1 Introduction
Chiral macrocyclic receptors are presently under intense study. Particularly suitable structures for construction of such moities are saccharide units. Due to their polyfunctional character leaving excessive room for all types of derivatives, their pronounced and broadly variable chirality, as well as their immanent ethylenoxy units, the ubiquitously accessible carbohydrate derivatives contain the primary structural elements as crown ethers.
The advantage of such starting materials, in contrast to other structures, was previously noted and used by Stoddart et al. who could obtain monosaccharide coronands from D-mannitol [1,2]. According to their studies carbohydrate precursors are suitable for construction of enzyme models [3–5]. Also Penades et al. could employ disaccharide crown ethers as chiral catalysts [6–8], and later reported on the complexation capacity of water-soluble carbohydrate-based macrocyles for neutral molecules [9–10]. The distinctive feature of these host molecules resides in the linkage of cyclophane and saccharide building units. Such hybrids of glycosides and aromatic building elements were suggested by Wilcox et al. to be termed “glycophanes” [11]. Previously, several groups were involved in the synthesis and characterisation of carbohydrate-based macrocycles [12–26], and some further examples were also reported on hetereoatom-substituted azacycles and bicyclic cryptandes [18,19,27–32].
2 Results and discussion
In this contribution a general synthetic approach for the selective construction of azamacrocycles from monosaccharide derivatives will be proposed. As a constraint, ring systems were required to contain at least two saccharide fragments. Thus, defined and adequately functionalised tethered monosaccharide precursors had to be synthesised. The most important reaction step in these sequences is the Richman–Atkins cyclisation [33,34]. Generally, in this method, alkali (preferred cesium) metal salts of sulfonamides are employed as excellent nucleophiles to react with good electrophiles in polar aprotic media such as dimethyl formamide. The conditions, applicability and scope of this condensation were broadly studied by various groups [35–42]. In comparison to the high dilution method [43,44] or template-mediated ring closing procedures [45,46], the Richman–Atkins approach is preparatively facile and advantageous. Even though the many polar functions in saccharide units may present problems for the required preorganisation of cycles, this approach seemed to be attractive and the preferred pathway.
As a suitable monosaccharide building unit the base stable derivative 1 [47] was identified. This carries benzyl ether protecting groups, the unblocked 4-OH position and the azido as a masked amino function. For construction of the 4,4’-tethered disaccharide mimetics the phase-transfer catalysed ether formation [48] employing various alkylation reagents was applied (Scheme 1). By using the two-phase system toluene and concentrated aqueous sodium hydroxide the 3-oxapentylene-bridged compound 3 was obtained with diethylene glycol ditosylate. Correspondingly, use of α,α’-dibromo p- and m-xylene resulted in formation of the p- and m-xylene-bridged compounds 6 and 9. Treatment of 1 with bis (2-chloroethyl)-ether gave 2 in high yields, which in turn with stoichoimetric amounts of p-toluene sulphonamide and cesium carbonate, led to formation of compound 12 bridged by a 3,9-oxa-6-N-tosyl-aza-undecane chain. Subsequently the azido functions were catalytically hydrogenated [49] to give the corresponding bis 6-amino derivatives 4, 7, 10 and 13 with palladium/charcoal in the presence of trietylamine to preserve the benzyloxy functions. Finally, low temperature tosylation led to the corresponding bis 6-tosylamides 5, 8, 11 and 14 via three steps in about 50% yield from 1.
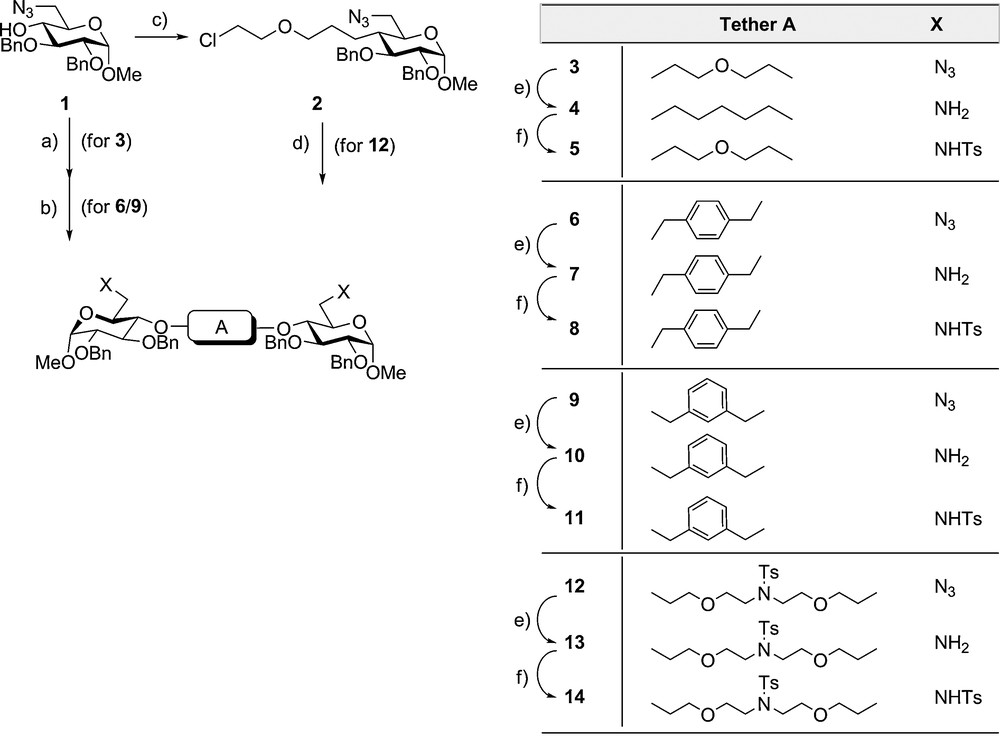
Synthesis of 4,4’-tetherod glucopyranosides: (a) (for 3) , 50% NaOH. Bu4nBr, Tol, 40X, (b) (for 6/9) 50% NaOH, Bu,NBr. Tol, 60–80°C. (c) , 50% NaOH. Bu4NBr. Tol. 60̊C; (d) (for 12) TsNH2 Cs2CO3 DMF. 50°C; (e) Pd/C (5%). Hj MeOH, Et3N; f) TsCI. Py, -20°C.
Further, cyclisation of the 4,4’-tethered derivatives 5 and 14 via their 6,6’-bis tosylamide functions was made by Richman–Atkins cyclisation employing various alkyl tosylates {ethylene glycol ditosylate, diethylene glycol ditosylate, diethanol amine tritosylate [50,51], and 1,8-bis-p-toluene sulfonyloxy-3,6-dithia-octane (15)} as well as α,α’-dibromo-p-xylene to give compounds 16–31 in good yields (Scheme 2). Treatment of the precursors 5 and 14 with 1,8-ditosyloxy-3,5-dithia-octane 15 as electrophilic partner could be performed at room temperature to give in somewhat reduced yields yet without elimination the carbohydrate coronands 19 and 31. The macrocyclic system 19 comprises a doubly tethered disaccharide component with a 23-membered ring, and 31 represents a novel class of carbohydrate coronands containing oxygen, nitrogen and sulphur donors within a 29-membered ring system. In all ring closing reactions dimethyl formamide as solvent and cesium carbonate as base were used advantageously exploiting the cesium effect [36–38,52,53]. Thus rings systems 16–31 containing two carbohydrate units and 17 to 29 chain links could be obtained in remarkable average yields above 50%.
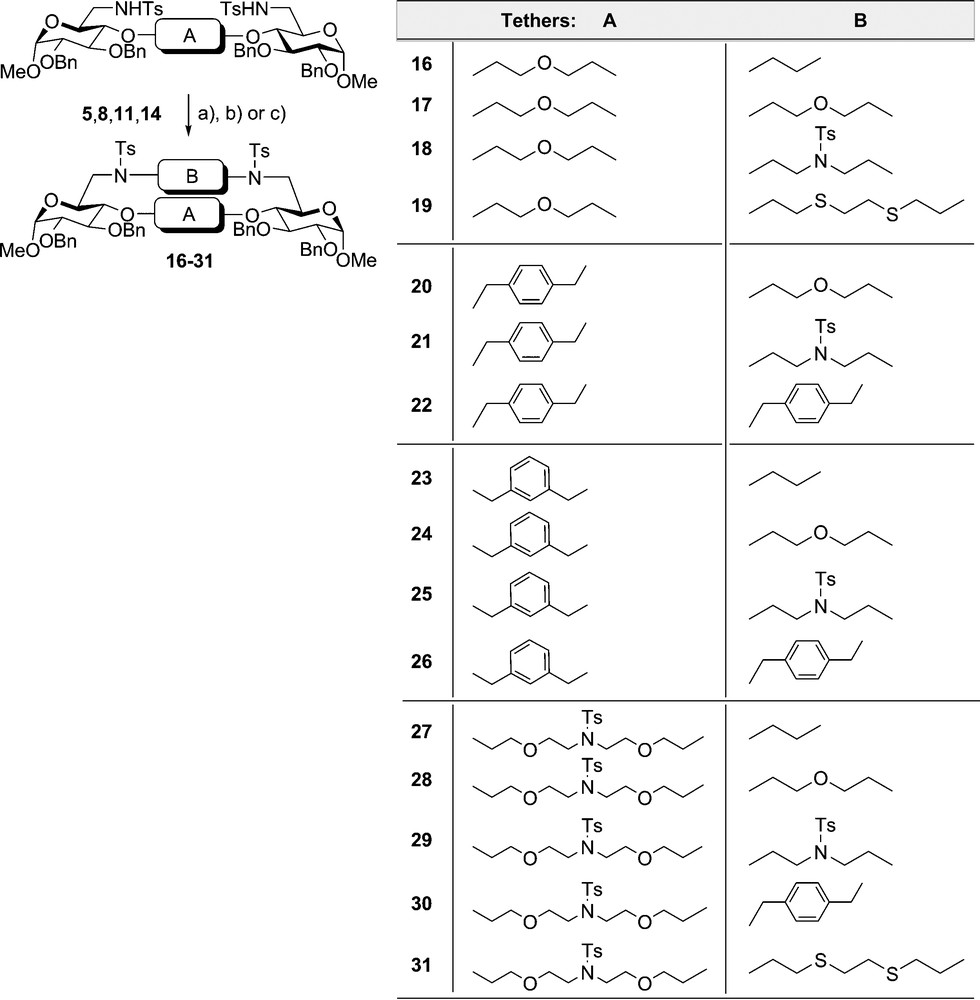
Richman- Atkins cydization via 6,6’-positions of 4,4-telhered glucopyranosides: (a) (for 16–18, 20–21, 23–25, 27–29) . C82CO3 DMF. 20–90’C. (b) (for 22, 26, 30) . Cs2CO, DMF. 20–90’C: (c) (for 19. 31) (15) CS2CO3. DMF. 20̊C.
It was of interest to achieve the synthesis of ring systems containing four saccharide fragments. Attempts to this endeavour via a [2+2] cyclisation could not be realised. Thus, by starting with the previously described 4,4’-tethered structures 5, 11 and 14 activated 6,6’-extended precursors had to be prepared (Scheme 3). By reaction of 5 or 11 with 2-(2-chloroethoxy)-tetrahydropyran [54] in excess and subsequent acid cleavage of the ether derivative gave compounds 32 and 34, respectively. By further low temperature tosylation the 6,6’-extended 4,4’-tethered glucopyranosides 33 and 35 could be obtained easily.
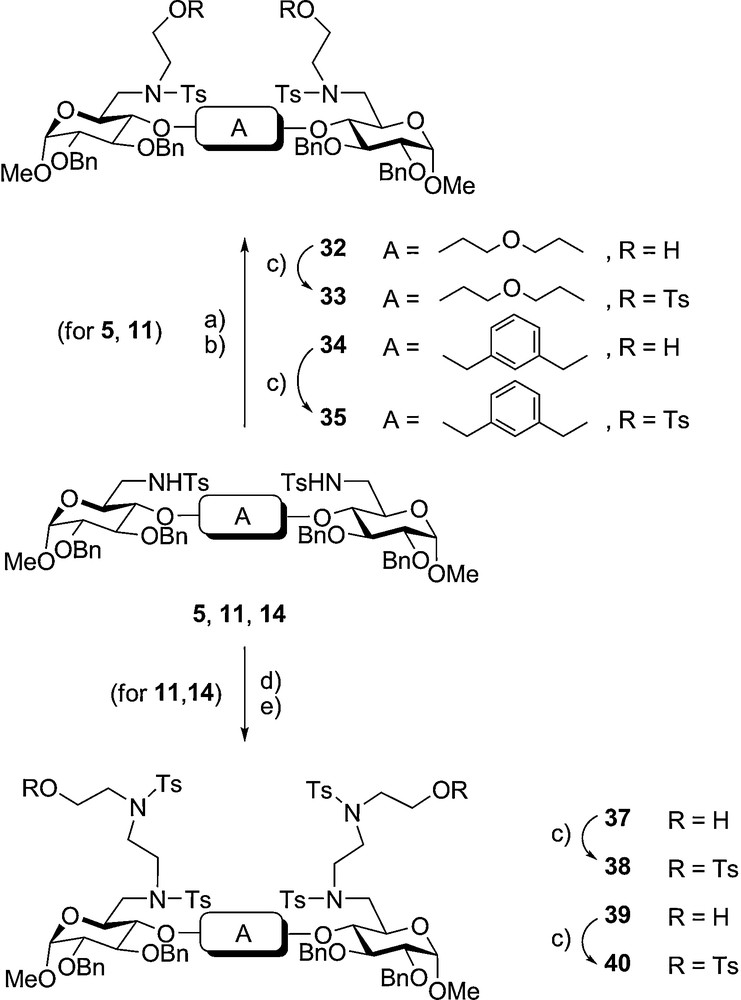
Preparation of 6,6’-extended 4.4’-tethered giucopyranosides (a) CS2CO3 DMF. 100̊C; (b) cone. HCI/MeOH, CH2CI2 20̊C; (c) TsCI, Py. –20°C; (d) (36), CS2CO3 DMF, 70°C; (e) Bu4NBr, THF, 20°C.
Using a corresponding approach the precursors 5 and 14 were transformed into other 6,6’-extended derivatives. The mono-silylated reagent 36 was obtained from diethanolamine by reaction with two equivalents of tosyl chloride at 0 °C and low temperature workup to give N-tosyl-diethanolamine [55] followed by selective silylation. After Cs2CO3-mediated reaction with compounds 11 and 14 the silylether groups were cleaved with tetrabutylammonium fluoride to give compounds 37 and 39, respectively. These in turn underwent monotosylations to give the final derivatives 38 and 40. Thus, in overall yields between 40–70% these readily available components are ideally suited for further reactions to function as electrophilic components in Richman–Atkins cyclisations (Scheme 3).
The final cyclisation towards the tetrasaccharide coronands 41–43 could be realised in around 50% yield. As previously mentioned [56] combinations of long-chain electrophiles and short nucleophiles would be preferred for condensations, in contrast to the opposite arrangement. However, in the present case the reactions of the 4,4’ tethered substrates 5 and 11 with the 6,6’-extended derivatives 33, 35 and 38 went as straight forward as in the previously described formation of the disaccharide analogs. These novel macrocycles with 34 to 40 ring atoms are novel representatives of [2+2]-glycophanes. Initially, smaller glycophanes made from trehalose in lower yields were reported by Penadés et al. [7,9,10]. In the present case the excellent operation of the Richman-Atkins approach allowed quite pleasing yields and very large components (Scheme 4).
