1 Introduction
Type I collagen molecules play a major role in tissue morphogenesis since they form the greater part of extracellular matrix (ECM) proteins in dense connective tissues. Cells produce triple-helical molecules that organize into cross-striated fibrils, further assembled into ordered 3D networks [1]. These hierarchical structures yield materials with high performances, in association with other matrix proteins, proteoglycans and sometimes a mineral phase. Tissue properties, as elasticity in the dermis, resistance to load in bone, resistance to stretch or shear in tendons, transparency in the cornea, all arise from specific matrix composition, fibril diameter or suprafibrillar organization.
Final structures as they appear in situ are well described from studies of tissue sections analysed in polarized light microscopy or transmission electron microscopy (TEM). Partially aligned collagen bundles appear in the derm [2], regular “crimps” corresponding to undulating fibrils are described in tendons [3], periodic rotations between fibrils are interpreted in cornea and compact bone [4,5]. Therefore, the polymorphism, observed within specific tissue collagen networks, largely originates from variations in collagen spatial order. The diversity arises from changes in fibril disposition: their degree of alignment, the presence of long-range undulations or the average angle between adjacent fibrils.
How do long-range ordered assemblies, throughout the body, arise from nanometer-size triple-helical collagen molecules? What determines the polymorphism found in living tissues? These questions still need to be addressed. A debate exists in the literature on whether the 3D organization of collagen fibrils in extracellular matrices is the result of cell-directed construction or of self-assembly processes. A recurrent hypothesis regarding fibril orientation controlled by cells originates from immuno-cytochemical data showing coalignments between the cytoskeleton and fibrous proteins of the ECM [1,6]. More recently, serial micrographs of tendons showed that plasma membrane channels between neighbouring cells contained small fibrils oriented parallel to extracellular fibril bundles [7]. This prompted the hypothesis that cellular protrusions running along the fibroblast were responsible for the parallelism of tendon. Opposite to these cell control hypotheses, the demonstration of collagen self-assembly properties, initiated by the pioneer work of Bouligand [8], gives clues to 3D packing in extracellular matrices [9,10].
The present short review focuses on the liquid crystalline properties of type I collagen. It will consider previous and recent data that characterize the structure of the mesomorphic state in vitro and demonstrate the link between fluid mesophases and ordered fibrillar networks. The possibility to form tissue-like collagen networks in vitro offers models to study cell–matrix interactions in a 3D context; adhesion and migration of connective tissue cells are thus studied on biomimetic matrices. The discussion will argue on what remains indirect evidences that matrix assembly in vivo must pass through self-assembly before fibrillogenesis.
2 Structure of the liquid crystalline phase of collagen
The evidence of a twisted assembly of collagen triple helices in vitro was first reported with sonicated solutions of type I collagen molecules [11]. The structure of a cholesteric phase, showing a long-range helical order with a pitch larger than 1 μm, was then resolved in polarized light microscopy. Highly concentrated collagen samples, placed between slide and coverslip, originated at that time from commercial bovine sources. Without previous sonication, the very high viscosity of the native samples hindered the formation of fingerprint patterns typical of cholesteric phases.
Improved purification is now performed with collagen I extracted from rat tail tendons. Shortly, tendons are placed in 0.5 M acetic acid and the resulting solution is filtrated and centrifuged at low speed until it is clear. The protein is then selectively precipitated with 700 mM NaCl and recovered by low-speed centrifugation. The pellets are dissolved and thoroughly dialysed against 0.5 M acetic acid. The solutions are used after a final centrifugation step at 50,000 g for 3 h. This controlled procedure, together with an optimized collagen concentration protocol (see Section 3, Fig. 2a), demonstrates that intact 300-nm-long collagen triple helices possess liquid crystalline properties, in a perfectly reproducible and homogeneous way. Observations in polarized light microscopy of collagen fluid phases within glass microchambers reveal a gradient from the less concentrated domains at the solution injection point to the most concentrated ones at the air/collagen interface (Fig. 1a) [12]. Along the concentration gradient, different situations are found: isotropic regions, loose cholesteric areas with high level of defects, and finally homogeneous cholesteric domains (Fig. 1b). The cholesteric pitch was observed to decrease continuously as concentration increases.
