus with a relatively high level and would characterize the essential oil of A. roseum var. odoratissimum. The identified compounds in our species can be classified as sulphur and non-sulphur compounds. It should be mentioned that, according to many other authors [6,7,24], the S-containing compounds are responsible for the appropriate smell and taste and also the health benefits of members of Liliaceae family. These characteristics indicate the important value of the studied species and its important use in traditional medicine.
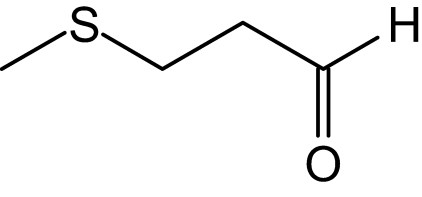
Chemical structure of methional.
It would be noteworthy to point out that the composition of any plant essential oil is influenced by the presence of several factors such as local climatic, seasonal and experimental conditions [28]. The thiosulfonates, which are very unstable, would further decompose to produce a series of degraded compounds including sulfides, according to the solvent used and the extraction conditions; such is the case of allicin (diallyl thiosulfinate). Under selective conditions, (crushing, cutting, extraction), the vacuolar enzyme alliinase, transforms “alliins” into the very unstable thiosulfonates, yielding 1-propenyl-containing sulfonates [24].
3.2.2 Phenolic and other compounds
The composition of the essential oil of A. roseum was characterized by the phenolic compounds, particularly alcohols, eugenol and its derivatives, representing 15% of the total compounds (Table 2). Besides, methyl benzoate was the second abundant compound present. Moreover, the essential oil of A. roseum was also rich in camphor (13.4%), having an antibacterial effect [28], and in pulegone, contributing to sensorial characteristics of this variety [29].
3.3 Antibacterial activity
The yield of A. roseum var. odoratissimum extracts presented in Table 1 shows the importance of the aqueous extract fraction followed by the methanolic extracts. However, the TOF extracts yielded the lowest value. The antibacterial activities of the different aerial extracts of A. roseum var. odoratissimum, i.e. aqueous, methanolic, ethyl acetate, petroleum ether, chloroform, TOF and the essential oil were determined against six bacterial strains, considered as among the common food-borne bacteria. The inhibition zone, measured in millimeters, including the diameter of the paper disk, was used as the criterion for measuring the antibacterial activity of A. roseum extracts and essential oil. Fig. 2 shows that the tested extracts could be classified according to their activity. The strongly active compounds included the essential oil with a mean antimicrobial growth inhibition zone of 10.0–12.0 mm against S. aureus, then S. epidermidis and M. luteus. The second most active extract against the same bacteria was that in chloroform. The third most active extract was that in petroleum ether with a growth inhibition zone of 10.0–15.0 mm against M. luteus, then S. aureus and finally S. typhimurium. The fourth most active extract was the ethyl acetate extract, which was strongly active against M. luteus (15 mm), then S. aureus and finally S. typhimurium (10.0 mm).
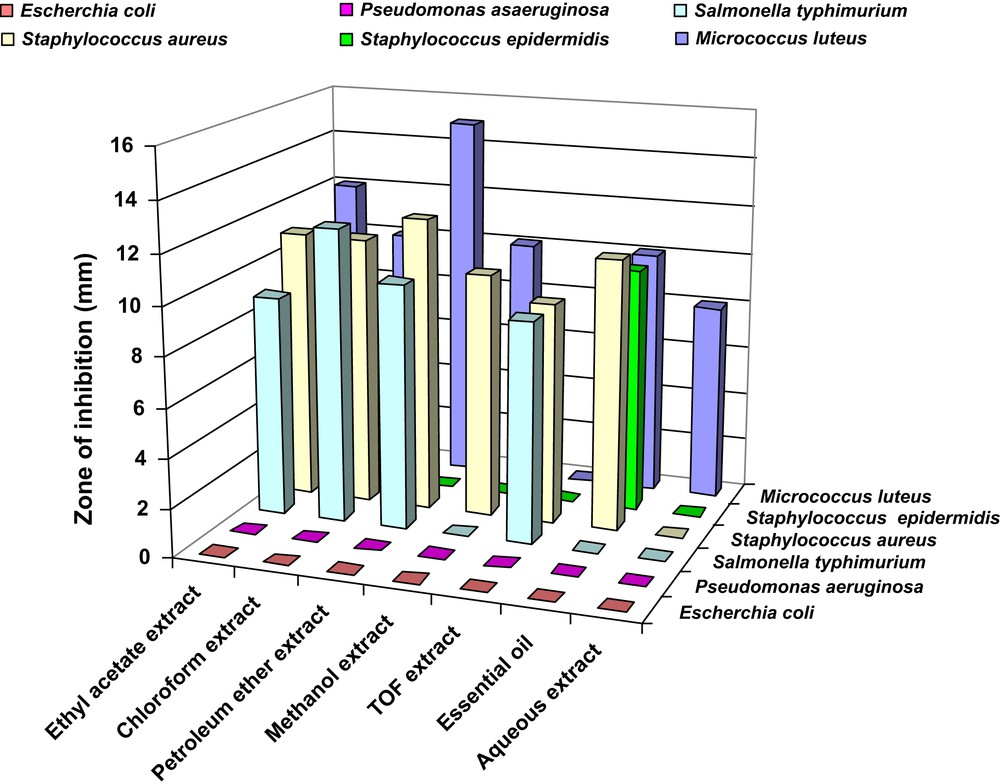
Antibacterial activity of different extracts (5 mg/ml) of Allium roseum var. odoratissimum, endemic species of southern Tunisia.