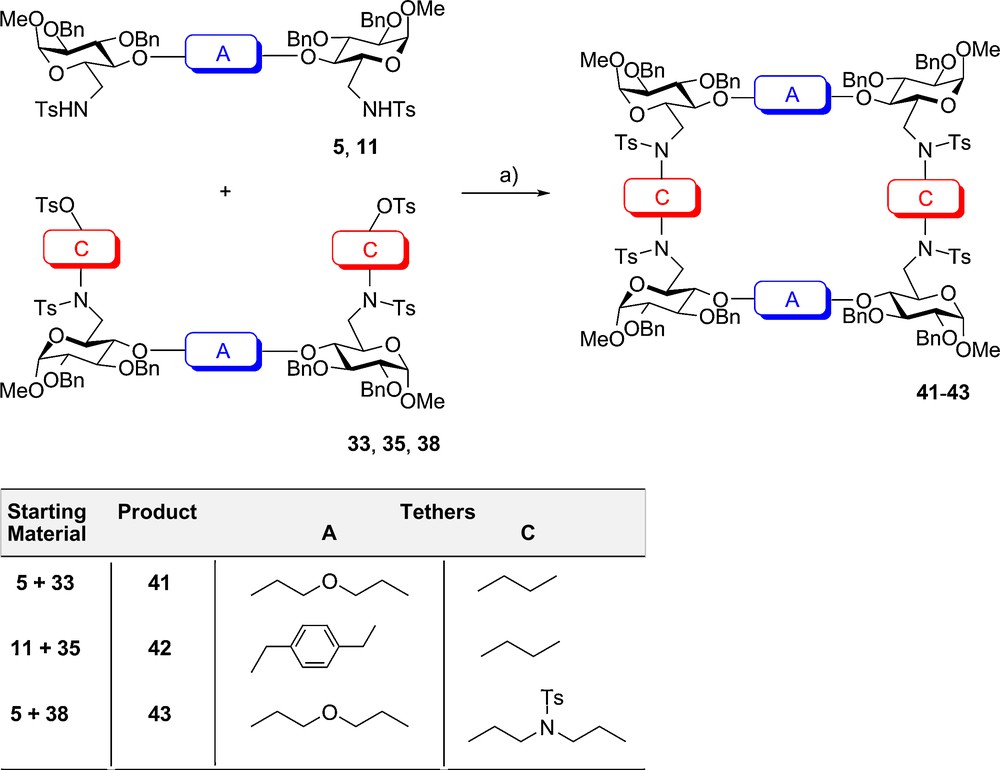
Synthesis of highly symmetric tetrasaccharide coronands: (a) Cs2CO3 DMF, 70–80X.
Generally difficult is the analysis of these coronands. Both by NMR and combustion analyses it remains ambiguous whether the products were obtained by a [1+1] or a [2+2] condensation due to the molecular symmetry. However, by Maldi-Tof an unequivocal structural assignment was at hand.
In conclusion, it was demonstrated that the Richman–Atkins cyclisation could be used advantageously to synthesize a number of structurally quite diverse aza-carbohydrate coronands in high yields. Based on single starting materials the present approach should be accepted as the preferred method for formation of flexibly variable glyophanes. Experiments are under way to address the de-blocking of these coronands. Further studies will be concerned with the properties such as stability of the cavities as well as their complexation capability for various metal ions.
3 Experimental part
3.1 General
All reactions were monitored by thin layer chromatography on silica gel foils GF254 (Merck). Detection was by UV or spraying with 20% ethanolic sulfuric acid and subsequent heating. Amines were detected with 10% ninhydrin in ethanol and further heating to 200 °C. Column chromatography was done on silica gel 60 (230–400 mesh, Merck) by the flash mode with the solvent mixture recorded. 1H NMR (400 MHz) and 13C NMR spectra (100 and 62 MHz) were done on Bruker AMX-400 and AC-250. Signal assignment was by 1H,1H- and 1H,13C-COSY measurements. CDCl3 (δ 77.0 for 13C) and TMS (δ 0.0 for 1H) were used as internal standards. FAB mass spectra were recorded on double focussing mass spectrometer VG 70-250 S (VG Analytical) with m-nitrobenzyl alcohol as matrix material and xenon as ionisation gas. Maldi-Tof mass spectra were done on Bruker Biflex with nitrogen laser 337 nm, puls width 5 ms and 4-hydroxy-α-cinnamic acid as matrix. Melting points and glass temperatures are uncorrected and were taken with a Leitz heating microscope or with an Olympus BH polarising microscope and Mettler FP82 heating table. Optical rotations were measured with Perkin-Elmer polarimeters 241 and 243 using sodium D line (589 nm), cuvette length 10 cm, and temperature 20 °C. Elemental analysis was performed by the Microanalytical Section of the Department of Chemistry.
3.1.1 General procedure 1
3.1.1.1 Alkylation by phase transfer catalysis (GP1)
The saccharide derivative was dissolved in the given solvent and alkylation reagent (0.55 eq) and phase transfer catalyst (1.5 eq) were added. Under vigorous stirring 20–50% aqueous sodium hydroxide was added and the reaction performed as recorded. For workup the aqueous phase was thoroughly extracted by an organic solvent, combined with the organic phase, dried over sodium sulfate, and after evaporation the residue purified or separated by chromatography.
3.1.2 General procedure 2
3.1.2.1 Hydrogenation of azides without cleavage of benzyl ethers (GP2)
The diazido derivatives (3–5 mmol) were dissolved in methanol (50–100 mL), triethylamine (2 mL) and 5% palladium on charcoal (500 mg) added. Hydrogenation was done at normal pressure for about 24 h, and workup was by filtration over celite and evaporation.
3.1.3 General procedure 3
3.1.3.1 Tosylation of amines and alcohols (GP3)
The starting material was dissolved in anhydrous pyridine (5–15 mL per hydroxyl or amino group) and cooled to –20 °C. Under stirring tosyl chloride (1.15 equ per hydroxyl or amino group) added. After termination of the reaction (1–5d) the solvent was evaporated in vacuo (temperature below 50 °C) and several times coevaporated with toluene. The raw material was purified by chromatography.
3.1.4 General procedure 4
3.1.4.1 Richman-Atkins cyclisation (GP4)
The ditosylate or dichloride (0.5–1.0 mmol) and the nucleophilic component (0.5–1.0 mmol) were dissolved in anhydrous dimethyl formamide (10 mL). After addition of base (3–5 eq of Na2CO3, K2CO3 of Cs2CO3) the reaction was kept as 60–80 °C for several days. Workup was by filtration over silica gel and evaporation. The remainder was dissolved in toluene/ethyl acetate and treated by ultra sonification for 5 min followed by filtration over silica gel, evaporation and purification of the raw material by chromatography.
4 Methyl 6-azido-2,3-di-O-benzyl-4-O-(5-chloro-3-oxapentyl)-6-deoxy-α-d-glucopyranoside (2)
The synthesis followed GP1 employing 1 (5.7 g,14.27 mmol), bis(2-chloro-ethyl)-ether (40.8 g, 33 mL, 285 mmol), (4.5 g, 14.2 mmol) tetrabutylammonium bromide, toluene (150 mL), 50% aqueous sodium hydroxide (60 mL), 60 °C, 3 h. Purification was by flash chromatography (dichloromethane/acetone 50:1). Yield 6.4 g (88%), colourless syrup; [α]D + 52.4 (c 1.0, CHCl3); 1H NMR (400 MHz, CDCl3): δ 7.36–7.28 (m, 10 H, Ph), 4.60 (d, H-1), 3.49 (dd, H-2), 3.90 (dd, H-3), 3.27 (dd, H-4), 3.75 (ddd, H-5), 3.56 (dd, H-6a), 3.48 (dd, H-6b), 3.40 (s, 3 H, OCH3), 4.95 (d, OCHABn), 4.79 (d, OCHA Bn), 4.77 (d, OCHBBn), 4.65 (d, OCHB Bn), 4.01–3.95 (m, 1 H, OCHA), 3.73–3.63 (m, 3 H, OCH2), 3.58 (dd, 2 H, CH2Cl), 3.56 (dd, 2 H, OCH2); J1,2 = 3.5, J2,3 = 9.5, J3,4 = 9.5, J4,5 = 9.5, J5,6a = 3.0, J5,6b= 5.0, 2J6a,6b = 12.0, JCH,CH = 4.5 and 6.0,2JBn = 10.5 and 12.0 Hz.; 13C NMR (100 MHz, CDCl3): δ 98.06 (C-1), 79.87 (C-2), 81.59 (C-3), 79.10 (C-4), 69.97 (C-5), 51.27 (C-6), 55.34 (OCH3), 75.62, 73.41 (2 C, OCH2Ph), 72.31, 71.27, 70.69 (3 C, OCH2), 42.73 (CH2C1), 138.73, 138.06 (2 C, q, Ph), 128.49–127.64 (10C, Ph).
4.1 Bis-1,5-[methyl-6-azido-2,3-di-O-benzyl-6-deoxy-4-yloxy-α-D-glucopyranoside]-(3-oxapentane) (3)
The synthesis followed GP1 employing 1 (8.3 g, 20.8 mmol), diethylene glycol ditosylate (4.74 g, 11.4 mmol), tetrabutylammonium bromide (3.3 g, 10.4 mmol), toluene (100 mL), 50% aqueous sodium hydroxide (70 mL), 40 °C, 3 d. Purification was by flash chromatography (dichloromethane/acetone 60:1). Yield 5.1 g (57%); glass temperature 83–87 °C; [α]D + 55.3 (c 1.0, CHCl3); 1H NMR (400 MHz, CDCl3): δ 7.34–7.25 (m, 20 H, Ph), 4.60 (d, 2 H, H-1,1’), 3.46 (dd, 2 H, H-2,2’), 3.87 (dd, 2 H, H-3,3’), 3.24 (dd, 2 H, H-4.4’), 3.73 (ddd, 2 H, H-5,5’), 3.48 (dd, 2 H, H-6a,6a’), 3.45 (dd, 2 H, H-6b,6b’), 3.39 (s, 6 H, OCH3, OCH3’), 4.92 (d, 2 H, OCHABn, Bn’), 4.76 (d, 2 H, OCHABn, Bn’), 4.75 (d, 2 H, OCHBBn, Bn’), 4.73 (d, 2 H, OCHB Bn, Bn’), 3.95–3.90 (m, 2 H, OCHA), 3.70–3.64 (m, 2 H, OCHA), 3.47 (s, 4 H, OCH2); J1,2 = 3.5, J2,3 = 9.0, J3,4 = 9.5, J4,5 = 9.5, J5,6a = 2.5, J5,6b = 5.5, 2J6a,6b = 13.2, 2JBn = 11.0 and 12.0 Hz; 13C NMR (100 MHz, CDCl3): δ 98.03 (C-1,1’), 79.86 (C-2,2’), 81.62 (C-3,3’), 79.05 (C-4,4’), 70.04 (C-5,5’), 51.23 (C-6,6’), 55.32 (OCH3, OCH3’), 75.56, 73.40 (4 C, OCH2Ph), 72.26, 70.60 (4 C, OCH2), 138.79, 138.12 (4 C, q, Ph), 128.50–127.65 (20 C, Ph).
4.2 Bis-1,5-[methyl-6-amino-2,3-di-O-benzyI-6-deoxy-4-yloxy-a-d-glucopyranoside]-(3-oxapentane) (4)
The synthesis followed GP2 using 3 (6.4 g, 7.36 mmol), Pd/C (5%, 1 g), triethylamine (4 mL), methanol (150 mL), 24 h. Yield 5.7 g (94%); colourless syrup; [α]D +38.7 (c 1.0, in CHCl3);1H NMR (400 MHz, CDCl3): δ 7.35–7.25 (m, 20 H, Ph), 4.54 (d, 2 H, H-1,1’), 3.43 (dd, 2 H, H-2,2’), 3.88 (dd, 2 H, H-3,3’), 3.19 (dd, 2 H, H-4,4’), 3.52 (ddd, 2 H, H-5,5’), 3.01 (dd, 2 H, H-6a,6a’), 2.78 (dd, 2 H, H-6b,6b’), 3.36 (s, 6 H, OCH3, OCH3’), 4.92 (d, 2 H, OCHABn, Bn’), 4.77 (d, 2 H, OCHABn, Bn’), 4.75 (d, 2 H, OCHBBn, Bn’), 4.62 (d, 2 H, OCHB Bn, Bn’), 3.94–3.86 (m, 2 H, OCHA), 3.75–3.68 (m, 2 H, OCHA), 3.55–3.45 (m, 4 H, OCH2), 1.72 (b, 4H,NH2); J1,2 = 3.5, J2,3 = 9.5, J3,4 = 9.5, J4,5 = 9.5, J5,6a = 3.0, J5,6b = 6.5, 2J6a,6b = 13.0, 2JBn = 11.0 and 12.0 Hz. 13C NMR (100 MHz, CDCI3): δ 97.94 (C-1,1’), 80.03 (C-2,2’), 81.91 (C-3,3’), 79.90 (C-4,4’), 71.62 (C-5,5’), 42.78 (C-6,6’), 55.05 (OCH3, OCH3’), 75.51, 73.34 (4 C, OCH2Ph), 72.23, 70.95 (4 C, OCH2), 138.80, 138.13 (4 C, q, Ph), 128.43–127.55 (20 C, Ph).
4.3 Bis-1,5-[methyl-2,3-di-O-benzyl-6-deoxy-6-tosylamino-4-yloxy-α-d-glucopyranoside]-(3-oxapentane) (5)
The preparation followed GP3 employing 4 (8.25 g, 10.1 mmol), p-toluene sulfonic acid chloride (4.43 g, 23.2 mmol), pyridine (250 mL), −20 °C, 48 h. Purification was by flash chromatography (dichloromethane/acetone 17:1). Yield 8.4 g (74%); glass temperature: 51 °C; [α]D + 38.7 (c 1.0, CHCl3); 1H NMR (400 MHz, CDCl3): δ 7.72–7.24 (m, 28 H, Ph), 4.39 (d, 2 H, H-1,1’), 3.34 (dd, 2 H, H-2,2’), 3.79 (dd, 2 H, H-3,3’), 3.25–3.15 (m, 6 H, H-4,4’, H-6a,6a’, H-6b,b’), 3.60–3.45 (m, 6 H, H-5,5’, OCH2), 3.20 (s, 6 H, OCH3, OCH3’), 5.25 (dd, 2 H, NHTs), 4.89 (d, 2 H, OCHABn, Bn’), 4.72 (d, 2 H, OCHABn, Bn’), 4.70 (d, 2 H, OCHBBn, Bn’), 4.56 (d, 2 H, OCHB Bn, Bn’), 3.97–3.90 (m, 2 H, OCHA), 3.78–3.71 (m, 2 H, OCHA), 2.38 (s, 6 H, CH3Ts); J1,2 = 3.5, J2,3 = 9.5, J3,4 = 9.5, J6,NH = 6.0, 2JBn = 11.0 and 12.0 Hz; 13C NMR (100 MHz, CDCl3): δ 98.05 (C-1,1’), 79.82 (C-2,2’), 81.59 (C-3,3’), 80.59 (C-4,4’), 68.52 (C-5,5’), 44.69 (C-6,6’), 55.28 (OCH3, OCH3’), 75.42, 73.47 (4 C, OCH2Ph), 72.31, 70.93 (4 C, OCH2), 21.55 (2 C, CH3Ts), 143.24–137.44 (8 C, q, Ph), 129.71–127.18 (28 C, Ph); Anal. calcd. for C60H72N2O15S2 (1125.4): C 64.04, H 6.45, N 2.49, S 5.70. Found: C 63.58, H 6.50, N 2.38, S 5.71.