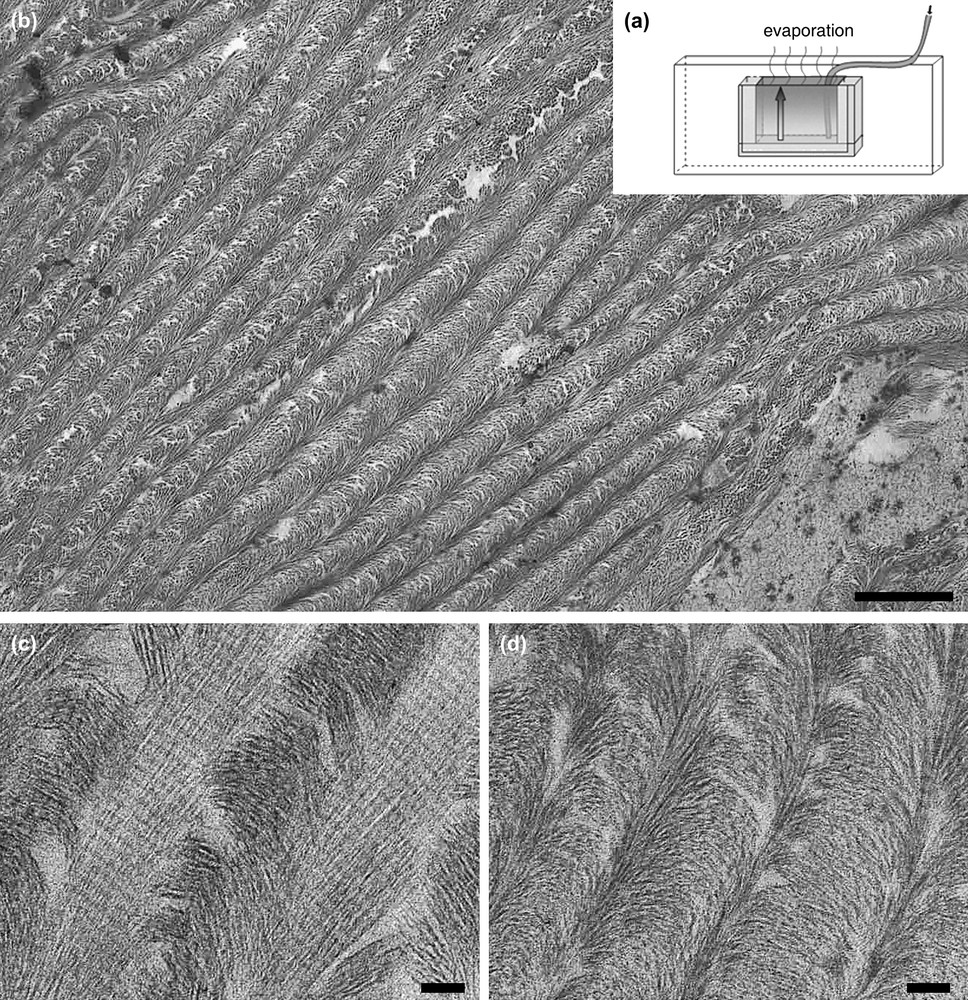
Ordered fibrillar networks of collagen. (a) Experimental microchamber opens on one side made out of glass slides, and spacers. Slow and continuous injection compensates evaporation so that the concentration progressively increases in the chamber. (b–d) Sections of collagen gels obtained by inducing fibrillogenesis in the microchamber. (b) At low magnification, tissue-like structure shows regular series of arced patterns, bar: 1 μm. (c, d) At high magnification, two different domains within the same gel allow us to correlate collagen fibril diameter and concentration, bar: 200 nm.