The TOF was moderately active against two food poisonous bacteria: S. typhimurium and S. aureus. The methanolic extract was active against S. aureus and M. luteus (10.0 mm). Finally, the aqueous extract was specific against the Micrococcus strain.
Under the same experimental conditions, 20 μl of all extracts (initially at 5 mg/ml) were ineffective against P. aeruginosa and E. coli; and both bacterial strains presented strong resistance against all the tested extracts, while M. luteus was the most sensitive bacterium.
These data indicate that Gram-positive bacteria are the most sensitive tested strain to the different extracts with the mean growth inhibition zone (8–15 mm), in contrast to A. sativum extracts, which exhibited their main antibacterial activity on Gram-negative bacteria [9]. Our results are in agreement with previous reports showing a weaker activity of essential oil of Salvia tomentosa, rich in camphor and carvacrol, against Gram-negative bacteria [30].
Therefore, the aqueous extract had a weak antibacterial activity against the tested bacterial strains, probably due to the absence of allicin or the inactivation of the allinase while treating the sample by heat. Indeed, allicin, which constitutes the main compound of aqueous garlic extract, is the principal bioactive compound. Siriponputikorn et al. [31] confirmed that fresh garlic has the highest antimicrobial property among the examined spices and some researchers believe that allicin is the main antimicrobial compound of fresh crushed garlic [32]. However, when garlic was heated, no inhibition zones were observed around the tested bacteria. The possibility that this compound is not heat stable or may be converted into some forms that have no or little antimicrobial activity would agree with the results of Wilkinson [33]. Furthermore, there was no antibacterial activity from shallot, even though this species belongs to the same family as garlic.
4 Conclusion
The qualitative and the quantitative analyses of essential oil showed the presence of 19 compounds in the whole essential oil of A. roseum var. odoratissimum. The number of sulphurous compounds was much higher than that of the non-sulphurous compounds. Most of the sulphurous compounds (2,4-dimethylthiophene, 2-propenyl methyl disulfide, 1-propenyl methyl disulfide and dimethyl trisulfide) were common to essential oil of A. sativum L. and A. fistulosum L. Among these compounds, methional was at the highest rate (17.1%). We report this compound for the first time in the essential oil of Allium genus, and hence it could be used as an important descriptor to characterize the essential oil of A. roseum var. odoratissimum and consequently this endemic species itself. Since the essential oil composition of the studied plant has not been reported before, the present data reveal for the first time the characteristic composition of the essential oil of this endemic species.
The antimicrobial activity of different extracts against six strains of bacteria performed by the disk agar diffusion showed that A. roseum var. odoratissimum has antibacterial activity which would differ according to the kind of extracts and the strain of bacteria tested; the technique of active compounds extraction would interfere, leading to more or less effective activities. The most active constituents of the extracted parts of A. roseum with a wide spectrum of antimicrobial effectiveness are the essential oil and the chloroform extract. Their antibacterial activity would be attributed to many factors such as phenological stage of plants, concentration and type of target microorganisms, extraction methods as well as extract types.
On the basis of the results of this study, A. roseum var. odoratissimum may be considered as a potential condiment with good flavour. At present, food safety is undoubtedly an important public health problem and there is a need to develop new methods that eliminate food pathogens. One way may consist in mixing the current practices with the addition of natural products. Therefore, the essential oil of A. roseum var. odoratissimum might be a valuable food additive. Further studies are needed for more extensive assessments of the processing methods in order to preserve the chemical, biological and sensorial properties.
Acknowledgments
Professor Mohamed Hammami from the Faculty of Medicine in Monastir is thanked for GC–MS analysis. Dr. Azaiez Ouled Belgacem from Arid Land Institute and Dr. El Hadi M. Yahia from the “Facultad de Ciencias Naturales Universidad Autónoma de Querétars”, Mexico, are thanked for their help with English.