4.4 Bis[methyl-6-azido-2,3-di-O-benzyl-6-deoxy-4-yloxy-α-d-glucopyranoside]-p-xylene (6)
The formation followed GP1 employing 1 (4.5 g, 11.3 mmol), α,α’-dibromo-p-xylene (1.6 g, 6.2 mmol), tetrabutylammonium bromide (3.6 g, 11.1 mmol), toluene (50 mL), 50% aqueous sodium hydroxide (40 mL), 80 °C, 24 h. Purification was by flash chromatography (dichloromethane/acetone 40:1). Yield 3.37 g (66%), colourless syrup; [α]D + 47.1 (c 1.0, CHCI3); 1H NMR (400 MHz, CDCl3): δ 7.37–7.20 (m, 24 H, Ph), 4.61 (d, 2 H, H-1,1’), 3.53 (dd, 2 H, H-2,2’), 3.96 (dd, 2 H, H-3,3’), 3.42 (dd, 2 H, H-4,4’), 3.76 (ddd, 2 H, H-5,5’), 3.43 (dd, 2 H, H-6a,6a’), 3.32 (dd, 2 H, H-6b,b’), 3.39 (s, 6 H, OCH3, OCH3’), 4.98 (d, 2 H, OCHABn, Bn’), 4.79 (d, 2 H, OCHABn, Bn’), 4.88 (d, 2 H, OCHBBn, Bn’), 4.55 (d, 2 H, OCHB Bn, Bn’), 4.78 (d, 2 H, OCHcBn, Bn”), 4.66 (d, 2 H, OCHc Bn, Bn’); J1,2 = 3.5, J2,3 = 9.5, J3,4 = 9.5, J4,5 = 9.5, J5,6a = 2.5, J5,6b = 5.5,2 J6a,6b = 13.0, 2JBn = 10.5, 11.0 and 12.0 Hz; 13C-NMR (100 MHz, CDCl3): δ 98.06 (C-1,1’), 80.01 (C-2,2’), 81.84 (C-3,3’), 78.37 (C-4,4’), 69.93 (C-5,5’), 51.40 (C-6,6’), 55.38 (OCH3, OCH3’), 75.73, 74.76, 73.42 (6 C, OCH2Ph), 138.63–137.69 (6 C, q, Ph), 128.50–127.67 (24 C, Ph); Anal. calcd. for C50H56N6O10 (901.0): C 66.65, H 6.26, N 9.33. Found: C 66.59, H 6.29, N 9.22.
4.5 Bis[methyl-6-amino-2,3-di-O-benzyl-6-deoxy-4-yloxy-α-d-glucopyranoside]-p-xylene (7)
The synthesis followed GP2 using 6 (3.1 g, 3.44 mmol), Pd/C (5%, 500 mg), triethylamine (2 mL), methanol (50 mL), ethyl acetate (30 mL), 24 h. Yield 2.73 g (94%); colourless syrup; [α]D + 34.6 (c 1.0, CHCl3); 1H NMR (400 MHz, CDCl3): δ 7.37–7.25 (m, 24 H, Ph), 4.56 (d, 2 H, H-1,1), 3.49 (dd, 2 H, H-2,2’), 3.98 (dd, 2 H, H-3,3’), 3.33 (dd, 2 H, H-4,4’), 3.56 (ddd, 2 H, H-5,5’), 2.97 (dd, 2 H, H-6a,6a’), 2.70 (dd, 2 H, H-6b,b’), 3.37 (s, 6 H, OCH3, OCH3’), 4.98 (d, 2 H, OCHABn, Bn’), 4.80 (d, 2 H, OCHABn, Bn’), 4.86 (d, 2 H, OCHBBn, Bn’), 4.59 (d, 2 H, OCHB Bn, Bn’), 4.78 (d, 2 H, OCHCBn, Bn’), 4.66 (d, 2 H, OCHCBn, Bn’), 1.49 (b, 4 H, NH2); J1,2 = 3.5, J2,3 = 9.5, J3,4 = 9.5, J4,5 = 9.5, J5,6a = 2.5, J5,6b = 6.5, 2J6a,6b = 13.0, 2JBn = 10.5, 11.0 and 12.0 Hz; 13C NMR (100 MHz, CDCl3): δ 97.94 (C-1,1), 80.17 (C-2,2’), 82.13 (C-3,3’), 78.77 (C-4,4’), 71.69 (C-5,5’), 42.86 (C-6,6’), 55.08 (OCH3, OCH3’), 75.69, 74.61, 73.35 (6 C, OCH2Ph), 138.79–137.83 (6 C, q, Ph), 128.46–127.60 (24 C, Ph).
4.6 Bis[methyl-2,3-di-O-benzyl-6-deoxy-6-tosylamino-4-yloxy-α-d-glucopyranoside]-p-xylene (8)
The synthesis followed GP3 using 7 (2.73 g, 3.22 mmol), p-toluene sulfonic acid chloride (1.42 g, 7.4 mmol), pyridine (75 mL), −20 °C, 48 h. Purification was by flash chromatography (toluene/ethyl acetate 1:1). Yield 3.14 g (84%); glass temperature 71–78 °C; [α]D − 3.2 (c 1.0, CHCl3); 1H NMR (400 MHz, CDCl3): δ 7.68–7.23 (m, 32 H, Ph), 4.43 (d, 2 H, H-1,1’), 3.42 (dd, 2 H, H-2,2’), 3.93 (dd, 2 H, H-3,3’), 3.41 (dd, 2 H, H-4,4’), 3.63 (ddd, 2 H, H-5,5’), 3.15–3.07 (m, 4 H, H-6a,6a’, H-6b,b’), 3.26 (s, 6 H, OCH3, OCH3’), 4.96 (d, 2 H, OCHABn, Bn’), 4.76 (d, 2 H, OCHABn, Bn’), 4.84 (d, 2 H, OCHBBn, Bn’), 4.58 (d, 2 H, OCHBBn, Bn’), 4.80 (d, 2 H, OCHCBn, Bn’), 4.61 (d, 2 H, OCHCBn, Bn’), 4.63 (dd, 2 H, NHTs), 2.38 (s, 6 H, CH3Ts); J1,2 = 3.5, J2,3 = 9.5, J3,4 = 9.5, J4,5 = 9.5, J5,6 = 4.0 and 5.0, J6,NH = 6.0 and 6.5, 2JBn = 10.5, 11.5 and 12.0 Hz; 13C NMR (100 MHz, CDCl3): δ 98.15 (C-1,1’), 81.76, 79.82, 78.06 (C-2,2’, C-3,3’, C-4,4’), 68.91 (C-5,5’), 43.58 (C-6,6’), 55.37 (OCH3, OCH3’), 75.70, 74.69, 73.51 (6 C, OCH2Ph), 21.50 (2 C, CH3Ts), 143.52–136.73 (10 C, q, Ph), 129.76–127.14 (32 C, Ph); Anal. calcd. for C64H72N2O14S2 (1157.4): C 66.42, H 6.27, N 2.42, S 5.54. Found: C 66.13, H 6.33, N 2.41, S 5.55.
4.7 Bis[methyl-6-azido-2,3-di-O-benzyl-6-deoxy-4-yloxy-α-d-glucopyranoside]-m-xylene (9)
The preparation followed GP1 using 1 (16.2 g, 40.5 mmol), α,α’-dibromo-m-xylene (5.88 g, 22.3 mmol), tetrabutylammonium bromide (6.4 g, 20 mmol), toluene (250 mL), 50% aqueous sodium hydroxide (100 mL), 60 °C, 24 h. Purification was by flash chromatography (dichloromethane/acetone 40:1). Yield 15.6 g (86%); colourless syrup; [α]D + 53.8 (c 1.0, CHCl3); 1H NMR (400 MHz, CHCI3): δ 7.38–7.18 (m, 24 H, Ph), 4.63 (d, 2 H, H-1,1’), 3.55 (dd, 2 H, H-2,2’), 3.98 (dd, 2 H, H-3,3’), 3.43 (dd, 2 H, H-4,4’), 3.78 (ddd, 2 H, H-5,5’), 3.42 (dd, 2 H, H-6a,6a’), 3.22 (dd, 2 H, H-6b,b’), 3.41 (s, 6 H, OCH3, OCH3’), 4.99 (d, 2 H, OCHABn, Bn’), 4.80 (d, 2 H, OCHABn, Bn’), 4.90 (d, 2 H, OCHBBn, Bn’), 4.56 (d, 2 H, OCHB Bn, Bn’), 4.79 (d, 2 H, OCH2Bn, Bn’), 4.67 (d, 2 H, OCHC Bn, Bn’); J1,2 = 3.5, J2,3 = 9.5, J3,4 = 9.5, J4,5 = 9.5, J5,6a = 2.5, J5,6b = 5.5,2J6a,6b = 13.0,2JBn = 10.5, 11.0 and 12.0 Hz; 13C NMR (100 MHz, CDCl3): δ 98.05 (C-1,1’), 80.01 (C-2,2’), 81.80 (C-3,3’), 78.46 (C-4,4’), 69.92 (C-5,5’), 51.39 (C-6,6’), 55.38 (OCH3, OCH3’), 75.69, 74.90, 73.41 (6 C, OCH2Ph), 138.61-138.04 (6 C, q, Ph), 129.04-127.08 (24 C, Ph); Anal. calcd. for C50H56N6O10 (901.0): C 66.65, H 6.26, N 9.33. Found: C 66.48, H 6.28, N 9.21.
4.8 Bis[methyl-6-amino-2,3-di-O-benzyl-6-deoxy-4-yloxy-α-d-glucopyranoside]-m-xylene (10)
The synthesis followed GP2 using 9 (15.4 g, 17.1 mmol), Pd/C (5%, 1.5 g), triethylamine (5 mL), methanol (300 mL), 5 d. Yield 14.0 g (96%); colourless syrup [a]D + 35.3 (c 1.0, CHCl3); 1H NMR (400 MHz, CDCl3): δ 7.35-7.17 (m, 24 H, Ph), 4.54 (d, 2 H, H-1,1’), 3.47 (dd, 2 H, H-2,2’), 3.96 (dd, 2 H, H-3,3’), 3.31 (dd, 2 H, H-4,4’), 3.53 (ddd, 2 H, H-5,5’), 2.94 (dd, 2 H, H-6a,6a’), 2.68 (dd, 2 H, H-6b,6b’), 3.35 (s, 6 H, OCH3, OCH3’), 4.95 (d, 2 H, OCHABn, Bn’), 4.77 (d, 2 H, OCHABn, Bn’), 4.84 (d, 2 H, OCHBBn, Bn’), 4.56 (d, 2 H, OCHB Bn, Bn’), 4.76 (d, 2 H, OCHCBn, Bn’), 4.63 (d, 2 H, OCHC Bn, Bn’), 1.39 (b, 4 H, NH2); J1,2 = 3.5, J2,3 = 9.5, J3,4 = 9.5, J4,5 = 9.5, J5,6a = 2.5, J5,6b = 6.5, 2J6a,6b = 13.0, 2JBn = 11.0, 11.0 and 12.0 Hz; 13C NMR (100 MHz, CDCl3): δ 97.94 (C-1,1’), 80.17 (C-2,2’), 82.11 (C-3,3’), 78.89 (C-4,4’), 71.82 (C-5,5’), 42.86 (C-6,6’), 55.08 (OCH3, OCH3’), 75.66, 74.78, 73.34 (6 C, OCH2Ph), 138.79-138.19 (6 C, q, Ph), 128.46-127.27 (24 C, Ph).
4.9 Bis[methyl-2,3-di-O-benzyl-6-deoxy-6-tosylamino-4-yloxy-α-d-glucopyranoside]-m-xylene (11)
The preparation followed GP3 employing 10 (13.9 g, 16.4 mmol), p-toluene suIfonic acid chloride (7.2 g, 37.7 mmol), pyridine (350 mL), –20 °C, 4 d. Purification was by flash chromatography (dichloromethane/acetone 20:1). Yield 14.4 g (76%); glass temperature: 58-60 °C; [α]D +9.9 (c 1.0, CHCl3); 1H NMR (400 MHz, CDCl3): δ 7.65–7.20 (m, 32 H, Ph), 4.42 (d, 2 H, H-1,1’), 3.41 (dd, 2 H, H-2,2’), 3.92 (dd, 2 H, H-3,3’), 3.40 (dd, 2 H, H-4,4’), 3.63 (ddd, 2 H, H-5,5’), 3.12-3.07 (m, 4 H, H-6a,6a’, H-6b, 6b’), 3.25 (s, 6 H, OCH3, OCH3’), 4.93 (d, 2 H, OCHABn, Bn’), 4.78 (d, 2 H, OCHA Bn, Bn’), 4.84 (d, 2 H, OCHBBn, Bn’), 4.62 (d, 2 H, OCHBBn, Bn’), 4.74 (d, 2 H, OCHCBn, Bn’), 4.59 (d, 2 H, OCHc Bn, Bn’), 4.60 (dd, 2 H, NHTs), 2.36 (s, 6 H, CH3Ts); J1,2 = 3.5, J2,3 = 9.5, J3,4 = 9.5, J4,5 = 9.5, J5,6 = 4.5 and 5.0, J6NH = 6.0 and 6.0, 2JBn = 10.5, 11.5 and 12.0 Hz; 13C NMR (100 MHz, CDCl3): δ 98.09 (C-1,1’), 78.26 (C-2,2’), 81.63 (C-3,3’), 79.82 (C-4,4’), 68.91 (C-5,5’), 43.69 (C-6,6’), 55.34 (OCH3, OCH3’), 75.65, 74.77, 73.46 (6 C, OCH2Ph), 21.49 (2 C, CH3Ts), 143.42-136.67 (10 C, q, Ph), 129.71–127.10 (32 C, Ph); Anal. calcd. for C64H72N2O4S2 (1157.4): C 66.42, H 6.27, N 2.42, S 5.54. Found: C 65.80, H 6.29, N 2.32, S 5.88.
4.10 Bis-1,11-[methyl-6-azido-2,3-di-O-benzyl-6-deoxy-4-yloxy-α-d-glucopyranoside]-(6-tosyl-3,9-dioxa-6-aza-undecane) (12)
The formation followed GP4 employing 2 (7.66 g, 15.1 mmol), p-toluene sulphonamide (1.43 g, 8.33 mmol), cesium carbonate (10 g, 30.7 mmol), DMF (150 mL), 80 °C, 3 d. Purification was by flash chromatography (dichloromethane/acetone 30:1). Yield 7.09 g (85%); colourless syrup; [α]D + 46.1 (c 1.0, CHCl3); 1H NMR (400 MHz, CDCl3): δ 7.66–7.24 (m, 24 H, Ph), 4.60 (d, 2 H, H-1,1’), 3.47 (dd, 2 H, H-2,2’), 3.88 (dd, 2 H, H-3,3’), 3.23 (dd, 2 H, H-4,4), 3.73 (ddd, 2 H, H-5,5’), 3.42 (dd, 2 H, H-6a,6a), 3.40 (dd, 2 H, H-6b,b), 3.39 (s, 6 H, OCH3, OCH3), 4.92 (d, 2 H, OCHABn, Bn’), 4.77 (d, 2 H, OCHABn, Bn’), 4.77 (d, 2 H, OCHBBn, Bn’), 4.64 (d, 2 H, OCHB Bn, Bn’), 3.93–3.89 (m, 2 H, OCHA), 3.66–3.60 (m, 2 H, OCHA), 3.55-3.40 (m, 8 H, OCH2), 3.30 (dd, 4 H, CH2NTs), 2.39 (s, 3 H, CH,Ts); J1,2 = 3.5, J2,3 = 9.5, J3,4 = 9.0, J4,5 = 9.5, J5,6a = 5.5, J5,6b = 2.5, 2J6a,6b = 12.0, JCH,CH = 6.0, 2JBn = 11.0 and 12.0 Hz; 13C NMR (100 MHz, CDC13): δ 98.05 (C-1,1’), 81.57, 79.87, 79.01 (C-2,2’, C-3,3’, C-4,4’), 70.46 (C-5,5’), 48.80 (C-6,6’), 55.35 (OCH3, OCH3’), 75.58, 73.40, 72.24, 70.46, 70.00 (10 C, OCH2, OCH2Ph), 48.80 (2 C, CH2NTs), 21.48 (CH3Ts), 138.70–138.06 (6 C, q, Ph), 129.64–127.18 (24 C, Ph).