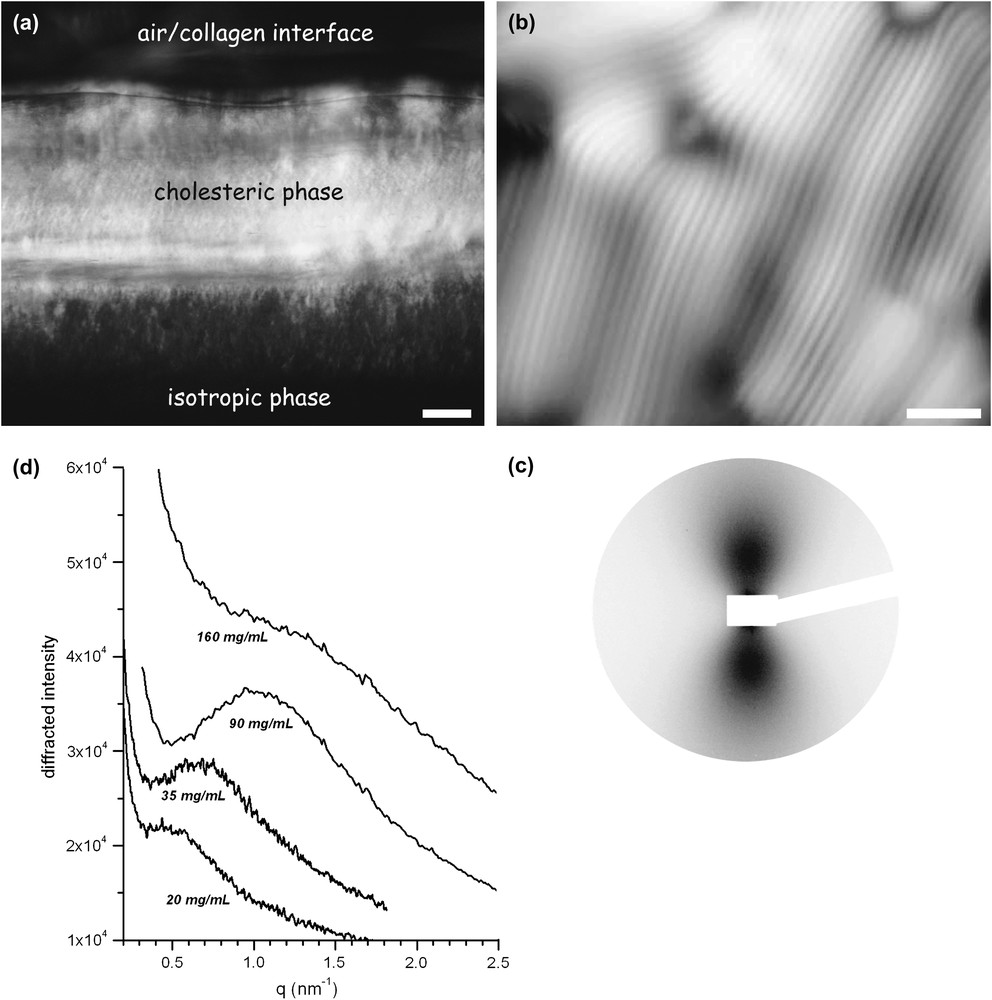
Collagen liquid crystalline phases observed in polarized light microscopy and small-angle X-ray scattering. (a) General view in polarized light microscopy of collagen injected within a glass microchamber. From bottom to top: the isotropic phase, the highly birefringent cholesteric phase and the air/water interface limit, bar: 100 μm. (b) Liquid crystalline fingerprint texture of collagen observed in polarized light microscopy between crossed polars. Molecular directions regularly rotate along a direction normal to the thin extinction bands with a helical pitch of a few μm, bar: 10 μm. (c) Small-angle X-ray scattering pattern of a collagen solution at 90 mg/ml recorded under moderate shearing. (d) Linear profiles I = f (q) taken along the vertical direction at concentrations ranging from 20 to 160 mg/ml. An interference peak is visible in both nematic and isotropic samples. The correlation length of the liquid-like order, proportional to the peak width, decreases as concentration increases, i.e. as the average distance between collagen molecules decreases.
The microscopic structure of the liquid crystalline collagen phase has only recently been characterized by small-angle X-ray scattering (ID02 station at the ESRF [13]). Anisotropic collagen at concentrations yielding cholesteric phases were placed in a cylindrical shear-cell. The moderate shear stress, generated by the flow, unwinds the chiral texture and produces uniaxially oriented monodomains. Strongly anisotropic SAXS patterns are then obtained with a scattered intensity higher in the direction perpendicular to the flow (Fig. 1c). This indicates a liquid-like order of elongated collagen molecules oriented along the flow direction. Interparticle scattering gives rise to a broad interference peak whose maximum is located at a q-value qmax, inversely proportional to the average distance between the triple helices (Fig. 1d). The mean distance between molecules is for example estimated at 6.45 nm for a concentration of 90 mg/ml.