4.11 Bis-1,11-[methyl-6-amino-2,3-di-O-benzyl-6-deoxy-4-yloxy-α-d-glucopyranoside]-(6-tosyl-3,9-dioxa-6-aza-undecane) (13)
The synthesis followed GP2 using 12 (6.95 g, 6.26 mmol), Pd/C (5%, 1.0 g), triethylamine (3 mL), methanol (120 mL), 3 d. Yield 6.22 g (94%); colourless syrup; [a]D + 36.4 (c 1.0, CHCl3); 1H NMR (400 MHz, CDCl3): δ 7.67-7.23 (m, 24 H, Ph), 4.55 (d, 2 H, H-1,1’), 3.44 (dd, 2 H, H-2,2’), 3.88 (dd, 2 H, H-3,3’), 3.18 (dd, 2 H, H-4,4’), 3.53 (ddd, 2 H, H-5,5’), 3.01 (dd, 2 H, H-6a,6a’), 2.77 (dd, 2 H, H-6b,b’), 3.36 (s, 6 H, OCH3, OCH3’), 4.92 (d, 2 H, OCHABn, Bn’), 4.77 (d, 2 H, OCHABn, Bn’), 4.75 (d, 2 H, OCHBBn, Bn’), 4.63 (d, 2 H, OCHB Bn, Bn’), 3.93-3.86 (m, 2 H, OCHA), 3.70-3.63 (m, 2 H, OCHA), 3.55-3.40 (m, 8 H, OCH2), 3.30 (dd, 4 H, CH2NTs), 2.39 (s, 3 H, CH3Ts), 1.88 (b, 4 H, NH2); J1,2 = 3.5, J2,3 = 9.0, J3,4 = 9.0, J4,5 = 9.5, J5,6a = 3.0, J5,6b = 6.0, 2J6a,6b = 13.0, JCH,CH = 6.5, 2JBn = 11.0 and 12.0 Hz; 13C NMR (100 MHz, CDCl3): δ 97.92 (C-1,1’), 80.03 (C-2,2’), 81.86 (C-3,3’), 79.91 (C-4,4’), 71.09 (C-5,5’), 42.80 (C-6,6’), 55.12 (OCH3, OCH3’), 75.52, 73.32 (4 C, OCH2Ph), 71.57, 70.66, 69.92 (6 C, OCH2), 48.85 (2 C, CH2NTs), 21.48 (CH3Ts), 138.80-136.66 (6 C, q, Ph), 129.66-126.96 (24 C, Ph).
4.12 Bis-1,11-[methyl-2,3-di-O-benzyl-6-deoxy-6-tosyIamino-4-yloxy-α-d-glucopyranoside]-(6-tosyl-3,9-dioxa-6-aza-undecane) (14)
The preparation followed GP3 employing 13 (6.22 g, 5.88 mmol), p-toluene sulfonic acid chloride (2.58 g, 13.5 mmol), pyridine (200 mL), −20 °C, 24 h. Purification was by flash-chromatography (dichloromethane/acetone 14:1). Yield 4.28 g (53%); glass temperature 46-49 °C; [α]D + 12.1 (c 1.0, CHCl3); 1H NMR (400 MHz, CDCl3): δ 7.75–7.23 (m, 32 H, Ph), 4.40 (d, 2 H, H-1,1’), 3.33 (dd, 2 H, H-2,2’), 3.79 (dd, 2 H, H-3,3’), 3.14 (dd, 2 H, H-4,4’), 3.70-3.30 (m, 16 H, H-5,5’, H-6a,6a’, H-6b,b’, OCH2), 3.21 (s, 6 H, OCH3, OCH3’), 5.33 (dd, 2 H, NHTs), 4.88 (d, 2 H, OCHABn, Bn’), 4.72 (d, 2 H, OCHABn, Bn’), 4.71 (d, 2 H, OCHBBn, Bn’), 4.57 (d, 2 H, OCHBBn, Bn’), 3.93–3.87 (m, 2 H, OCH2), 3.23–3.10 (m, 4 H, CH2NTs), 2.37, 2.36 (s, 9 H, CH3Ts); J1,2 = 3.5, J2,3 = 9.0, J3,4 = 9.0, J4,5 = 9.5, J6,NH = 6.0 and 6.5, 2JBn = 11.5 and 12.0 Hz. 13C NMR (100 MHz, CDCl3): δ 98.03 (C-1,1’), 79.77 (C-2,2’), 81.46 (C-3,3’), 80.64 (C-4,4’), 68.34 (C-5,5’), 44.71 (C-6,6’), 55.24 (OCH3, OCH3’), 75.44, 73.40 (4 C, OCH2Ph), 72.27, 70.49, 69.81 (6 C, OCH2), 48.58 (2 C, CH2NTs), 21.49 (3 C, CH3Ts), 138.65–137.10 (10 C, q, Ph), 129.69–127.13 (32 C, Ph).
4.13 1,8-Bis-p-toluenesulfonyloxy-3,6-dithia-octane (15)
1,8-Dihydroxy-3,6-dithia-octane (15 g, 82 mmol) were dissolved in pyridine (200 mL) cooled to −20 °C and p-toluene sulfonic acid chloride (34.5 g, 181 mmol) added in portions. After 4 h the solution was poured onto iced water (300 mL) and diethylether (300 mL). The combined organic phases were extracted twice with cold 2 N hydrochloric acid (300 mL) and ice water (300 mL), dried over Na2SO4 and evaporated to give 29. Yield 28.6 g (71%), mp 71 °C; 1H NMR (400 MHz, CDCl3): δ 7.78 (m, 4 H, Ph), 7.36 (m, 4 H, Ph), 4.14 (dd, 4 H, CH2OTs), 2.79 (dd, 4 H, CH2CH2OTs), 2.67 (s, 6 H, CH2S), 2.45 (s, 6 H, CH3Ts); JCH,CH = 7.0 Hz; Anal. calcd. for C20H26O6 (490.7): C 48.96, H 5.34, S 26.14. Found: C 48.76, H 5.38, S 26.21.
4.14 Cyclo-(N,N’)-1,2- {bis-1,5-(methyl-2,3-di-O-benzyl-6-deoxy-6-tosylamino-4-yloxy-α-d-glucopyranoside]-(3-oxapentane)}-ethane (16)
The preparation followed GP4 using 5 (495 mg, 0.44 mmol), ethylene glycol ditosylate (163 mg, 0.44 mmol), cesium carbonate (717 mg, 2.2 mmol), DMF (10 mL), 80 °C, 24 h. Purification was by flash chromatography (toluene/ethyl acetate 4:1). Yield 362 mg (71%); mp 145 °C; [α]D + 5.0 (c 0.5, CHCl3); 1HMR (400 MHz, CDCl3): δ 7.73–7.19 (m, 28 H, Ph), 4.24 (d, 2 H, H-1,1’), 3.42 (dd, 2 H, H-2,2’), 3.83 (dd, 2 H, H-3,3’), 3.09 (dd, 2 H, H-4,4’), 3.85–3.60 (m, 8 H, H-5,5’, H-6a,6a’, OCH2), 3.23 (dd, 2 H, H-6b,6b’), 2.75 (s, 6 H, OCH3, OCH3’), 4.91 (d, 2 H, OCHABn, Bn’), 4.72 (d, 2 H, OCHABn, Bn’), 4.74 (d, 2 H, OCHBBn, Bn’), 4.58 (d, 2 H, OCHB Bn, Bn’), 3.54–3.36 (m, 8 H, OCH2, CH2NTs), 2.40 (s, 6 H, CH3Ts); J1,2 = 3.5, J2,3 = 9.5, J3,4 = 9.5, J4,5 = 9.5, J5,6b = 9.0, 2J6a,6b = 15.0, 2JBn = 11.0 and 12.0 Hz; 13C NMR (100 MHz, CDCl3): δ 97.16 (C-1,1’), 80.18 (C-2,2’), 82.00 (C-3,3’), 79.88 (C-4,4’), 68.38 (C-5,5’), 49.79 (C-6,6’), 54.48 (OCH3 OCH3’) 75.57, 73.49 (4 C, OCH2Ph), 71.67, 71.16 (4 C, OCH2), 46.21 (2 C, CH2NTs), 21.47 (2 C, CH3Ts), 142.85–137.65 (8 C, q, Ph), 129.17–127.51 (28 C, Ph); Anal. calcd. for C62H74N2O15S2 (1151.4): C 64.68, H 6.48, N 2.43, S 5.57. Found: C 63.89, H 6.44, N 2.39, S 5.66. FAB-MS: m/z (%) = 1174 (90) [M+Na+], 996 (100) [M+-CH3C6H4SO2].
4.15 Cyclo-(N,N’)-1,5-{bis-1,5-[methyl-2,3-di-O-benzyl-6-deoxy-6-tosylamino-4-yloxy-α-d-glucopyranoside]-(3-oxapentane)}-(3-oxapentane) (17)
The synthesis followed GP4 using 5 (680 mg, 0.61 mmol), diethylene glycol ditosylate (252 mg, 0.61 mmol), cesium carbonate (977 mg, 3.0 mmol), DMF (10 mL), 80 °C, 3 d. Purification was by flash chromatography (toluene/ethyl acetate 4:1). Yield 392 mg (54%); glass temperature 68–70 °C; [α]D + 9.9 (c 1.0, CHCl3); 1H NMR (400 MHz, CDCl3): δ 7.73–7.22 (m, 28 H, Ph), 4.36 (d, 2 H, H-1,1’), 3.40 (dd, 2 H, H-2,2’), 3.66 (dd, 2 H, H-3,3’), 3.06 (dd, 2 H, H-4,4’), 3.73–3.44 (m, 16 H, H-5,5’, H-6a,6a’ H-6b,6b’, OCH2, CH2NTs), 2.88 (s, 6 H, OCH3, OCH3’), 4.88 (d, 2 H, OCHABn, Bn’), 4.68 (d, 2 H, OCHA Bn, Bn’), 4.72 (d, 2 H, OCHBBn, Bn’), 4.55 (d, 2 H, OCHB Bn, Bn’), 4.00–3.89 (m, 4 H, OCH2), 4.40–4.33 (m, 2 H, CH2NTs), 2.37 (s, 6 H, CH3Ts); J1,2 = 3.5, J2,3 = 9.0, J3,4 = 9.0, J4,5 = 9.0, 2JBn = 11.0 and 12.0 Hz; 13C NMR (100 MHz, CDCl3): δ 97.72 (C-1,1’), 79.75 (C-2,2’), 82.04 (C-3,3’), 80.53 (C-4,4’), 70.84 (C-5,5’), 54.77 (OCH3, OCH3’), 75.24, 73.39 (4 C, OCH2Ph), 73.16, 70.94, 70.08 (6 C, OCH2), 47.94, 47.68 (4 C, C-6,6’, CH2NTs), 21.46 (2 C, CH3Ts), 142.79–138.18 (8 C, q, Ph), 129.48–127.36 (28 C, Ph); Anal. calcd. for C64H78N2O16S2 (1195.5): C 64.30, H 6.58, N 2.34, S 5.36. Found: C 64.06, H 6.59, N 2.26, S 5.44. FAB-MS: m/z (%) =1218 (100) [M+Na+].
4.16 Cyclo-(N,N’)-1,5-{bis-1,5-[methyl-2,3-di-O-benzyl-6-deoxy-6-tosylamino-4-yloxy-α-d-glucopyranoside]-(3-oxapentane)}-(3-tosyl-3-azapentane) (18)
This preparation was by GP4 employing 5 (450 mg, 0.40 mmol), diethanol amine tritosylate (228 mg, 0.40 mmol), cesium carbonate (652 mg, 2.0 mmol), DMF (10 mL), 80 °C, 3 d. Purification was by flash chromatography (toluene/ ethyl acetate 5:1). Yield 390 mg (73%); glass temperature 85–86 °C; [α]D = + 34.2 (c 1.0, CHCl3); 1H NMR (400 MHz, CDCl3): δ 7.75–7.16 (m, 32 H, Ph), 4.35 (d, 2 H, H-1,1’), 3.48 (dd, 2 H, H-2,2’), 3.78 (dd, 2 H, H-3,3’), 3.20 (dd, 2 H, H-4,4’), 3.60 (ddd≈dd, 2 H, H-5,5’), 3.76–3.27 (m, 18 H, H-6a,6a’, H-6b,6b’, OCH2, CH2NTs), 2.84 (s, 6 H, OCH3, OCH3’), 4.88 (d, 2 H, OCHABn, Bn’), 4.78 (d, 2 H, OCHABn, Bn’), 4.70 (d, 2 H, OCHBBn, Bn’), 4.55 (d, 2 H, OCHB Bn, Bn’), 3.93–3.86 (m, 2 H, OCH2), 2.44, 2.43 (s, 9 H, CH3Ts); J1,2 = 3.5, J2,3 = 9.0, J3,4 = 9.0, J4,5 = 9.5, J5,6 = 9.5, 2JBn = 11.0 and 12.0 Hz; 13C NMR (100 MHz, CDCl3): δ 97.65 (C-1,1’), 80.36 (C-2,2’), 82.21 (C-3,3’), 79.60 (C-4,4’), 68.55 (C-5,5’), 54.98 (OCH3, OCH3’), 75.43, 73.50 (4 C, OCH2Ph), 71.54, 71.13 (4 C, OCH2), 50.17, 49.74, 47.84 (6 C, C-6,6’, CH2NTs), 21.47 (3 C, CH3Ts), 143.11–137.18 (10 C, q, Ph), 129.83–127.38 (32 C, Ph); Anal. calcd. for C71H85N3O7S3 (1348.7): C 63.23, H 6.35, N 3.12, S 7.13: Found: C 63.12, H 6.44, N 3.12, S 7.01. FAB-MS: m/z (%) = 1370 (100) [M+Na+], 1193 (75) [M+-CH3C6H3SO2].
4.17 Cyclo-(N,N’)-1,8-{bis-1,5-[methyl-2,3-di-O-benzyI-6-deoxy-6-tosylamino-4-yloxy-α-d-glucopyranoside]-(3-oxapentane)}-(3,6-dithia-octane) (19)
The synthesis was done by GP4 employing 5 (310 mg, 0.28 mmol), 1,8-bis-p-toluene sulfonyloxy-3,6-dithia-octane (15, 135 mg, 0.28 mmol), cesium carbonate (450 mg, 1.38 mmol), DMF (10 mL), 20 °C, 5 d. Purification was by flash chromatography (toluene/ethyl acetate 4:1). Yield 132 mg (38%); glass temperature 59–62 °C; [α]D + 12.5 (c 1.0, CHCl3); 1H NMR (400 MHz, CDCl3): δ 7.71–7.19 (m, 28 H, Ph), 4.32 (d, 2 H, H-1,1’), 3.37 (dd, 2 H, H-2,2’), 3.73 (dd, 2 H, H-3,3’), 2.99 (dd, 2 H, H-4,4’), 3.74–3.34 (m, 16 H, H-5,5’, H-6a,6a’, H-6b,6b’, OCH2, CH2NTs), 2.93 (s, 6 H, OCH3, OCH3’), 4.89 (d, 2 H, OCHABn, Bn’), 4.71 (d, 2 H, OCHABn, Bn’), 4.70 (d, 2 H, OCHBBn, Bn’), 4.55 (d, 2 H, OCHB Bn, Bn’), 3.93–3.85 (m, 2 H, OCH2), 2.86–2.72 (m, 8 H, CH2S), 2.36 (s, 6 H, CH3Ts); J1,2 = 3.5, J2,3 = 9.5, J3,4 = 9.0, J4,5 = 9.5, 2JBn = 11.0 and 12.0 Hz; 13C NMR (100 MHz, CDCl3): δ 97.59 (C-1,1’), 79.70 (C-2,2’), 81.83 (C-3,3’), 80.42 (C-4,4’), 68.90 (C-5,5’), 54.98 (OCH3, OCH3’), 75.48, 73.46 (4 C, OCH2Ph), 72.41, 70.83 (4 C, OCH2), 48.74, 47.57 (4 C, C-6,6’, CH2NTs), 32.50, 30.83 (4 C, CH2S), 21.46 (2 C, CH3Ts), 143.03–137.82 (8 C, q, Ph), 129.38–127.53 (28 C, Ph); C66H82N2O15S4 (1271.7); FAB-MS: m/z (%) = 1293 (100) [M+Na+], 1240 (80) [M+ -OCH3].
4.18 Cyclo-(N,N’)-1,5-{bis[methyl-2,3-di-O-benzyI-6-deoxy-6-tosylamino-4-yloxy-α-d-glucopyranoside]-p-xylene}-(3-oxapentane) (20)
The preparation followed GP4 using 8 (500 mg, 0.43 mmol), diethylene glycol ditosylate (210 mg, 0.5 mmol), cesium carbonate (750 mg, 2.3 mmol), DMF (10 mL), 70 °C, 24 h. Purification was by flash chromatography (toluene/ethyl acetate 1:1). Yield 382 mg (72%); glass temperature 105 °C; [α]D + 49.4 (c 1.0, CHCl3); 1H NMR (400 MHz, CDCl3): δ 7.69–7.22 (m, 32 H, Ph), 4.41 (d, 2 H, H-1,1’), 3.46 (dd, 2 H, H-2,2’), 3.88 (dd, 2 H, H-3,3’), 3.05 (dd, 2 H, H-4,4’), 3.52–3.41 (m, 6 H, H-5,5’, OCH2), 3.39 (dd≈d, 2 H, H-6a,6a’), 2.92 (dd, 2 H, H-6b,6b’), 2.95 (s, 6 H, OCH3, OCH3’), 5.01 (d, 2 H, OCHABn, Bn’), 4.84 (d, 2 H, OCHABn, Bn’), 4.87 (d, 2 H, OCHBBn, Bn’), 4.52 (d, 2 H, OCHB Bn, Bn’), 4.73 (d, 2 H, OCHCBn, Bn’), 4.60 (d, 2 H, OCHCBn, Bn’), 3.33–3.25 (m, 4 H, CH2NTs), 2.41 (s, 6 H, CH3Ts); J1,2 = 3.5, J2,3 = 9.5, J3,4 = 9.5, J4,5 = 9.5, J5,6a ≈ 0, J5,6b = 10.0, 2J6a,6b = 15.5, 2JBn = 11.0, 11.5 and 12.0 Hz; 13C NMR (100 MHz, CDCl3): δ 97.48 (C-1,1’), 77.53 (C-2,2’), 79.76 (C-3,3’), 82.43 (C-4,4’), 69.74 (C-5,5’), 48.50 (C-6,6’), 54.84 (OCH3, OCH3’), 75.67, 74.14, 73.36 (6 C, OCH2Ph), 69.68 (2 C, OCH2), 46.40 (2 C, CH2NTs), 21.50 (2 C, CH3Ts), 142.94–137.54 (10 C, q. Ph), 129.52–127.41 (32 C, Ph); Anal. calcd. for C68H78N2O15S2 (1227.5): C 66.54, H 6.40, N 2.28, S 5.22. Found: C 65.89, H 6.45, N 2.25, 5.24.
4.19 Cyclo-(N,N’)-15-{bis[methyl-2,3-di-O-benzyl-6-deoxy-6-tosylamino-4-yloxy-α-d-glucopyranoside]-p-xylene}-(3-tosyl-3-aza-pentane) (21)
The synthesis followed GP4 using 8 (512 mg, 0.44 mmol), diethanol amine tritosylate (290 mg, 0.51 mmol), cesium carbonate (750 mg, 2.3 mmol), DMF (10 mL), 70 °C, 24 h. Purification was by flash chromatography (toluene/ethyl acetate 2:1). Yield 445 mg (73%); glass temperature 85–87 °C; [α]D + 46.3 (c 1.0, CHCl3); 1H NMR (400 MHz, CDCl3): δ 7.73–7.10 (m, 36 H, Ph), 4.39 (d, 2 H, H-1,1’), 3.42 (dd, 2 H, H-2,2’), 3.92 (dd, 2 H, H-3,3’), 2.94 (dd, 2 H, H-4,4’), 3.62 (ddd, 2 H, H-5,5’), 3.36 (dd, 2 H, H-6a,6a’), 2.53 (dd, 2 H, H-6b,6b’), 2.96 (s, 6 H, OCH3, OCH3’), 5.04 (d, 2 H, OCHABn, Bn’), 4.88 (d, 2 H, OCHA Bn, Bn’), 4.84 (d, 2 H, OCHBBn, Bn’), 4.50 (d, 2 H, OCHB Bn, Bn’), 4.74 (d, 2 H, OCHCBn, Bn’), 4.63 (d, 2 H, OCHcBn, Bn’), 3.40–3.32 (m, 4 H, CH2NTs), 3.23–3.08 (m, 4 H, CH2NTs), 2.43, 2.40 (s, 9 H, CH3Ts); J1,2 = 3.5, J2,3 = 9.5, J3,4 = 9.0, J4,5 = 9.5, J5,6a = 2.5, J5,6b = 9.0, 2J6a,6b = 15.0, 2JBn =11.0, 12.0 and 12.0 Hz; 13C NMR (100 MHz, CDCl3): δ 97.38 (C-1,1’), 79.67 (C-2,2’), 82.25 (C-3,3’), 78.44 (C-4,4’), 68.19 (C-5,5’), 50.98 (C-6,6’), 54.95 (OCH3, OCH3’), 75.74, 74.21, 73.20 (6 C, OCH2Ph), 49.08, 47.58 (4 C, CH2NTs), 21.56, 21.53 (3 C, CH3Ts), 143.35–135.84 (12 C, q, Ph), 129.72–127.64 (36 C, Ph); Anal. calcd. for C75H85N3O16S3 (1380.7): C 65.24, H 6.21, N 3.04, S 6.97. Found: C 64.85, H 6.32, N 3.00, S 7.08. FAB-MS: m/z (%) = 1402 (100) [M+Na+], 1225 (75) [M+-CH3C6H4SO2].
4.20 Cyclo-(N,N’)-{bis[methyl-2,3-di-O-benzyl-6-deoxy-6-tosylamino-4-yloxy-α-d-glucopyranoside)-p-xylene}-p-xylene (22)
The synthesis followed GP4 using 8 (450 mg, 0.39 mmol), α,α’-dibromo-p-xylene (113 mg, 0.43 mmol), cesium carbonate (650 mg, 2.0 mmol), DMF (10 mL), 70 °C, 24 h. Purification was by flash chromatography (toluene/ethyl acetate 3:1). Yield 325 mg (67%); mp 100 °C; [α]D – 1.5 (c 1.0, CHCl3); 1H NMR (400 MHz, CDCl3): δ 7.68–6.96 (m, 36 H, Ph), 4.24 (d, 2 H, H-1,1’), 3.41 (dd, 2 H, H-2,2’), 3.89 (dd, 2 H, H-3,3’), 3.02 (dd, 2 H, H-4,4’), 3.74 (ddd, 2 H, H-5,5’), 3.42 (dd, 2 H, H-6a,6a’), 3.33 (dd, 2 H, H-6b,6b’), 2.91 (s, 6 H, OCH3, OCH3’), 4.97 (d, 2 H, OCHABn, Bn’), 4.78 (d, 2 H, OCHABn, Bn’), 4.82 (d, 2 H, OCHBBn, Bn’), 4.36 (d, 2 H, OCHBBn, Bn’), 4.78 (d, 2 H, OCHCBn, Bn’), 4.60 (d, 2 H, OCHCBn, Bn’), 4.59 (d, 2 H, NCHABn, Bn’), 4.21 (d, 2 H, NCHABn, Bn’), 2.41 (s, 6 H, CH3Ts); J1,2 = 3.5, J2,3 = 9.5, J3,4 = 9.0, J4,5 = 9.0, J5,6b = 2.0, J5,6b = 9.5, 2J6a,6b = 15.0, 2JNBn = 15.0, 2JBn = 10.5, 11.5 and 12.0 Hz; 13C NMR (100 MHz, CDCl3): δ 97.45 (C-1,1’), 80.06 (C-2,2’), 81.98 (C-3,3’), 78.83 (C-4,4’), 67.54 (C-5,5’), 46.52 (C-6,6’), 54.90 (OCH3, OCH3’), 75.70, 74.33, 73.51 (6 C, OCH2Ph), 49.10 (2 C, NCH2Ph), 21.49 (2 C, CH3Ts), 142.78–135.07 (12 C, q, Ph), 129.13–127.61 (36 C, Ph); Anal. calcd. for C72H78N2O14S2 (1259.5): C 68.66, H 6.24, N 2.22, S 5.09. Found: C 68.32, H 6.35, N 2.23, S 5.13. FAB-MS: m/z (%) = 1282 (100) [M+Na+], 1103 (30) [M+-CH3C6H4SC2].
4.21 Cyclo-(N,N’)-1,2-{bis[methyl-2,3-di-O-benzyl-6-deoxy-6-tosylamino-4-yloxy-α-d-glucopyranoside]-m-xylene}-ethane (23)
The preparation followed GP4 using 11 (1.09 mg, 0.94 mmol), ethylene glycol ditosylate (348 mg, 0.94 mmol), cesium carbonate (1.63 mg, 5.0 mmol), DMF (20 mL), 80 °C, 48 h. Purification was by flash chromatography (toluene/ethyl acetate 5:1). Yield 604 mg (54%); glass temperature 91–96 °C; [α]D – 17.3 (c 1.0, CHCl3); 1H NMR (400 MHz, CDCl3): δ 7.71–6.93 (m, 32 H, Ph), 4.13 (d, 2 H, H-1,1’), 3.42 (dd, 2 H, H-2,2’), 3.89 (dd, 2 H, H-3,3’), 3.08 (dd, 2 H, H-4,4’), 3.62–3.58 (m, 4 H, H 5,5’, H-6a,6a’), 3.33 (dd≈d, 2 H, H-6b,6b’), 2.62 (s, 6 H, OCH3, OCH3’), 4.96 (d, 2 H, OCHABn, Bn’), 4.72 (d, 2 H, OCHABn, Bn’), 4.90 (d, 2 H, OCHBBn, Bn’), 4.46 (d, 2 H, OCHB Bn, Bn’), 4.79 (d, 2 H, OCHCBn, Bn’), 4.60 (d, 2 H, OCHC Bn, Bn’), 3.84–3.77 (m, 2 H, CH2NTs), 3.15–3.06 (m, 2 H, CH2NTs), 2.39 (s, 6 H, CH3Ts); J1,2 = 3.5, J2,3= 9.5, J3,4 = 9.0, J4,5 = 9.0, J5,6b ≈ 0, 2J6a,6b = 14.0, 2JBn = 11.0, 11.5 and 12.0 Hz; 13C NMR (100 MHz, CDCl3): δ 96.94 (C-1,1’), 80.03 (C-2,2’), 81.94 (C-3,3’), 79.54 (C-4,4’), 67.95 (C-5,5’), 46.32 (C-6,6’), 54.33 (OCH3, OCH3’), 75.69, 74.57, 73.53 (6 C, OCH2Ph), 50.38 (2 C, CH2NTs), 21.45 (2 C, CH3Ts), 142.81–137.54 (10 C, q, Ph), 129.04–126.00 (32 C, Ph); Anal. calcd. for C66H74N2O14S2 (1183.5): C 66.98, H 6.30, N 2.37, S 5.42. Found: C 66.97, H 6.33, N 2.29, S 5.60. FAB-MS: m/z (%) = 1205 (100) [M+Na+].
4.22 Cyclo-(N,N’)-1,5-{bis[methyl-2,3-di-O-benzyl-6-deoxy-6-tosylamino-4-yloxy-α-d-glucopyranoside]-m-xylene}-(3-oxapentane) (24)
The synthesis followed GP4 using 11 (1.0 g, 0.89 mmol), diethylene glycol ditosylate (393 mg, 0.95 mmol), cesium carbonate (1.36 g, 4.2 mmol), DMF (20 mL), 70 °C, 24 h. Purification was by flash chromatography (toluene/ethyl acetate 3:1). Yield 626 mg (57%); glass temperature 79–81 °C; [α]D + 40.6 (c 1.0, CHCl3); 1H NMR (400 MHz, CDCl3): δ 7.60–7.16 (m, 32 H, Ph), 4.39 (d, 2 H, H-1,1’), 3.49 (dd, 2 H, H-2,2’), 3.80 (dd, 2 H, H-3,3’), 3.21 (dd, 2 H, H-4,4’), 3.65–3.44 (m, 8 H, H-5,5’, OCH2, NCH2Ts), 3.91 (dd≈d, 2 H, H-6a,6a), 3.25 (dd, 2 H, H-6b,6b’), 2.90 (s, 6 H, OCH3, OCH3’), 4.97 (d, 2 H, OCHABn, Bn’), 4.80 (d, 2 H, OCHA Bn, Bn’), 4.89 (d, 2 H, OCHBBn, Bn’) 4.66 (d, 2 H, OCHB Bn, Bn’), 4.72 (d, 2 H, OCHCBn, Bn’), 4.59 (d, 2 H, OCHC Bn, Bn’), 3.16–3.10 (m, 2 H, CH2NTs), 2.36 (s, 6 H, CH3Ts); J1,2 = 3.5, J1,3 = 9.5, J3,4 = 9.5, J4,5 = 9.5, J5,6a = 0, J5,6b = 9.5, 2J6a,6b = 15.5, 2JBn = 11.0, 11.5 and 12.0 Hz; 13C NMR (100 MHz, CDCl3): δ 97.56 (C-1,1’), 79.71 (C-2,2’), 82.37 (C-3,3’), 79.16 (C-4,4’), 69.81 (C-5,5’), 48.37 (C-6,6’), 54.72 (OCH3, OCH3’), 75.49, 74.92, 73.40 (6 C, OCH2Ph), 70.02 (2 C, OCH2), 46.98 (2 C, CH2NTs), 21.47 (2 C, CH3Ts), 142.89–138.10 (10 C, q, Ph), 129.43–127.26 (32 C, Ph); Anal. calcd. for C68H78N2O15S2 (1227.5): C 66.54, H 6.40, N 2.28, S 5.22. Found: C 66.37, H 6.44, N 2.22, S 5.39. FAB-MS: m/z (%) = 1250 (100) [M+Na+].
4.23 Cyclo-(N,N’)-1,5-{bis[methyl-2,3-di-O-benzyl-6-deoxy-6-tosyIamino-4-yloxy-α-d-glucopyranoside]-m-xylol}-(3-tosyl-3-azapentane) (25)
The synthesis followed GP4 using 11 (1.0 g, 0.89 mmol), diethanolamine tritosylate (539 mg, 0.95 mmol), cesium carbonate (1.36 g, 4.2 mmol), DMF (20 mL), 70 °C, 24 h. Purification was by flash chromatography (toluene/ethyl acetate 3:1). Yield 498 mg (41%); glass temperature 87–90 °C; [α]D + 20.2 (c 1.0, CHCl3); 1H NMR (400 MHz, CDCl3): δ 7.68–7.03 (m, 36 H, Ph), 4.36 (d, 2 H, H-1,1’), 3.46 (dd, 2 H, H-2,2’), 3.94 (dd, 2 H, H-3,3’), 3.17 (dd, 2 H, H-4,4’), 3.67 (ddd, 2 H, H-5,5’), 3.48–3.03 (m, 12 H, H-6a,6a’, H-6b,6b’, NCH2Ts), 2.94 (s, 6 H, OCH3, OCH3’), 4.99 (d, 2 H, OCHABn, Bn’), 4.81 (d, 2 H, OCHABn, Bn’), 4.86 (d, 2 H, OCHBBn, Bn’), 4.52 (d, 2 H, OCHD Bn, Bn’), 4.74 (d, 2 H, OCHCBn, Bn’), 4.53 (d, 2 H, OCHc Bn, Bn”), 2.43, 2.41 (s, 9 H, CH3Ts); J1,2 = 3.5, J2,3 = 9.5, J3,4 = 9.5, J4,5 = 9.5, J4,6 = 2.5 and 9.5, 3JBn = 10.5, 11.0 and 12.0 Hz; 13C NMR (100 MHz, CDCl3): δ 97.58 (C-1,1’), 79.83 (C-2,2’), 82.06 (C-3,3’), 79.62 (C-4,4’), 67.86 (C-5,5’), 54.72 (OCH3, OCH3’), 75.73, 74.58, 73.37 (6 C, OCH3Ph), 50.86, 49.08, 47.81 (6 C, C-6,6’, CH3NTs), 21.56, 21.50 (3 C, CH3Ts), 143.18–136.73 (12 C, q, Ph), 129.78–127.50(36 C, Ph); Anal. calcd. for C75H85N3O16S3 (1380.7): C 65.24, H 6.21, N 3.04, S 6.97. Found: C 64.51, H 6.21, N 2.97, S 7.04. FAB-MS: m/z (%) = 1403 (100) [M+Na+], 1225 (40) [M+ -CH3C6H4SO2].