The ionic strength influence on the isotropic (I)/cholesteric (chiral nematic, N∗) transition was also investigated as a function of the concentration of acetic acid used as a solvent. Collagen samples of known concentrations are sucked into capillary tubes and observed in polarized light. This method is widely used to discriminate between anisotropic optically birefringent samples and dark isotropic ones, and map out a phase diagram. As the initial acetic acid concentration is increased from 5 to 500 mM, the critical collagen concentration of the I/N∗ transition increases from 50–60 to 80–85 and to 100 mg/ml [13]. This increase is mostly due to the higher ionic strength that leads to a stronger screening of the repulsive electrostatic potential between triple helices. Such experimental data on ordered transitions, and their good agreement with statistical physics theories, assess the nature of collagen self-assembly processes. In our experiments, the pH roughly ranges from 2.5 to 3.5, which provides a large enough excess of positive charges to ensure a complete solubility of collagen well beyond the I/N∗ threshold. Although such a pH value is hardly physiologic, the resulting solutions serve as models to study the behaviour of collagen triple helices in the dense fluid state. When hypothesizing what might happen in biological situations, we propose that the critical threshold, necessary for the phase transition to occur, can be reached when soluble procollagen molecules accumulate in secretory vesicles or in the space directly outside the cells.
3 Collagen fibrillogenesis in condensed media
The process of forming fibrils from acid-soluble collagen solutions was described by biochemists already in the years 1960 and gave rise, since then, to an abundant literature [14–17]. Fibrils self-assemble in vitro at room temperature when the pH is modified from acid to neutral, molecules form lateral associations due to minimized electrostatic repulsions. Observed in TEM, after staining with heavy metal ions, reconstituted fibrils exhibit the typical periodic striation of 67 nm along their principal axis. Extensive studies in the literature brought up extremely valuable data about the structure of fibrils and their assembly mechanisms. However, in all fibrillogenesis published experiments, the collagen concentration is kept well below 1 mg/ml. Consequently, micrographs of reconstituted fibrils deposited on TEM grids systematically appear randomly disposed.
Relevant comparisons with biological structures appeared only when fibrillogenesis was processed in condensed media, in the concentration range where liquid crystalline assembly occurs. It appears that these values are close to the concentrations of collagen in tissues, that is between 50 and 200 mg/ml. It was experimentally possible to form fibrils within highly viscous mesophases by applying a protocol first described for cell culture studies by Ehrman and Gey [18]. Ammonia vapour diffusion is used to raise the pH of highly viscoelastic collagen solutions without disturbing the long-range molecular order. The sol/gel transition results in dense gels that can be fixed with glutaraldehyde, dehydrated and embedded in a resin. Cross-striated fibrils observed in TEM throughout the sample demonstrate that very high concentrations do not prevent the fibril formation process. Ordered fibrillar networks exhibit architectures close to those of connective tissues, namely of compact bone or of tendon [19,20].
When first obtained between slide and coverslip, such biomimetic ordered assemblies only developed over μm distance ranges. A new protocol, using controlled evaporation, compensated by a slow and continuous injection of acid-soluble collagen into glass microchambers, was recently designed. The initially rather low protein concentration, of 5 mg/ml or less, slowly increases as evaporation proceeds (Fig. 2a) [12]. This procedure greatly improves the homogeneity of the resulting fibrillar networks. Tissue-like matrices are now obtained, regularly organized over lateral distances of centimeters, showing regular series of arced patterns in TEM (Fig. 2b). Similar patterns have largely been described in extracellular matrices. The advantage of experimental microchambers is also to work with reduced amounts of protein, which allows assays with molecules difficult to purify in large quantities, as procollagen, or allows one to study the effect of other added components of ECM.
An unexpected result with the microchamber device was to establish a relationship between collagen fibril diameters and collagen concentration. The experimental setup induces a concentration gradient between the air interface, at the top chamber, and the collagen injection point, at the bottom of the chamber (Fig. 2a). When the sample is exposed to ammonia vapours, the gas dissolves in the hydration water and fibrillogenesis occurs. It is then possible to cut pieces of the dense gel, and correlate TEM observations to collagen concentration evaluated by hydroxyproline titration [12]. Two different regions of the same gel are illustrated in Fig. 2, showing large fibril diameters of 300 nm or more in a concentration range of 100–150 mg/ml (Fig. 2c) and much smaller fibrils of diameters below 50 nm at concentrations two to three times higher (200–450 mg/ml) (Fig. 2d). This shows that, in vitro, as simple a physico-chemical parameter as concentration can account for variations in fibril diameter. This result was further confirmed in vitro in experimental conditions where the effect of concentration and pH were dissociated (to be published).