4.24 Cyclo-(N,N’)-{bis[methyl-2,3-di-O-benzyl-6-deoxy-6-tosylamino-4-yloxy-α-d-glucopyranoside]-m-xylene}-p-xylene (26)
The preparation followed GP4 using 11 (1.0 g, 0.89 mmol), α,α’-dibromo-p-xylene (250 mg, 0.95 mmol), cesium carbonate (1.36 g, 4.2 mmol), DMF (20 mL), 70 °C, 24 h. Purification was by flash chromatography (toluene/ ethyl acetate 7:1). Yield 553 mg (49%); glass temperature 89–91 °C; [α]D – 1.0 (c 1.0, CHCl3); 1H NMR (400 MHz, CDCl3): δ 7.68–7.01 (m, 36 H, Ph), 4.26 (d, 2 H, H-1,1’), 3.40 (dd, 2 H, H-2,2’), 3.85 (dd, 2 H, H-3,3’), 3.02 (dd, 2 H, H-4,4’), 3.67 (ddd, 2 H, H-5,5’), 3.28–3.17 (m, 4 H, H-6a,6a’, H-6b,6b’), 3.00 (s, 6 H, OCH3, OCH3’), 4.94 (d, 2 H, OCHABn, Bn’), 4.72 (d, 2 H, OCHABn, Bn’), 4.76 (d, 2 H, OCHBBn, Bn’), 4.59 (d, 2 H, OCHBBn, Bn’), 4.65 (d, 2 H, OCHCBn, Bn’), 4.34 (d, 2 H, OCHCBn, Bn’), 4.54 (d, 2 H, NCHABn, Bn’), 4.16 (d, 2 H, NCHA Bn, Bn’), 2.41 (s, 6 H, CH3Ts); J1,2 = 3.5, J2,3 = 9.5, J3,4 = 9.5, J4,5 = 9.5, J5,6 = 2.5 and 9.5, 2JNBn =15.0, 2JBn = 11.0, 11.5 and 12.0 Hz; 13C NMR (100 MHz, CDCl3): δ 97.46 (C-1,1’), 80.07 (C-2,2’), 81.81 (C-3,3’), 79.17 (C-4,4’), 67.17 (C-5,5’), 46.64 (C-6,6’), 55.13 (OCH3, OCH3’), 75.70, 74.47, 73.43 (6 C, OCH2Ph), 49.66 (2 C, NCH2Ph), 21.50 (2 C, CH3Ts), 142.76–134.82 (12 C, q, Ph), 129.11–125.30 (36 C,Ph); Anal. calcd. for C72H78N2O14S2 (1259.5): C 68.66, H 6.24, N 2.22, S 5.09. Found: C 68.15, H 6.27, N 2.20, S 4.97. FAB-MS: m/z (%) = 1281 (100) [M+Na+], 1103 (40) [M+-CH3C6H4SO2].
4.25 Cyclo-(N,N’)-1,2-{bis-1,11-[methyl-2,3-di-O-benzyl-6-deoxy-6-tosylamino-4-yloxy-α-d-glucopyranoside]-(6-tosyl-3,9-dioxa-6-aza-undecane)}-ethane (27)
The preparation followed GP4 using 14 (432 mg, 0.32 mmol), ethylene glycol ditosylate (117 mg, 0.32 mmol), cesium carbonate (488 mg, 1.5 mmol), DMF (10 mL), 80 °C, 2 d. Purification was by flash chromatography (toluene/ethyl acetate 4:1). Yield 202 mg (45%); glass temperature 74–77 °C; [α]D + 28.0 (c 1.0, CHC13); 1H NMR (400 MHz, CDCl3): δ 7.72–7.22 (m, 32 H, Ph), 4.53 (d, 2 H, H-1,1’), 3.46 (dd, 2 H, H-2,2’), 3.76 (dd, 2 H, H-3,3’), 2.94 (dd, 2 H, H-4,4’), 3.66–3.62 (m, 2 H, H-5,5’), 3.58–3.17 (m, 22 H, H-6a,6a’, H-6b,6b’, OCH2, CH2NTs), 3.02 (s, 6 H, OCH3, OCH3’), 4.92 (d, 2 H, OCHABn, Bn’), 4.70 (d, 2 H, OCHABn, Bn’), 4.69 (d, 2 H, OCHBBn, Bn’), 4.60 (d, 2 H, OCHB Bn, Bn’), 3.95–3.87 (m, 2 H, OCH2), 2.40, 2.39 (s, 9 H, CH3Ts); J1,2 = 3.5, J2,3 = 9.0, J3,4 = 9.0, J4,5 = 9.5, 1JBn = 11.0 and 12.0 Hz; 13C NMR (100 MHz, CDCl3): δ 97.50 (C-1,1’), 80.49 (C-2,2’), 82.05 (C-3,3’), 80.23 (C-4,4’), 69.24 (C-5,5’), 55.00 (OCH3, OCH3’), 75.52, 73.22 (4 C, OCH2Ph), 72.45, 70.98, 70.55 (6 C, OCH2), 50.21, 49.97, 47.73 (6 C, C-6,6’, CH2NTs), 21.49 (3 C, CH3Ts), 143.18–136.90 (10 C, q, Ph), 129.71–126.98 (32 C, Ph); Anal. calcd. for C3H89N3O18S3 (1392.7): C 62.96, H 6.44, N 3.02, S 6.9. Found: C 62.43, H 6.45, N 3.08, S 7.17. FAB-MS: m/z(%) = 1414 (95) [M+Na+], 1237 (100) [M+ -CH3C6H4SO2].
4.26 Cyclo-(N,N’)-1,5-{bis-1,11-(methyl-2,3-di-O-benzyl-6-deoxy-6-tosylamino-4-yloxy-α-d-gIucopyranoside]-(6-tosyl-3,9-dioxa-6-aza-undecane)}-(3-oxapentane) (28)
The preparation followed GP4 using 14 (480 mg, 0.35 mmol), diethylene glycol ditosylate (146 mg, 0.35 mmol), cesium carbonate (570 mg, 1.8 mmol), DMF (10 mL), 80 °C, 2 d. Purification was by flash chromatography (toluene/ethyl acetate 3:1). Yield 304 mg (61%); glass temperature 62–65 °C; [α]D + 16.9 (c 1.0, CHCl3); 1H NMR (400 MHz, CDCl3): δ 7.71–7.16 (m, 32 H, Ph), 4.39 (d, 2 H, H-1,1’), 3.40 (dd, 2 H, H-2,2’), 3.75 (dd, 2 H, H-3,3’), 3.95–3.28 (m, 32 H, H-4,4’, H-5,5’, H-6a,6a’ H-6b,6b’, OCH2, CH2NTs), 2.94 (s, 6 H, OCH3, OCH3’), 4.90 (d, 2 H, OCHABn, Bn’), 4.72 (d, 2 H, OCHA Bn, Bn’), 4.69 (d, 2 H, OCHBBn, Bn’), 4.56 (d, 2 H, OCHB Bn, Bn’), 2.38, 2.35 (s, 9 H, CH3Ts); J1,2 = 3.5, J2,3 = 9.5, J3,4 = 9.5, 2JBn = 11.0 and 12.0 Hz; 13C NMR (100 MHz, CDCl3): δ 97.60 (C-1,1’), 81.84, 80.47, 79.78 (6 C, C-2,2’, C-3,3’, C-4,4’), 69.62 (C-5,5’), 54.93 (OCH3, OCH3’), 75.54, 73.34 (4 C, OCH2Ph), 72.24, 70.73, 70.08, 69.54 (8 C, OCH2), 49.06, 48.97, 47.17 (6 C, C-6,6’, CH2NTs), 21.47 (3 C, CH3Ts), 142.90–138.14 (10 C, q, Ph), 129.76–127.08 (32 C, Ph); Anal. calcd. for C75H93N3O10S3 (1436.7): C 62.70, H 6.52, N 2.92, S 6.69. Found: C 62.69, H 6.60, N 2.84, S 6.6. FAB-MS: m/z (%) = 1458 (100) [M+Na+], 1280 (35) [M+-CH3C6H4SO2].
4.27 Cyclo-(N,N’)-1,5-{bis-1,11-[methyl-2,3-di-O-benzyl-6-deoxy-6-tosylamino-4-yloxy-α-d-glucopyranoside-(6-tosyl-3,9-dioxa-6-aza-undecane)}-(3-tosyl-3-azapentane) (29)
The synthesis followed GP4 employing 14 (290 mg, 0.21 mmol), diethanol amine tritosylate (120 mg, 0.21 mmol), cesium carbonate (325 mg, 1.0 mmol), DMF (10 mL), 80 °C, 3 d. Purification was by flash chromatography (toluene/ethyl acetate 3:1). Yield 204 mg (60%); glass temperature 93 °C; [α]D + 20.7 (c 1.0, CHCl3); 1H NMR (400 MHz, CDCl3): δ 7.72–7.20 (m, 36 H, Ph), 4.39 (d, 2 H, H-1,1’), 3.42 (dd, 2 H, H-2,2’), 3.74 (dd, 2 H, H-3,3’), 2.94 (dd, 2 H, H-4,4’), 3.94–3.25 (m, 30 H, H-5,5’, H-6a,6a’ H-6b,6b’, OCH2, CH2NTs), 2.84 (s, 6 H, OCH3, OCH3’), 4.90 (d, 2 H, OCHABn, Bn’), 4.72 (d, 2 H, OCHABn, Bn’), 4.70 (d, 2 H, OCHBBn, Bn’), 4.58 (d, 2 H, OCHB Bn, Bn’), 2.44, 2.41, 2.39 (s, 12 H, CH3Ts); J1,2 = 3.5, J2,3 = 9.5, J3,4 = 9.0, J4,5 = 9.5, 2JBn = 11.0 and 12.0 Hz; 13C NMR (100 MHz, CDCl3): δ 97.54 (C-1,1’), 79.88 (C-2,2’), 81.86 (C-3,3’), 80.27 (C-4,4’), 69.22 (C-5,5’), 54.87 (OCH3, OCH3’), 75.52, 73.33 (4 C, OCH2Ph), 72.29, 70.63, 69.75 (6 C, OCHj), 49.85, 49.22, 48.71, 47.73 (8 C, C-6,6’, CH2NTs), 21.50 (4 C, CH3Ts), 143.20–138.17 (12 C, q, Ph), 129.78–127.10 (36 C, Ph); Anal. calcd. for C82H100N4O20S4 (1590.0): C 61.95, H 6.34, N 3.52, S 8.07. Found: C 61.68, H 6.43, N 3.59, S 8.27. FAB-MS: m/z (%) = 1612 (100) [M+Na+].
4.28 Cyclo-(N,N’)-{bis-1,11-[methyl-2,3-di-O-benzyl-6-deoxy-6-tosylamino-4-yloxy-α-d-glucopyranoside]-(6-tosyl-3,9-dioxa-6-aza-undecane)}-p-xylene (30)
The synthesis followed GP4 using 14 (479 mg, 0.35 mmol), α,α’-dibromo-p-xylene (93 mg, 0.35 mmol), cesium carbonate (488 mg, 1.5 mmol), DMF (10 mL), 80 °C, 3 d. Purification was by flash chromatography (toluene/ethyl acetate 4:1). Yield 278 mg (54%); glass temperature 75–82 °C; [α]D + 3.6 (c 1.0, CHCl3); 1H NMR (400 MHz, CDCl3): δ 7.74–7.21 (m, 36 H, Ph), 4.27 (d, 2 H, H-1,1’), 3.36 (dd, 2 H, H-2,2’), 3.70 (dd, 2 H, H-3,3’), 3.87–3.22 (m, 24 H, H-4,4’, H-5,5’, H-6a,6a’, H-6b,6b’, OCH2, CH2NTs), 2.88 (s, 6 H, OCH3, OCH3’), 4.90 (d, 2 H, OCHABn, Bn’), 4.69 (d, 2 H, OCHABn, Bn’), 4.72 (d, 2 H, OCHBBn, Bn’), 4.56 (d, 2 H, OCHBBn, Bn’), 4.50 (d, 2 H, NCHABn, Bn’), 4.35 (d, 2 H, NCHA Bn, Bn’), 2.41, 2.38 (s, 9 H, CH3Ts); J1,2 = 3.5, J2,3 = 9.5, J3,4 = 9.5, 2JNBn = 15.0, 2JBn= 11.0 and 12.0 Hz; 13C NMR (100 MHz, CDCl3): δ 97.57 (C-1,1’), 79.82 (C-2,2’), 81.91 (C-3,3’), 80.94 (C-4,4’), 68.29 (C-5,5’), 54.97 (OCH3, OCH3’), 75.47, 73.43 (4 C, OCH2Ph), 72.50, 70.88, 70.11 (6 C, OCH2), 50.11 (2 C, NCH2Bn), 49.42, 46.74 (4 C, C-6,6’, CH2NTs), 21.49 (3 C, CH3Ts), 142.87–135.66 (12 C, q, Ph), 129.80–127.27 (36 C, Ph).
4.29 Cyclo-(N,N’)-1,8-{bis-1,11-[methyl-2,3-di-O-benzyl-6-deoxy-6-tosylamino-4-yloxy-α-d-glucopyranoside]-(6-tosyl-3,9-dioxa-6-aza-undecane)}-(3,6-dithia-octane) (31)
The preparation followed GP4 using 14 (275 mg, 0.20 mmol), 1,8-ditosyloxy-3,6-dithia-octane (15, 108 mg, 0.22 mmol), cesium carbonate (325 mg, 1.0 mmol), DMF (10 mL), 20 °C, 3 d. Purification was by flash chromatography (toluene/ethyl acetate 5:1). Yield 88 mg (29%); glass temperature 54–58 °C; [α]D + 19.8 (c 1.0, CHCl3); 1H NMR (400 MHz, CDCl3): δ 7.70–7.20 (m, 32 H, Ph), 4.35 (d, 2 H, H-1,1’), 3.34 (dd, 2 H, H-2,2’), 3.75 (dd, 2 H, H-3,3’), 2.92 (dd, 2 H, H-4,4’), 3.58–3.53 (m, 2 H, H-5,5’), 3.83–3.77 (m, 2 H, H-6a,6a’), 2.98 (s, 6 H, OCH3, OCH3’), 4.89 (d, 2 H, OCHABn, Bn’), 4.71 (d, 2 H, OCHA Bn, Bn’), 4.69 (d. 2 H, OCHBBn, Bn’), 4.56 (d, 2 H, OCHB Bn, Bn’), 3.90–3.84 (m, 2 H, OCHA), 3.64–3.59 (m, 2 H, OCHA), 3.55–3.38 (m, 8 H, OCH2), 3.37–3.28 (m, 10 H, H-6b,6b’, CH2NTs), 2.80–2.72 (m, 4 H, CH2S), 2.69 (s, 4 H, H2S), 2.37, 2.36 (s, 9 H, CH3Ts); J1,2 = 3.5, J2,3 = 9.0, J3,4 = 9.5, J4,5 = 9.5, 2JBn = 10.5 and 12.0 Hz; 13C NMR (100 MHz, CDCl3): δ 97.56 (C-1,1’), 79.75 (C-2,2’), 81.70 (C-3,3’), 80.21 (C-4,4’), 69.22 (C-5,5’), 55.03 (OCH3, OCH3’), 75.46, 73.31 (4 C, OCH2Ph), 72.12, 70.55, 69.80 (6 C, OCH2), 48.67, 48.66, 48.01 (6 C, C-6,6, CH2NTs), 30.26, 28.89 (4 C, CH2S), 21.42 (3 C, CH3Ts), 143.17–137.45 (10 C, q, Ph), 129.75–127.01 (32 C, Ph); C77H97N3O18S5 (1513.0);FAB-MS: m/z (%) = 1534 (100) [M+Na+], 1480 (70) [M+-OCH3].
4.30 Bis-1,5-{methyl-2,3-di-O-benzyl-6-deoxy-6-[N-tosyl-N-(2-hydroxyethyl)amino-4-yloxy-α-d-glucopyranoside]}-(3-oxapentane) (32)
The synthesis followed GP4 using 5 (1.01 g, 0.9 mmol), 2-(2-chloroethoxy)-tetrahydropyran (4.43 g, 4.5 mL, 26.9 mmol), cesium carbonate (1.47 g, 4.5 mmol), DMF (10 mL), 100 °C, 24 h. The suspension was filtered over silica gel, evaporated dissolved in dichlormethane/methanol 1:1 (20 mL) and treated with conc HCl (0.5 mL) for 3 h. Following neutralisationwith NaHCO3 filtration and evaporation the raw material was purified by chromatography (dichlormethane/acetone 10:1). Yield 710 mg (66%); glass temperature 56–58 °C; [α]D + 44.5 (c 1.0, CHCl3); 1H NMR (400 MHz, CDCl3): δ 7.68–7.24 (m, 28 H, Ph), 4.47 (d, 2 H, H-1,1’), 3.40 (dd, 2 H, H-2,2’), 3.85 (dd, 2 H, H-3,3’), 3.02 (dd, 2 H, H-4,4’), 3.89 (ddd, 2 H, H-5,5’), 3.81 (dd, 2 H, H-6a,6a’), 3.16 (dd, 2 H, H-6b,6b’), 3.19 (s, 6 H, OCH3, OCH3’), 4.89 (d, 2 H, OCHABn, Bn’), 4.73 (d, 2 H, OCHABn, Bn’), 4.71 (d, 2 H, OCHABn, Bn’), 4.58 (d, 2 H, OCHB Bn, Bn’), 3.95–3.65 (m, 4 H, OCH2), 3.73–3.64 (m, 4 H, CH2OH), 3.58–3.53 (m, 2 H, CHANTs). 3.53–3.45 (m, 4 H, OCH2), 3.05–2.98 (m, 2 H, CHANTs), 3.27 (b, 2 H, OH,OH’), 2.37 (s. 6 H, CH3Ts); J1,2 = 3.5, J2,3 = 9.0, J3,4 = 9.0, J4,5 = 9.5, J5,6a = 2.5, J5,6b = 9.0, 2J6a,6b = 15.0, 2JBn = 10.5 Hz; 13C NMR (100 MHz, CDCl3): δ 97.76 (C-1,1’), 79.76 (C-2,2’), 81.54 (C-3,3’), 80.68 (C-4.4’), 69.98 (C-5,5’), 50.78 (C-6,6’), 55.27 (OCH3, OCH3’), 75.55, 73.36 (4 C, OCH2Ph), 72.06. 71.02 (4 C, OCH2), 61.63 (2 C, CH2OH), 52.21 (2 C, CH2NTs), 21.47 (2 C, CH3Ts), 143.41–136.17 (8 C, q, Ph), 129.62–127.58 (28 C, Ph).
4.31 Bis-1,5-{methyl-2,3-di-O-benzyl-6-deoxy-6-[N-tosyl-N-(2-tosyloxyethyl)amino-4-yloxy-α-d-glucopyranoside]}-(3-oxapentane) (33)
The synthesis followed GP4 using 32 (700 mg, 0.58 mmol), p-toluene sulfonic acid chloride (242 mg, 1.27 mmol), pyridine (10 mL), -20 °C, 48 h. Purification was by flash chromatography (toluene/ethyl acetate 30:1). Yield 520 mg (59%); glass temperature 55–57 °C; [α]D + 39.5 (c 1.0, CHCl3); 1H NMR (400 MHz, CDCl3): δ 7.73–7.22 (m, 36 H, Ph), 4.47 (d, 2 H, H-1,1’), 3.41 (dd, 2 H, H-2,2’), 3.82 (dd, 2 H, H-3,3’), 3.05 (dd, 2 H, H-4,4’), 3.33 (dd, 2 H, H-6a,6a’), 3.22 (dd, 2 H, H-6b,6b’), 3.11 (s, 6 H, OCH3, OCH3’), 4.87 (d, 2 H, OCHABn, Bn’), 4.73 (d, 2 H, OCHABn, Bn’), 4.72 (d, 2 H, OCHBBn, Bn’), 4.60 (d, 2 H, OCHBBn, Bn’), 4.19 (dd, 4 H, CH2OTs), 4.00–3.93 (m, 2 H, OCH2), 3.76–3.44 (m, 12 H, H-5,5’, OCH2, CH2NTs), 2.42, 2.37 (s, 12 H, CH2Ts); J1,2 = 3.5, J2,3 = 9.0, J3,4 ≈ 9.0, J4,5 = 9.0, J5,6a = 6.5, J5,6b = 7.5, 2J6a,6b = 15.0, JCH,CH= 3.5 and 6.5, 2JBn = 11.0 and 12.0 Hz; 13C NMR (100 MHz, CDCl3): δ 97.73 (C-1,1’), 79.85 (C-2,2’), 81.53 (C-3,3’), 79.74 (C-4,4’), 70.36 (C-5,5’), 50.54 (C-6,6’), 55.23 (OCH3, OCH3’), 75.47, 73.30 (4 C, OCH2Ph), 72.11, 70.95 (4 C, OCH2), 68.09 (2 C, CH2OTs), 48.20 (2 C, CH2NTs), 21.65, 21.48 (4 C, CH3Ts), 143.93–132.85 (12 C, q, Ph), 129.93–127.49 (36 C, Ph); Anal. calcd. for C78H92N2O21S4 (1521.9): C 61.56, H 6.09, N 1.84, S 8.43. Found: C 61.37, H 6.10, N 1.87, S 8.29. FAB-MS: m/z (%) = 1543 (100) [M+Na+].
4.32 Bis{methyl-2,3-di-O-benzyl-6-deoxy-6-[N-tosyl-N-(2-hydroxyethyl)amino-4-yloxy-α-d-glucopyranoside]}-m-xylene (34)
The synthesis followed GP4 using 11 (2.77 g, 39 mmol), 2-(2-chlorethoxy)-tetrahydropyran (7.8 g, 7.8 mL, 47.9 mmol), cesium carbonate (3.9 g, 12 mmol), DMF (30 mL), 80 °C, 2 d. The suspension was filtered over silica gel, evaporated dissolved in dichloromethane/methanol 1:1 (40 mL) and treated with conc HCl (0.5 mL) for 30 min. Following neutralisation with NaHCO3, filtration and evaporation the raw material was purified by chromatography (dichloromethane/acetone 1:1). Yield 2.1 g (71%); glass temperature 53–55 °C; [α]D + 44.5 (c 1.0, in CHCl3); 1H NMR (400 MHz, CDCl3): δ 7.59–7.15 (m, 32 H, Ph), 4.51 (d, 2 H, H-1,1’), 3.46 (dd, 2 H, H-2,2’), 3.96 (dd, 2 H, H-3,3’), 3.17 (dd, 2 H, H-4,4’), 4.00 (ddd, 2 H, H-5,5’), 3.73 (dd, 2 H, H-6a,6a’), 2.99 (dd, 2 H, H-6b,6b’), 3.23 (s, 6 H, OCH3, OCH3’), 4.95 (d, 2 H, OCHABn, Bn’), 4.76 (d, 2 H, OCHABn, Bn’), 4.91 (d, 2 H, OCHBBn, Bn’), 4.56 (d, 2 H, OCHB Bn, Bn’), 4.74 (d, 2 H, OCHCBn, Bn’), 4.62 (d, 2 H, OCHCBn, Bn’), 3.74 (ddd, 2 H, CHAOH), 3.65 (ddd, 2 H, CHA OH), 3.53 (ddd, 2 H, CHANTs), 2.94 (ddd, 2 H, CHANTs), 2.52 (b, 2 H, OH,OH), 2.35 (s, 6 H, CH3Ts); J1,2 = 3.5, J2,3 = 9.5, J3,4 = 9.5, J4,5 = 9.5, J5,6a = 2.5, J5,6b = 9.5, 2J6a,6b = 15.0, JCH,CH = 3.0 and 6.0, 2JCH,CH = 11.5 and 14.5, 2JBn = 11.0 and 12.0 Hz; 13C NMR (100 MHz, CDCl3): δ 97.81 (C-1,1’), 79.88 (C-2,2’), 81.61 (C-3,3’), 79.88 (C-4,4’), 70.28 (C-5,5’), 51.11 (C-6,6’), 55.34 (OCH3, OCH3’). 75.69, 74.52, 73.36 (6 C, OCH2Ph), 61.74 (2 C, CH2OH), 52.77 (2 C, CH2NTs), 21.47 (2 C, CH3Ts), 143.45–135.71 (10 C, q, Ph), 129.64–125.31 (32 C, Ph); Anal. calcd. for C6gH80N2O16S3 (1245.5): C 65.58, H 6.47, N 2.25, S 5.15. Found: C 65.16, H 6.52, N 2.13, S 5.20.
4.33 Bis{methyl-2,3-di-O-benzyl-6-deoxy-6-[N-tosyl-N-(2-tosyloxyethyl)]amino-4-yloxy-α-d-glucopyranoside]}-m-xylene (35)
The synthesis followed GP4 using 34 (1.93 g, 1.55 mmol), p-toluene sulfonic acid chloride, pyridine (40 mL), -20 °C, 5 d. Purification was by flash chromatography (toluene/ethyl acetate 5:1). Yield 2.06 g (85%); glass temperature 49–51 °C; [α]D + 27.4 (c 1.0, CHCl3); 1H NMR (400 MHz, CDCl3): δ 7.72–7.15 (m, 40 H, Ph), 4.51 (d, 2 H, H-1,1’), 3.47 (dd, 2 H, H-2,2’), 3.93 (dd, 2 H, H-3,3’), 3.21 (dd, 2 H, H-4,4’), 3.72 (ddd, 2 H, H-5,5’), 3.50 (dd, 2 H, H-6a,6a’), 3.12 (dd, 2 H, H-6b,6b’), 3.14 (s, 6 H, OCH3, OCH3’), 4.94 (d, 2 H, OCHABn, Bn’), 4.74 (d, 2 H, OCHABn, Bn’), 4.91 (d, 2 H, OCHBBn, Bn’), 4.63 (d, 2 H, OCHBBn, Bn’), 4.74 (d, 2 H, OCHCBn, Bn’), 4.63 (d, 2 H, OCHCBn, Bn’), 4.18 (dd, 4 H, CH2OTs), 3.51–3.44 (m, 2 H, CHANTs), 3.31 (ddd, 2 H, CHANTs), 2.42, 2.35 (s, 12 H, CH3Ts); J1,2 = 3.5, J2,3 = 9.5, J3,4 = 9.5, J4,5 = 9.5, J5,6a = 1.5, J5,6b = 8.0, 2J6a,6b = 15.0, JCH,CH = 6.0 and 7.5, JCH,CH = 14.5, 2JBn = 10.5. 11.0 and 12.0 Hz; 13C NMR (100 MHz, CDCl3): δ 97.72 (C-1,1’), 79.96 (C-2,2’), 81.70 (C-3,3’), 79.02 (C-4,4’), 70.51 (C-5,5’), 50.69 (C-6,6’), 55.29 (OCH3, OCH3’), 75.63, 74.50, 73.27 (6 C, OCH2Ph), 68.11 (2 C, CH2OTs), 48.40 (2 C, CH2NTs), 21.65, 21.47 (4 C, CH3Ts), 144.95–132.83 (14 C, q, Ph), 130.23–127.01 (32 C, Ph); Anal. calcd. for C82H92N2O20S4 (1553.9): C 63.38, H 5.97, N 1.80, S 8.25. Found: C 62.47, H 5.91, N 1.79, S 8.35.
4.34 1-tert-Butyldimethylsilyloxy-6-tosyloxy-3-tosyl-3-azapentane (36)
1-Tosyloxy-3-tosyl-3-azapentane (8.92 g 21.6 mmol) was dissolved in pyridine (100 mL) and cooled to -20 °C. In portions tert-butyldimethylsilylchloride (3.58 g, 23.7 mmol) was added and stirring continued for 24 h at -20 °C. Then co-distillation with toluene and evaporation to dryness was followed by chromatographic purification (dichloromethane). Yield 7.8 g (69%); colourless syrup; 1H NMR (400 MHz, CDCl3): δ 7.75, 7.64, 7.34, 7.27 (m, 8 H, Ph), 4.17 (t, 2 H, CH2OTs), 3.68 (t. 2 H, CH2OSi), 3.47, 3.22 (t, 4 H, CH2NTs), 2.45, 2.42 (s, 6 H, CH3Ts), 0.84 (s, 9 H, CH3-t-Bu), 0.20 (s. 6 H, CH,Si), JCH,CH = 6.0 and 6.5 Hz; Anal. calcd. for C24H37NO6S2Si (527.8): C 54.62, H 7.07, N 2.65, S 12.15. Found: C 54.51, H 7.15, N 2.62, S 12.15.
4.35 Bis-1,5-{methyl-2,3-di-O-benzyl-6-deoxy-6-[N-tosyl-N-(5-hydroxy-3-tosyl-3-azapentyl)]amino-4-yloxy-α-d-glucopyranoside}-(3-oxapentane) (37)
The synthesis followed GP4 using 5 (1.51 g, 1.35 mmol), 36 (2.85 g, 5.4 mmol), cesium carbonate (1.76 g, 5.4 mmol), DMF (25 mL), 80 °C, 24 h. The raw material was purified by chromatography (toluene/ethyl acetate 6:1) then dissolved in THF (50 mL) and stirred with tetrabutylammonium fluoride (2.46 g, 7.8 mmol) for 3 h at RT. After evaporation the raw material was purified by chromatography (dichloromethane/acetone 7:1). Yield 1.35 g (62%); glass temperature 51–53 °C; [α]D + 28.9 (c 1.0, in CHCl3); 1H NMR (400 MHz, CDCl3): δ 7.73–7.23 (m, 36 H, Ph), 4.45 (d, 2 H, H-1,1’), 3.40 (dd, 2 H, H-2,2’), 3.83 (dd, 2 H, H-3,3’), 3.11 (dd, 2 H, H-4,4’), 3.62 (m, 2 H, H-5,5’), 3.05 (s, 6 H, OCH3, OCH3’), 4.88 (d, 2 H, OCHABn, Bn’), 4.73 (d, 2 H, OCHABn, Bn’), 4.70 (d, 2 H, OCHBBn, Bn’), 4.57 (d, 2 H, OCHB Bn, Bn’), 4.05–3.15 (m, 28 H, H-6a,6a’, H-6b,b’, OCH2, CH2NTs), 2.40, 2.37 (s, 12 H, CH3Ts); J1,2 = 3.5, J2,3 = 9.5, J3,4 = 9.5, J4,5 = 9.5, 2JBn = 11.0 and 12.0 Hz; 13C NMR (100 MHz, CDCl3): δ 97.67 (C-1,1’), 79.91 (C-2,2’), 81.70 (C-3,3’), 79.71 (C-4,4’), 69.82 (C-5,5’), 55.18 (OCH3, OCH3’), 75.48, 73.28 (4 C, OCH2Ph), 71.99, 70.95 (4 C, OCH2), 61.41 (2 C, CH2OH), 52.40, 51.01, 49.41, 49.01 (8 C, C-6,6’, CH2NTs), 21.52, 21.49 (4 C, CH3Ts), 143.58–137.75 (12 C, q, Ph), 129.83–127.31 (36 C, Ph).