4 Cell–matrix interactions in a biomimetic context
Cell biologists have shown that cells secrete and assemble an extracellular matrix which provides structural support for cell adhesion, migration and tissue organization, as well as external regulation of cellular functions [21]. A further step, involving the liquid crystalline properties of collagen and their perspectives in tissue morphogenesis, was therefore to analyse the phenotype of connective tissue cells, in interaction with ordered collagen fibrillar networks. Cell culture experiments were undertaken with collagen matrices concentrated at 5, 40 and 80 mg/ml. To this purpose, collagen stock solutions are poured into crystallizing dishes and submitted to slow solvent evaporation in a laminar flow bench, in sterile conditions. Gelated fibrillar matrices are obtained by placing the samples under ammonia vapours at room temperature and rinsed in culture media until neutrality is reached. Cell suspensions are seeded at the dense matrix surface and maintained in a controlled atmosphere.
Different cell types have been seeded on dense collagen matrices, primary fibroblasts with cells issued from healthy skin biopsies, fibroblasts from “knock-out” mice which did not express certain collagen receptors (integrins) and osteoblasts from transformed or primary cell lines. Once a week, until 28 days, matrix samples are fixed in paraformaldehyde, dehydrated, embedded in paraffin and prepared for microscopy analysis. The corresponding culture media are collected and frozen for biochemical analyses. A common observation to all cell types is that they attach, proliferate and survive on dense collagen matrices during the 28 days of culture. Differences appear, however, as a function of the cell origin or transformation, in their ability to migrate on the surface or inside the matrices. The most achieved analysis concerned primary fibroblasts, their migration was followed and density measured on serial sections of 500-μm-thick matrices. At day 28 the migration distance attains 320 μm and the cell density reaches 5500 cells/mm3 [22]. The morphology of fibroblasts located at the surface of the matrices show a spindle shape appearance typical of a cellular monolayer. Once inside the collagen matrices pioneer cells found at the migration front present a stellate appearance (arrow) and tracks are seen (*) corresponding to a local hydrolysis of the collagen network (Fig. 3a). The migration hydrolysis process in vivo involves enzymes (collagenase 1 or MMP1) which initiate the degradation of fibrillar type I collagen and membrane-bound MT1-MMP [23]. Collagen denaturated into gelatin at physiological temperature further degrades into small fragments by the action of gelatinase A (MMP2) and gelatinase B (MMP9) [24]. In our 3D culture conditions, immunohistochemical staining of the dense gels and other biology techniques (zymography, western blots) showed that only membrane-bounded MT1-MMP and gelatinase A (MMP2) appeared active [25]. Proliferation curves show a delay of the migration process as a function of concentration, as at day 7 cells are already present inside the 5 mg/ml matrices, whereas it takes over two weeks for the first leading cells to penetrate the much denser 40 mg/ml matrices (Fig. 3b). Immunohistochemical labelling showed that cells within the matrices were active in synthesizing collagen and fibronectin. Furthermore, they appear to both die by apoptosis (Tunel test) and proliferate (BrdU labelling) so that the mean cell density at one month corresponds to 2000 cells/mm3 (Fig. 3c) [25].
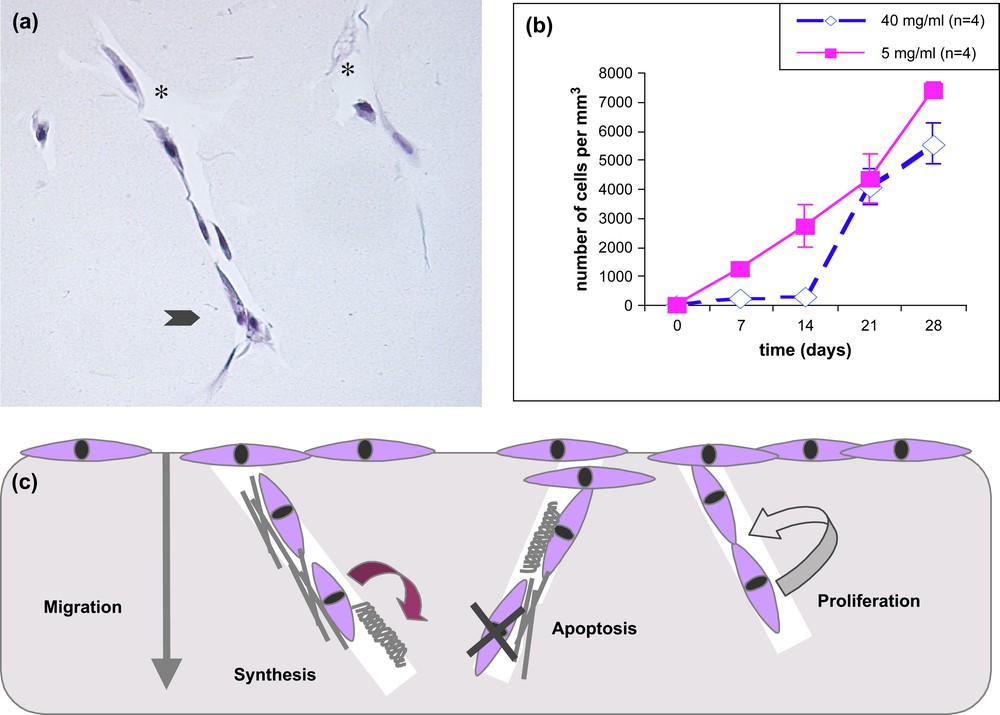
Cell–matrix interactions. (a) Morphology of fibroblasts migrating within 3D collagen matrices. A leading fibroblast, at the front, has a stellate appearance (arrow), tracks are seen due to collagen hydrolysis by metalloproteinases (star). (b) Density of dermal fibroblasts within collagen matrices concentrated at 5 and 40 mg/ml. At first a delay is observed for cells to migrate inside the more compact collagen networks but at day 28 the cell number catches up the density observed in the more diluted ones. (c) Diagram of fibroblast activities in interaction with a biomimetic collagen matrix, the cells migrate by hydrolysing the matrix, synthesize collagen and fibronectin, some die by apoptosis but others proliferate, so the final density is close to tissue evaluations [22].