4.36 Bis-1,5-{methyl-2,3-di-O-benzyl-6-deoxy-6-[N-tosyl-N-(5-tosyloxy-3-tosyl-3-azapentyl)]amino-4-yloxy-α-d-glucopyranoside}-(3-oxapentane) (38)
The synthesis followed GP4 using 37 (950 mg, 0.59 mmol), p-toluene sulfonic acid chloride (260 mg, 1.35 mmol), pyridine (10 mL), −20 °C, 5 d. Purification was by flash chromatography (dichloromethane/acetone 30:1). Yield 782 mg (70%); glass temperature 67–69 °C; [α]D + 35.3 (c 1.0, CHCl3); 1H NMR (400 MHz, CDCl3): δ 7.74–7.24 (m, 44 H, Ph), 4.59 (d, 2 H, H-1,1’), 3.43 (dd, 2 H, H-2,2’), 3.86 (dd, 2 H, H-3,3’), 3.11 (dd, 2 H, H-4,4’), 3.68 (m, 2 H, H-5,5’), 3.14 (s, 6 H, OCH3, OCH3’), 4.88 (d, 2 H, OCHABn, Bn’), 4.76 (d, 2 H, OCHABn, Bn’), 4.70 (d. 2 H, OCHBBn, Bn’), 4.62 (d, 2 H, OCHBBn, Bn’), 4.11 (dd, 4 H, CH2OTs), 4.05–3.97 (m, 2 H, OCHA), 3.81–3.75 (m, 2 H, OCHA), 3.63–3.10 (m, 20 H, H-6a,6a, H-6b,b,2, CH2NTs), 2.42, 2.40. 2.38 (s, 18 H, CH2Ts); J1,2 = 3.5, J2,3 = 9.0, J3,4 = 9.5, J4,5 = 9.5, JCH, CH = 5.5 and 6.0, 2JBn = 11.0 and 12.0 Hz; 13C NMR (100 MHz, CDCl3): δ 97.65 (C-1,1’), 79.86 (C-2,2’), 81.59 (C-3,3’), 79.62 (C-4,4’), 70.40 (C-5,5’), 55.25 (OCH3, OCH3’), 75.48, 73.12 (4 C, OCH3Ph), 72.12, 70.96 (4 C, OCH3), 68.42 (2 C, CH3OTs), 50.74, 49.58, 49.41, 48.76 (8 C, C-6,6’, CH2NTs), 21.66, 21.54, 21.50 (6 C, CH3Ts), 143.86–132.50 (16 C, q, Ph), 129.98–127.33 (44 C, Ph); C96H114N4O25S6 (1916.4); FAB-MS: m/z (%) = 1939 (100) [M+Na+].
4.37 Bis{methyl-2,3-di-O-benzyl-6-deoxy-6-[N-tosyl-N-(5-hydroxy-3-tosyl-3-azapentyl)]amino-4-yloxy-α-d-glucopyranoside}-m-xylene (39)
The synthesis followed GP4 using 11 (1.5 g, 1.3 mmol), 36 (1.76 g, 3.3 mmol), cesium carbonate (2.1 g, 6.5 mmol), DMF (15 mL), 80 °C, 24 h. The raw material was purified by chromatography (toluene/ethyl acetate 7:1) then dissolved in THF (50 mL) and stirred with tetrabutylammoniumfluoride (2.2 g, 7.1 mmol) for 24 h at RT. After evaporation the raw material was purified by chromatography (toluene/ethyl acetate 1:1). Yield 1.34 g (63%); glass temperature 63–65 °C; [α]D + 23.1 (c 1.0, CHCl3); 1H NMR (400 MHz, CDCl3): δ 7.66–7.16 (m, 40 H, Ph), 4.51 (d, 2 H, H-1,1’), 3.46 (dd, 2 H, H-2,2’), 3.94 (dd, 2 H, H-3,3’), 3.27 (dd, 2 H, H-4,4’), 3.71 (ddd, 2 H, H-5,5’), 3.10 (s, 6 H, OCH3, OCH3’), 4.94 (d, 2 H, OCHABn, Bn’), 4.76 (d, 2 H, OCHABn, Bn’), 4.92 (d, 2 H, OCHBBn, Bn’), 4.68 (d, 2 H, OCHB Bn, Bn’), 4.72 (d, 2 H, OCHCBn, Bn’), 4.61 (d, 2 H, OCHC Bn, Bn’), 3.65 (dd, 4 H, CH2OH), 3.50–3.12 (m, 16 H, H-6a,6a’, H-6b,b’, OCH2, CH2NTs), 2.40, 2.35 (s, 12 H, CH3Ts); J1,2 = 3.5, J2,3 = 9.5. J3,4 = 9.5, J4,5 = 9.5, J5,6 = 2.5 and 9.5, JCH,CH = 5.0 and 5.5, 2JBn =0.5, 11.0 und 12.0 Hz; 13C NMR (100 MHz, CDC13): δ 97.72 (C-1,1’), 80.01 (C-2,2’), 81.88 (C-3,3)’, 78.96 (C-4,4’), 70.12 (C-5,5’), 55.27 (OCH3, OCH3’), 75.64, 74.58, 73.26 (6 C, OCH2Ph), 61.28 (2 C, CH2OH), 52.37, 50.63, 49.45, 49.44 (8 C, C-6,6’, CH2NTs), 21.52, 21.48 (4 C, CH3Ts), 143.61–135.73 (14 C, q, Ph), 129.83–127.29 (40 C, Ph); Anal. calcd. for C86H102N4O20S4 (1640.0): C 62.98, H 6.27, N 3.42, S 7.82. Found: C 62.86, H 6.41, N 3.39, S 7.84.
4.38 Bis{methyl-2,3-di-O-benzyl-6-deoxy-6-[N-tosyl-N-(5-tosyloxy-3-tosyl-3-azapentyl)]amino-4-yloxy-α-d-glucopyranoside}-m-xylene (40)
The synthesis followed GP4 using 39 (1.2 g, 0.73 mmol), p- toluene sulfonic acid chloride (350 mg, 1.83 mmol), pyridine (40 mL), -20 °C, 6 d. Purification was by flash chromatography (dichloromethane/acetone 35:1). Yield 905 mg (64%); glass temperature 57–61 °C; [α]D + 21.3 (c 1.0, CHCl3); 1H NMR (400 MHz, CDCl3): δ 7.73–7.19 (m, 48 H, Ph), 4.63 (d, 2 H, H-1,1’), 3.49 (dd, 2 H, H-2,2’), 3.97 (dd, 2 H, H-3,3’), 3.29 (dd, 2 H, H-4,4’), 3.75 (m, 2 H, H-5,5’), 3.16 (s, 6 H, OCH3, OCH3’), 4.94 (d, 2 H, OCHABn, Bn’), 4.76 (d, 2 H, OCHABn, Bn’), 4.94 (d, 2 H, OCHBBn, Bn’), 4.70 (d, 2 H, OCHB Bn, Bn’), 4.74 (d, 2 H, OCHCBn, Bn’), 4.67 (d, 2 H, OCHCBn, Bn’), 4.11 (dd, 4 H, CH2OTs), 3.50–3.15 (m, 16 H, H-6a,6a’, H-6b,6b’, OCH2, CH2NTs), 2.42, 2.41, 2.36 (s, 18 H, CH3Ts); J1,2 = 3.5, J2,3 = 9.5, J3,4 = 9.5, J4,5 = 9.5, JCH,CH = 5.5 and 6.0, 2JBn = 10.5, 11.0 and 11.5 Hz; 13C NMR (100 MHz, CDCl3): δ 97.65 (C-1,1’), 80.00 (C-2,2’), 81.82 (C-3,3’), 79.01 (C-4,4’), 70.51 (C-5,5’), 55.31 (OCH3, OCH3’), 75.63, 74.51, 73.11 (6 C, OCH2Ph), 68.46 (2 C, CH2OTs), 50.96, 49.63, 48.79, 46.02 (8 C, C-6,6’, CH2NTs), 21.66, 21.54, 21.49 (6 C, CH,Ts), 145.10–135.59 (18 C, q, Ph), 129.99–127.07 (48 C, Ph); Anal. calcd. for C100H114N4O24S6 (1948.4): C 61.65, H 5.90, N 2.88, S 9.87. Found: C 60.87, H 5.89, N 3.00, S 9.89.
4.39 Cyclo-(N,N’)-bis-1,2-{bis-1,5-[methyl-2,3-di-O-benzyl-6-deoxy-6-(tosyl)amino-4-yloxy-α-d-glucopyranoside]-(3-oxapentane)}-ethane (41)
The synthesis followed GP4 using 5 (200 mg, 0.18 mmol), 33 (270 mg, 0.17 mmol), cesium carbonate (357 mg, 1.1 mmol), DMF (10 mL), 70 °C, 3 d. Purification was by flash chromatography (toluene/ethyl acetate 3:1). Yield 211 mg (52%); glass temperature 83 °C; [α]D + 34.2 (c 1.0, CHCl3); 1H NMR (400 MHz, CDCl3): δ 7.67–7.16 (m, 56 H, Ph), 4.45 (d, 4 H, H-1), 3.40 (dd, 4 H, H-2), 3.77 (dd, 4 H, H-3), 2.95 (dd, 4 H, H-4), 3.57 (ddd≈dd, 4 H, H-5), 3.75 (dd≈d, 4 H, H-6a), 3.17 (dd, 4 H, H-6b), 2.97 (s, 12 H, OCH3), 4.89 (d, 4 H, OCHABn), 4.71 (d, 4 H, OCHaBn), 4.69 (d, 4 H, OCHBBn), 4.59 (d, 4 H, OCHBBn), 3.90–3.84 (m, 4 H, OCHA), 3.70–3.63 (m, 4 H, OCHA), 3.50–3.29 (m, 16 H, OCH2, CH2NTs), 2.34 (s, 12 H, CH3Ts); J1,2 = 3.5, J2,3 = 9.5, J3,4 = 9.5, J4,5 = 9.5, J5,6 = 0, J5,6b = 9.5, 2J6a,6b = 15.0, 2JBn =11.0 and 12.0 Hz; 13C NMR (100 MHz, CDC13): δ 97.49 (4 C, C-1), 79.93 (4 C, C-2), 81.77 (4 C, C-3), 80.49 (4 C, C-4), 69.86 (4 C, C-5), 49.80 (4 C, C-6), 55.00 (4 C, OCH3), 75.51, 73.22 (8 C, OCH2Ph), 72.09, 70.45 (8 C, OCH2), 47.49 (4 C, CH2NTs), 21.50 (4 C, CH3Ts), 143.01–137.13 (16 C, q, Ph), 129.46–127.13 (56 C, Ph); Anal. calcd. for C124H148N4O30S4 (2302.8): C 64.68, H 6.48, N 2.43, S 5.57. Found: C 64.55, H 6.42, N 2.43, S 5.54. FAB-MS: m/z (%) = 2323 (100) [M+Na+], MALD1-MS: m/z = 2322.5 [M+Na+].
4.40 Cyclo-(N,N’)-bis-1,2-{bis[methyl-2,3-di-O-benzyl-6-deoxy-6-tosylamino-4-yloxy-α-d-glucopyranoside)-m-xylene}-ethane (42)
The synthesis followed GP4 using 11 (1.0 g, 0.87 mmol), 35 (1.34 g, 0.87 mmol), cesium carbonate (1.41 g, 4.4 mmol), DMF (20 mL), 80 °C, 3 d. Purification was by flash chromatography (toluene/ethyl acetate 4:1). Yield 892 mg (43%); glass temperature 78–85 °C; [α]D + 23.3 (c 1.0, CHCl3); 1H NMR (400 MHz, CDCl3): δ 7.56–7.09 (m, 64 H, Ph), 4.44 (d, 4 H, H-1), 3.38 (dd, 4 H, H-2), 3.84 (dd, 4 H, H-3), 3.03 (dd, 4 H, H-4), 3.66–3.59 (m, 12 H, H-5, H-6a, H-6b), 2.95 (s, 12 H, OCH3), 4.92 (d, 4 H, OCHABn), 4.73 (d, 4 H, OCHABn), 4.82 (d, 4 H, OCHBBn), 4.56 (d, 4 H, OCHB Bn), 4.66 (d, 4 H, OCHCBn), 4.58 (d, 4 H, OCHC Bn), 3.41–3.35 (m, 4 H, CHANTs), 3.18–3.12 (m, 4 H, CHA NTs), 2.33 (s, 12 H, CH,Ts); J1,2 = 3.5, J2,3 = 9.5, J3,4 = 9.0, J4,5 = 9.5, 2JBn= 10.5, 11.0 and 11.5 Hz; 13C NMR (100 MHz, CDCl3): δ 97.52 (4 C, C-1), 79.99 (4 C, C-2), 81.88 (4 C, C-3), 79.62 (4 C, C-4). 69.77 (4 C, C-5), 50.05 (4 C, C-6), 54.95 (4 C, OCH3), 75.56, 74.81, 73.25 (12 C, OCH2Ph), 47.76 (4 C, CH2NTs). 21.51 (4 C, CH3Ts), 143.02–136.89 (20 C, q, Ph), 129.48–127.55 (64 C, Ph)¸ Anal. calcd. for C132H148N4O28S4 (2366.9): C 66.98, H 6.30, N 2.37, S 5.42. Found: C 66.00, H 6.29, N 2.31, S 5.60. FAB-MS: m/z (%) = 2502 (100) [M+Cs+], 2451 (30) [M+Rb+], 2389 (50) [M+Na+], 2211 (40) [M+-CH3C6H4SO2].
4.41 Cyclo-(N,N’)-bis-1,5-{bis-l,5-[methyl-2,3-di-O-benzyl-6-deoxy-6-tosylamino-4-yloxy-α-d-glucopyranoside]-(3-oxapentane)}-(3-tosyl-3-azapentane) (43)
The synthesis followed GP4 using 5 (200 mg, 0.17 mmol), 38 (308 mg, 0.16 mmol), cesium carbonate (325 mg, 1.0 mmol), DMF (10 mL), 80 °C, 3 d. Purification was by flash chromatography (toluene/ethyl acetate 3:1). Yield 196 mg (45%); glass temperature 75–78 °C; [α]D + 32.2 (c 1.0, CHCl3); 1H NMR (400 MHz, CDCl3): δ 7.69–7.24 (m, 64 H, Ph), 4.57 (d, 4 H, H-1), 3.38 (dd, 4 H, H-2), 3.79 (dd, 4 H, H-3), 3.00 (dd, 4 H, H-4), 3.85–3.10 (m, 44 H, H-5, H-6a, H-6b, OCH2, CH2NTs), 3.06 (s, 12 H, OCH3), 4.89 (d, 4 H, OCHABn), 4.62 (d, 4 H, OCHA Bn), 4.71 (d, 4 H, OCHABn), 4.55 (d, 4 H, OCHB Bn), 2.34, 2.28 (s, 18 H, CH3Ts); J1,2 = 3.5, J2,3 = 9.5, J3,4 = 9.0, J4,5 = 9.0, 2JBn =11.0 and 12.0 Hz; 13C NMR (100 MHz, CDCl3): δ 97.45 (4 C, C-1), 81.78, 80.37, 80.02 (12 C, C-2, C-3, C-4), 69.81 (4 C, C-5), 55.13 (4 C, OCH,), 75.41, 73.05 (8 C, OCH2Ph), 72.14, 70.70 (8 C, OCH2), 50.92, 49.13, 48.68 (12 C, C-6,6’, CH2NTs), 21.43 (6 C, CH,Ts), 143.19–136.64 (20 C, q, Ph), 129.81–127.35 (64 C, Ph).
Acknowledgement
Support of this work by the Deutsche Forschungsgemeinschaft and the Fonds der Chemischen Industrie is gratefully acknowledged.