Seeding bone cells instead of fibroblasts demonstrates the versatility of dense collagen matrice as interesting 3D culture models. Observations with transformed osteoblasts seeded on 50 mg/ml matrices suggests that cells attach, survive and form an epithelium-like morphology quite similar to in vivo observations (to be published). Future prospects will also need to consider the behaviour of stem cells on biomimetic collagen matrices as convergent data relate that complex extracellular matrices promote tissue-specific stem cell differentiation [26].
5 Conclusion
It is now clearly established that soluble collagen exhibits liquid crystalline properties. Native full-length type I helical molecules form cholesteric phases beyond a critical concentration. The exact location of the transition boundary depends on the solvent composition, increasing from 50–60 to 80–85 mg/ml as the initial acetic acid concentration is increased from 5 to 500 mM. The relatively good agreement between experimental data and statistical physics theories inspired by Onsager's work indicates that the transition is mostly driven by excluded-volume effects owing to the elongated conformation of the protein. When a sol/gel transition is pH-triggered, the highly viscoelastic collagen phase transforms into a fibrillar gel mimicking a variety of tissue networks as a function of concentration. An accurate control of ordered assemblies occurring at different hierarchical levels is possible by the use of the microchamber setup. Analogues of extracellular matrix networks produced in vitro, with a structure close to that of biological tissues, offers good models for the culture of cells in 3D, the study of their migration and signalling activities, in a manner close to physiological conditions. It also brings new perspectives in tissue engineering, biomimetic collagen networks, associated or not with specific cells, being good candidates for soft tissue repair. In the case of hard tissues it will be necessary to control association with an adequate apatite mineral phase, in particular to reach the required mechanical properties. The structuration of amorphous silica by a collagen liquid crystal template has already been obtained [27], demonstrating the ability of collagen to support the long-range organization of a mineral phase.
Fundamental questions, however, remain unsolved about the self-assembly properties of collagen and their implication in tissue morphogenesis. Our hypothesis is that mesophases play important roles in ECM 3D organizations. A possible scenario implies a liquid crystalline ordering of precursor procollagen molecules prior to fibrillogenesis. Some data support this hypothesis that came from the study in polarized light of purified procollagen molecules soluble at physiological pH, and observed at high concentrations in polarized light [28]. The relevance to physiological mechanisms can thus attribute the initial mesophase to procollagen during intracellular transport; the formation of banded collagen fibrils then proceeds within the liquid crystalline phase by enzymatic cleavage of N- and C-terminal regions in the extracellular matrix. Recent success in processing human collagen in transgenic tobacco plants [29], and the production of recombinant (pro)collagen in yeasts or bacteria [30], open new perspectives for liquid crystalline collagen research, both fundamental aspects and medical applications.
Acknowledgments
The authors are grateful to Anny Anglo for technical assistance, to Patrick Davidson, Bernard Coulomb and Pierre Marie for enjoyable discussions and collaborations, and to. Yves Bouligand for opening the route to fascinating research topics.


